Abstract
Purpose
Dysfunctional lymphangiogenesis is pivotal for various pathological processes including tumor lymph node metastasis which is a crucial cause of therapeutic failure for ESCC. In this study, we aim to elucidate the molecular mechanisms and clinical relevance of Zinc-finger protein ZNF468 in lymphangiogenesis and lymphatic metastasis in ESCC.
Methods
Immunohistochemistry, Western blot, Kaplan-Meier plotter analysis and Gene Set Enrichment Analysis were preformed to detect the association of ZNF468 with lymphangiogenesis and poor prognosis in ESCC patients. Foot-pads lymph node metastasis model, tube formation assay, 3D-culture assay and invasion assay were preformed to verify the effect of ZNF468 on lymphangiogenesis and lymph node metastasis. CUT&Tag analysis, immunoprecipitation and mass spectrometry analysis and ChIP-PCR assay were preformed to study the molecular mechanisms of ZNF468 in lymphangiogenesis.
Results
We found that ectopic expression of ZNF468 was correlated with higher microlymphatic vessel density in ESCC tissues, leading to poorer prognosis of ESCC patients. ZNF468 enhanced the capacity of lymphangiogenesis and promoted lymphatic metastasis in ESCC both in vitro and in vivo. However, silencing ZNF468 reversed these phenotypes in ESCC. Mechanically, we demonstrated that ZNF468 recruits the histone modification factors (PRMT1/HAT1) to increase the levels of H4R2me2a and H3K9ac, which then leads to the recruitment of the transcription initiation complex on the VEGF-C promoter, ultimately promoting the upregulation of VEGF-C transcription. Strikingly, the promoting effect of lymphatic metastasis induced by ZNF468 in ESCC was abrogated by targeting PRMT1 using Arginine methyltransferase inhibitor-1 or silencing VEGF-C. Furthermore, we found that the activation of PI3K/AKT and ERK1/2 signaling is required for ZNF468-medicated lymphatic metastasis in ESCC. Importantly, the clinical relevance between ZNF468 and VEGF-C were confirmed not only in ESCC samples and but also in multiple cancer types.
Conclusion
Our results identified a precise mechanism underlying ZNF468-induced epigenetic upregulation of VEGF-C in facilitating lymphangiogenesis and lymph node metastasis of ESCC, which might provide a novel prognostic biomarker and potential therapeutic for ESCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Esophageal cancer ranks the sixth leading cause of cancer-related deaths worldwide (544,000 deaths), which has proved to be a significant global health challenge according to the 2020 Global Cancer Statistics [1, 2]. Esophageal squamous cell carcinoma (ESCC) accounts for almost 90% of esophageal cancer cases in middle and low-income high-risk areas [3, 4]. Currently, esophagectomy was the primary curative treatment for resectable locally advanced esophageal cancer. However, the diagnoses of esophageal cancer often occured at an advanced stage with metastasis, leading to less than 20% for the 5-year survival rates of esophageal cancer patients [5]. The lack of predictive biomarker for ESCC diagnosis has grown to be the most challenging obstacle for improving the prognosis of esophageal cancer patients. It is well known that lymphangiogenesis in ESCC contributed to lymph node metastasis and subsequently stands for a large burden for the malignant development and poor prognosis of ESCC [6,7,8]. Hence, it is an urgent need to unveil the molecular mechanism underlying ESCC lymphangiogenesis as well as lymph node metastasis, which might offer a practical therapeutic target to ameliorate ESCC prognosis in clinical practice.
The lymphatic system plays a crucial role in maintaining cellular homeostasis. However, abnormal lymphangiogenesis is known to contribute to a range of pathological processes, such as inflammatory diseases and tumor metastasis [9, 10]. Deregulation of vascular endothelial growth factors, including VEGF-C, has been reported to play an important independent role for lymphangiogenesis and correlated with the dissemination of tumour cells to regional lymph nodes. Mandriota et al. firstly demonstrated that VEGF-C effectively activated lymphangiogenesis growth factor signaling by interacting with the corresponding receptor (VEGFR-3) in a RipVEGF-C transgenics mice [11]. Multiple studies have demonstrated that elevated VEGF-C are associated with lymph-node metastasis and contributed to poor prognosis of cancers patients [12]. Tang and colleagues found 80.4% of cancer tissues exhibit strong VEGF-C immunoreactivity in 51 cases of pancreatic cancer tissues by utilizing immunohistochemical analysis [13]. Furthermore, higher VEGF-C-positive staining was significantly correlated with poorer histologic grade, lymphatic invasion, liver metastasis and Duke’s stage in advanced colorectal carcinoma [14]. The level of VEGF-C expression is linked to lower 5-year survival rates in ESCC [15]. Importantly, Kaplan-Meier analyses revealed that high expression of VEGF-C promotes lymphangiogenesis and has been shown to closely relate with shorter disease-free survival (DFS) (P = 0.006) and overall survival (OS) (P = 0.017) in ESCC patients [16]. These studies emphasized the significant role of VEGF-C, which could potentially serve as a reliable marker to predict the prognostic characteristics of various types of cancer. Therefore, elucidating the definite mechanism of VEGF-C dysregulation might provide a new therapeutic target for inhibiting lymph node metastasis and offer a more efficacious strategy for cancer clinical intervention.
Histone modification is an important mechanism for regulating protein stability and play crucial role in disease development, including carcinogenesis, immune disorders cell and proliferation [17, 18]. Histone methylation is one of the epigenetic modifications in tumor development by controlling gene repression and gene activation rely on adding or removing lysine and arginine residues within proteins respectively [19]. It is usually believed that demethylation or trimethylation of H3K4 is associated with transcriptional activation, while demethylation or trimethylation of H3K9 and H3K27 are linked to transcriptional inhibition [20, 21]. For example, PRMT1 suppresses the protein levels of cGAS by inducing asymmetric dimethylation on the arginine residues, subsequently suppressing anti-tumor immunity [22]. Additionally, overexpression of enhancer of zeste homolog 2 (EZH2) fosters levels of H3K27me3 and promotes KRT14 gene expression by reprogramming the regulatory mode of transcription factor SP1, which fosters metastasis ability of triple-negative breast cancer [23]. These studies demonstrated that aberrant histone modification participates in regulating gene expression and contribute to malignant development of tumors. Nowadays, an increasing body of evidence suggests that drugs targeting epigenetics have shown promising therapeutic effects in combination with other therapies, holding tremendous potential for the prevention and treatment of tumors.
ZNF468 is a novel member of the zinc finger protein family which constitutes the largest transcription factor family with finger domain and plays a significant role in multiple biological processes [24]. Recent studies have identified diverse zinc-finger proteins as critical regulators for cancer initiation and progression [25, 26]. For instance, Zhang et al. reported that the potential tumor suppressor ZNF671 is downregulated in nasopharyngeal carcinoma and promotes cell proliferation and tumorigenicity [27]. Another study has showed that ectopic expression of ZNF575 inhibited colorectal cancer cell proliferation, promoted cell apoptosis, and impaired tumor growth [28]. Furthermore, Hu and colleagues demonstrated that ZNF668 can inhibit cancer development through the p53 pathway and act as a potential tumor suppressor in breast cancer [29]. He and colleagues showed that ZNF154 functions as a tumor suppressor in ESCC and correlates with poor prognosis [30]. The above studies suggest the important role of zinc finger protein family members in the development of cancer. Whereas the associated biological function and molecular mechanism remains of ZNF468 underlying progression of ESCC remain largely unknown. Exploring the potential role of ZNF468 as a therapeutic target for ESCC may contribute to the development of innovative therapeutic strategies and improve the prognosis of patients with ESCC.
2 Materials and methods
2.1 Cell lines and Cell Culture
ECA109 and KYSE30 cell lines were obtained from the German Resource Centre for Biological Material. The culture conditions for cell lines are Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum (Life Technologies, USA) and cultured under 5% CO2 in air at 37 °C [31]. All cell lines were identified by short tandem repeat (STR) fingerprinting. Cell transfection experiment was performed by using ProFect-3K Transfection Reagent Kit (Celfolio, Cat No:82-300015, Macrollbiotechnology Ltd.)
2.2 Western blot
Western blot analysis was performed according to our previous published studies [32]. All the antibody using in our study was: anti-flag (Sigma, F3165), anti-ZNF468 (Abcam, ab88862), anti-VEGF-C (Abcam, ab9546), anti-HA (Abcam, ab9110), anti-myc (Abcam, ab32072) anti-GST (Abcam, ab111947). After incubation with the target protein, the membrane was re-incubated with GAPDH as a control.
2.3 Immunohistochemistry (IHC)
Immunohistochemistry analysis was performed according to our published studies [32]. Anti-ZNF468, anti-VEGF-C, anti-Lyve-1 antibody was used in this study. According to the staining intensity of the membrane, nucleus, or cytoplasm, cells are classified into different levels, and the proportion of cell count in each level is counted (for example, level 1 (0–10% intensity) is 1 point, level 2 (11–40% intensity) is 2 points, level 3 (41–70% intensity) is 3 points, and level 4 (71–100% intensity) is 4 points). Finally, all scores are added up to obtain the total score [33].
2.4 Invasion assay
1 × 104 ESCC indicated cells were cultured in Transwell filter (pre-coated with Matrigel) (BD Biosciences). After fixed and soak in hematoxylin for 5 min, the cells passing through the Matrigel were count and take the average value. The number of cells passing through 10 visual fields was calculated for statistics.
2.5 CUT&tag analysis
CUT&Tag assay was performed by using Hyperactive® Universal CUT&Tag Assay Kit for Illumina (TD904, Vazyme) and the experimental steps are strictly carried out according to the manufacturer’s instructions. In short, we collected 1 × 106 indicated cells and centrifuge at 2500 rpm× g for 5 min, discard the supernatant and add NE buffer and centrifuge at 2500 rpm× g for 5 min, and discard all the supernatant. Then add 100 µl activated ConA Beads mix with the cell nucleus them incubate at room temperature for 10 min. Subsequently, join 50 µl precooled ZNF468 Antibody resuspended cells (nuclei)-magnetic bead complexes overnight. Add a secondary antibody to mix the antibody with the cell (nucleus) magnetic bead complex evenly and incubate by rotating at room temperature for 30–60 min. Subsequently incubate with pA/G-Tnp transposons for 1 h then incubate with Extract DNA Beads at 55 ℃ for 10 min. The fragmented DNA will be used for qPCR quantitative detection. Finally, library quality testing and CUT&Tag -seq analysis were performed. The raw sequencing data in the current study can be downloaded from the NCBI’s Sequence Read Archive (SRA) using accession code PRJNA1017969.
2.6 Streptavidin affinity purification of dCas9-captured proteins
The biotinylated dCas9-based method was performed according to our published studies [34]. A total of 5 × 107 FB-dCas9-expressing Eca109 cells transduced with VEGF-C-specific sgRNA were used for detecting potential proteins which located on the VEGF-C promoter and analyzed by western blot and IP-MS analysis (Shanghai Applied Protein Technology Co.Ltd.). VEGF-C-specific sgRNA was: GTGTCCGTCTACAGATGTGGNGG.
2.7 Immunoprecipitation and mass spectrometry (MS) analysis
Firstly, cell lysates from were incubated with anti-HA (Abcam, ab9110), anti-myc (Abcam, ab32072), or anti-flag (Sigma, F3165) conjugated agarose beads, respectively. The washing solution runs through SDS-PAGE gel for dyeing and decolorization, all bands are cut and decolorized with the decolorization solution. Proteomics grade trypsin treatment was centrifugally dried and re dissolved in Nano LC mobile phase A (0.1% formic acid/water) for bottle loading and subjected to LC-MS/MS analysis (Wininnovate Bio (Shenzhen, China).
2.8 Chemical reagents
PRMT1 inhibitor (Arginine methyltransferase inhibitor-1, AMI-1, S7884), PI3K inhibitor (LY294002, S1105), anti-VEGFR-3 antibodies (Axitinib, S1005) were purchased from Selleck.
2.9 Statistics
Univariate and multivariate analysis of different prognostic parameters in patients with ESCC was analyzed with Cox- proportional hazards model. Student’s 2-tailed t test was used to compare whether the difference between two averages is significant. SPSS 21.0 statistical software was used in our study. Data represent mean ± SD.
3 Result
3.1 Upregulation of ZNF468 associates with microlymphatic vessel density (MLD) and closely related to poor prognosis in ESCC patients
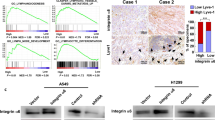
The relationship between ZNF468 and lymphatic metastasis was firstly investigated in ESCC tissues and found that ZNF468 was markedly upregulated in ESCC with lymph node metastasis (LN+), compared to ESCC tissues without lymph node metastasis (LN−) (Fig. 1A). Strikingly, the protein levels of ZNF468 were closely related to Lyve1, markers of microlymphatic vessel density in ESCC (Fig. 1B). The high level of both ZNF468 expression and Lyve1 staining indicated shorter metastasis-free survival time compared with lower level of ZNF468 expression and Lyve1 in ESCC patients (P = 0.017, HR (95% CI) = 1.78 (1.17–2.40)) (Fig. 1C). Consistent with this result, the mRNA levels of ZNF468 were significantly higher in ESCC with lymph node metastasis (N1), compared to ESCC tissues without lymph node metastasis (N0) (Fig. 1D). Further, we found that high level of ZNF468 was closely related to metastasis-free survival (P = 0.008, HR (95% CI) = 3.23 (2.74–3.73)) in ESCC patients in public dataset, suggesting that ZNF468 might act as an independent prognostic factor in ESCC (Fig. 1E and Supplementary Table 2). Moreover, ZNF468 overexpression significantly correlated with gene signatures associated with lymphangiogenesis and metastasis (GO_LYMPHANGIOGENESIS, CLASPER _ LYMPHATIC _ VESSELS _ DURING _ METASTASIS _ DN, BIDUS _ METASTASIS _ UP, BIDUS _ METASTASIS _ DN) in ESCC tissues generated from the Cancer Genome Atlas (TCGA) dataset (Fig. 1F). Collectively, these results suggest that ZNF468 was upregulated in lymph node metastatic-ESCC tissues, and overexpression of ZNF468 associates with lymphangiogenesis and poor prognosis in ESCC patient.
Upregulation of ZNF468 associates with microlymphatic vessel density (MLD) and correlates with poor prognosis in ESCC patients. A Immunohistochemistry staining analysis ZNF468 expression in ESCC tissues with lymph node metastasis (LN−) or without lymph node metastasis (LN+). ***P < 0.001. B Immunohistochemistry staining analysis ZNF468 and Lyve1 expression in ESCC tissues. **P < 0.01. C The Kaplan-Meier survival curves compare ESCC patients with both higher ZNF468 and Lyve1 or lower ZNF468 and Lyve1 expression levels (n = 74; P = 0.017). D mRNA expression of ZNF468 in ESCC tissues with lymph node metastasis (LN1 + 2+3) or without lymph node metastasis (LN0) in TCGA dataset. E Survival analysis compare ESCC patients with higher ZNF468 and lower ZNF468 expression levels in TCGA dataset (n = 32; P = 0.008). F GSEA analysis the correlation analysis between the mRNA levels of ZNF468 and lymphangiogenesis and metastasis in TCGA datasets
3.2 Ectopic expression of ZNF468 promotes lymphangiogenesis and lymph node metastasis in vivo
Next, a foot-pads lymph node metastasis model was used to verify effect of ZNF468 on lymph node metastasis in vivo. ECA109/Vector, ECA109/ZNF468, ECA109/Control and ECA109/ZNF468-shRNA stable cell lines has been established perspectively and injected the foot pads separately into nude mice (n = 5/group; Fig. 2A and Supplementary Fig. 1). We firstly found that the volumes of the popliteal lymph nodes were significantly smaller in ZNF468-shRNA cohort but significantly enlarged in ZNF468-overexpression group compared to control (Fig. 2B). Furthermore, the Lyve-1-positive microvessels is abnormally elevated in tumors formed by exogenous high expression of ZNF468, but significantly decreased in the group with low expression of ZNF468 (Fig. 2C). Meanwhile, we found that lymph nodes volume and metastatic ratios of popliteal lymph nodes were higher in mice burdened with ZNF468-positive tumor cells, but lower in mice with ZNF468-shRNA cells than control cells (Fig. 2D). Strikingly, the tumors formed by ECA109/ZNF468 cells were larger and heavier while formed by ECA109/ZNF468-shRNA cells were smaller and lighter compared to the corresponding control group (Fig. 2E-G). However, there was not significantly effects on body weight of mice both in ECA109/ZNF468 and ECA109/ZNF468-shRNA group compared with control groups (Supplementary Fig. 2).
Ectopic expression of ZNF468 promotes lymphangiogenesis and lymph node metastasis in vivo. A Popliteal lymph node metastasis model used in our study, **P < 0.01. B Lymph nodes in indicated group (left) and quantification of lymph nodes volume (right), **P < 0.01. C Lyve-l expression in the indicated group, **P < 0.01. D ZNF468 expression in indicated group by performing IHC assay (left). Lymph node metastasis ratio in the indicated cells (Right). E ESCC cells were subcutaneous injected into nude mice (n = 5/group). Images of the tumors from all mice in each group. F Changes in tumor volume of mice in different groups. G Mouse tumor tumors in different groups, **P < 0.01
3.3 Overexpression of ZNF468 promotes lymphangiogenesis and increases invasiveness of ESCC cells in vitro
Furthermore, the biological function of ZNF468 on lymphangiogenesis and ESCC cell invasiveness has been investigated in vitro. The culture medium derived from ZNF468 exogenous high expression ESCC cells has a significant ability to induce tube formation in human lymphatic endothelial cells (HLEC) (Fig. 3A). In addition, the migration ability of HLEC was significantly improved after treated with the medium produced by ZNF468-overexpressing ESCC cells, while reduced after treated with ZNF468-inhibting ESCC cells (Fig. 3B). Meanwhile, ZNF468-overexpressed cells significantly enhanced the invasion and migration capacity of ESCC cells, as displayed by 3D-culture and transwell assays (Fig. 3C and Supplementary Fig. 3). The above results suggest that ectopic expression of ZNF468 plays a crucial role in promoting lymphatic vessel formation and metastasis of ESCC cells in vitro.
3.4 ZNF468 binds to the VEGF-C promoter and upregulates the expression of VEGF-C
CUT&Tag-seq has been conducted by using ZNF468 antibody (Fig. 4A) and demonstrated that genes with GO biological process terms “lymphangiogenesis”, “lymph vessel morphogenesis” and “lymph node development” were enriched (Fig. 4B). Multiple reports have suggested that VEGF-C expression is biologically and clinically associated with lymphangiogenesis and contributed to lymphatic metastatic of tumor cells [35, 36]. Interestingly, 3 potential ZNF468 binding sites were predicted in the promoter of VEGF-C in the JASPAR database (Fig. 4C). ZNF468 was found to be greatly enriched in the VEGF-C promoter sequence by performing ChIP-PCR assay (Fig. 4D). The dual luciferase assay showed that exogenous overexpression of ZNF468 significantly enhanced the luciferase activity of the VEGF-C promoter, but inhibition of ZNF468 expression showed the opposite (Fig. 4E). In addition, the mutation of the ZNF468 binding site did not affect the luciferase activity of the VEGF-C promoter (Fig. 4E). Meanwhile, significant upregulation of VEGF-C expression was observed in ESCC cells which overexpressing ZNF468, but downregulation was observed in ESCC cells with ZNF468 silencing expression (Supplementary Fig. 4A and 4B). Additionally, ChIP-PCR assay revealed that ZNF468 bound to the region 3 within the VEGF-C promoter region (Fig. 4F). Furthermore, as illustrated in Fig. 4G and Supplementary Fig. 4C, we found that the luciferase activities driven by serial DNA fragments cloned from the VEGF-C promoter region, including fragments covering nucleotides − 1886 to -105 (P1), -942 to -105 (P2), -956 to -508 (P5), were significantly increased in the ZNF468 upregulation group but decreased in the ZNF468 knockdown group. Thus, these results indicate that ZNF468 can bind to the promoter of VEGF-C and transcriptionally upregulate VEGF-C expression in ESCC cells.
ZNF468 binds to the VEGF-C promoter and upregulates the levels of VEGF-C. A Distribution of ZNF468 binding sites in the promoter (− 5 kb to + 5 kb), gene body, and intergenic regions (left). ZNF468 binding regions were enriched in the gene promoters (middle). Heatmap of the ChIP enrichment signal in ZNF468-binding regions (right). B GO Enrichment of ZNF468 reuglated Genes (% count). C ZNF468 binding sites in VEGF-C promoter were predicted using the JASPAR database. D ChIP-PCR assay performed by IgG or ZNF468 antibody. **P < 0.01. E Relative luciferase activities in the indicated cells (left). **P < 0.01. F ChIP-PCR analysis ZNF468 located in the F5 region of VEGF-C promoter. **P < 0.01. G Relative luciferase assay analyzes the specific region of ZNF468 located in VEGF-C promoter in the indicated cells. **P < 0.01
3.5 Histone modification factors: PRMT1 and HAT1 are involved in ZNF468-mediated transcriptional upregulation of VEGF-C
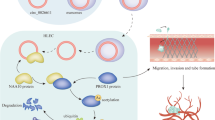
A biotinylated dCas9-based method combined with mass spectrometry analysis were used to explore the mechanism of ZNF468 upregulating VEGF-C level in ESCC. It was found that PRMT1 may be a potential factor for VEGF-C transcription regulation in ESCC (Fig. 5A). Interestingly, we found that ZNF468 directly interacts with PRMT1 by performing co-immunoprecipitation and far-western blotting assay in ESCC cells (Fig. 5B-C). We further identified specific ZNF468 regions that interact with PRMT1. We found that ZNF468-Full, ZNF468-F1, ZNF468-F2 and ZNF468-F3, but not ZNF468-F4, was interacted with PRMT1 in ESCC cells (Fig. 5D and Supplementary Fig. 5A). Moreover, we demonstrated that silencing PRMT1 or ZNF468 significantly reduced the levels of H4R3me2a but have no effect on H4R3me1 levels of the VEGF-C promoter (Fig. 5E). It has been reported that dimethyl H4R3 modification could induce H3K9/14 acetylation or H4K8/12 acetylation, resulting in the transcription initiation complex binding to promoters and initiating target gene transcription [37]. In line with these reports, we found that inhibition of PRMT1 by siRNA or with PRMT1 inhibitor (2.5mM AMI-1 treatment for 48 h) not only reduced the acetylation levels of H3K9 on the VEGF-C promoter but also decrease the luciferase activity of the VEGF-C promoter and mRNA/protein levels of VEGF-C (Fig. 5F and Supplementary Fig. 5C-E). HAT1 is the histone acetyltransferase discovered which is critical for maintenance of H3K9ac, H4K5ac and H4K12ac at target genes. Interestingly, we found that HAT1 has also been predicted to be a potential factor for VEGF-C transcription regulation in ESCC and clarified the direct interaction between ZNF468 and HAT1 by performing co-immunoprecipitation assay in ESCC cells (Fig. 5A and Supplementary Fig. 5B). Inhibition of HAT1 by siRNA not only reduced the acetylation levels of H3K9 on the VEGF-C promoter but also decrease the luciferase activity of the VEGF-C promoter and mRNA/protein levels of VEGF-C (Fig. 5F and Supplementary Fig. 5C-E). Furthermore, inhibition of PRMT1 or HAT1 significantly decreased the abundance of transcription initiation complex such as TBP, TAF1, and POII on the VEGF-C promoter (Fig. 5G). Functionally, silencing PRMT1/HAT1 or treatment with AMI-1 significantly impaired the ability of ZNF468-induced HLEC tube formation and migration and invasive of ESCC cells (Fig. 5H and I and Supplementary Fig. 5F). Collectively, these results demonstrate that the combination of PRMT1-induced H4R3 methylation and HAT1-induced H3K9 histone acetylation jointly promotes VEGF-C transcription in ZNF468-overexpressing ESCC cells.
Histone modification factors: PRMT1 and HAT1 are involved in ZNF468-mediated transcriptional upregulation of VEGF-C. A Schematic of searching for key factors regulating VEGF-C. B Endogenous ZNF468 interacted with endogenous PRMT1 in ESCC cells. C Far-western blot showing that ZNF468 interacted with PRMT1. D PRMT1 interacted with F2 region between aa 8 and aa 217 of ZNF468. E Enrichments of H4R2me2a and H4R3me1 on VEGF-C promoter region in the indicated group. **P < 0.01. F Enrichments of H3K9ac, H3K14ac, H4K5ac, H4K8ac, H4K12ac on VEGF-C promoter region in the indicated ESCC cells. G Enrichments of TBP, POII, TAF-1 on VEGF-C promoter region in the indicated ESCC cells. **P < 0.01. H The migration ability of HLECs in the indicated ESCC cells. **P < 0.01. I The tube formation ability of HLECs in the indicated ESCC cells. **P < 0.01
3.6 PRMT1/VEGF-C is required in ZNF468 induced lymph node metastasis in ESCC
We next studied whether PRMT1 and VEGF-C are required for ZNF468-induced lymphatic metastasis in ESCC. Inhibition of PRMT1 with AMI-1 or silencing VEGF-C dramatically reduced the tube formation ability of HLECs (Fig. 6A). The migration ability of ESCC cells was also significantly repressed when treated with PRMT1 inhibition or silencing VEGF-C (Fig. 6B). Furthermore, inhibition of PRMT1 with AMI-1 or silencing VEGF-C dramatically reduced lymph nodes volumes and decreased the number of ZNF468-positive tumor cells in the lymph nodes in the indicated mice (Fig. 6C). Meanwhile, we found that inhibition of PRMT1 with AMI-1 or silencing VEGF-C significantly decreased the Lyve-1 staining in tumors formed by ECA109/ZNF468 (Fig. 6D). Together, PRMT1 and VEGF-C play an important role in ZNF468 induced lymphangiogenesis and lymphatic metastasis of ESCC, both in vitro and in vivo.
PRMT1/VEGF-C is required in ZNF468 induced lymph node metastasis in ESCC. A The tube formation ability of HLECs in the indicated ESCC cells. **P < 0.01. B The migration ability of ESCC cells in the indicated group. **P < 0.01. C Lymph node metastasis ratio in the indicated cells. D Lyve-l expression in the indicated group, **P < 0.01
3.7 ZNF468-mediated lymphangiogenesis and lymphatic metastasis is dependent on the PI3K/AKT and ERK1/2 signaling pathway
It is well known that abnormal activation of PI3K/AKT and ERK1/2 signaling pathway was contributed to lymphangiogenesis and eventually leading to lymphatic metastasis in tumor [38, 39]. Interestingly, we found that phosphorylation of PI3K, AKT and ERK1/2 was markedly increased in HLECs transfected with ZNF468 but reduced in HLECs transfected with ZNF468-shRNA (Fig. 7A). However, increase levels of phosphorylation of PI3K, AKT and ERK1/2 induced by ZNF468 was significantly repressed in HLECs when treatment with VEGF-C siRNA or with anti-VEGFR-3(Fig. 7A). Furthermore, the tube formation ability of HLECs cells, and the migration ability of HLECs was significantly repressed by using the PI3K inhibitor (10 µg/ml LY294002 treatment for 48 h) or Axitinib, which is a potent and selective inhibitor of vascular endothelial growth factor (VEGF) receptor tyrosine kinases 1 to 3 (100 ng/mL treatment for 45 min) in HLECs (Fig. 7B-C). Therefore, these results suggest that ZNF468 induces lymphangiogenesis and lymphatic metastasis in ESCC via the PI3K/AKT and ERK1/2 signaling pathway.
ZNF468-mediated lymphangiogenesis and lymphatic metastasis is dependent on the PI3K/AKT and ERK1/2 signaling pathway. A Western blotting analysis of p-PI3K, p-AKT, p-ERK1/2 protein in the indicated cells. GAPDH served as a loading control. B The tube formation ability of HLECs in the indicated groups. **P < 0.01. C The migration ability of HLECs in the indicated groups
3.8 Clinical significance of ZNF468 and VEGF-C in ESCC
As shown in Fig. 8A and B, the expression level of ZNF468 was closely related to the expression of VEGF-C in 9 newly collected clinical ESCC samples (r = 0.692, P = 0.024) and in paraffin tissues (P < 0.01). In order to further prove the clinical relevance, we verified the clinical relevance of ZNF468 and VEGF-C in multiple published profiles: Esophageal cancer (GSE20347; r = 0.556; p < 0.001; n = 34; GSE 23400; r = 0.401; p < 0.001; n = 106); Pancreatic Cancer (GSE17891; r = 0.791; p < 0.001; n = 47); Gastric cancer (GSE29272; r = 0.419; p < 0.001; n = 268); Liver hepatocellular carcinoma(TCGA; r = 0.303; p = 0.027; n = 358) (Fig. 8C). Furthermore, we found that ZNF468 was overexpressed in multiple cancers (Supplementary Fig. 6A) and Kaplan-Meier analyses revealed that ZNF468 were associated with shorter overall survival and disease-free survival not only in ESCC (Supplementary Fig. 6B) but also in other cancer types such as PAAD, LIHC, LGG, LUSC, BRCA, Sarcoma in TCGA public dataset (Supplementary Fig. 6C). Therefore, the clinical significance of ZNF468 and VEGF-C expression extends beyond ESCC to include various other types of cancer.
Clinical significance of ZNF468 and VEGF-C in ESCC. A ZNF468 levels were positively associated with VEGF-C expression in human ESCC specimens. B Analysis of expression (upper) and correlation (down) of VEGF-C with ZNF468 expression in 9 freshly collected ESCC samples. C ZNF468 expression correlated positively with VEGF-C expression in published profiles of multiple cancers. **P < 0.01. D Schematic diagram illustrating that ZNF468 promotes lymphangiogenesis and lymphatic metastasis via epigenetic upregulation of VEGF-C and that targeting ZNF468/PRMT1/HAT1/VEGF-C axis prohibits tumor-associated lymphagiogenesis and suppresses lymphatic metastasis in ESCC.
4 Discussion
Clinically, regional lymph node metastasis plays a key role in the diagnosis, staging and treatment of tumors. Malignant tumors of epithelial origin tend to develop local lymph node metastasis, which has great influence on clinical prognosis of tumor patients [40]. Although the phenomenon of tumor cells metastasized through lymphatic vessels has been discovered for years, the exact mechanism is still not completely clear. In our study, we firstly provide evidence that overexpression of ZNF468 was associated with high microlymphatic vessel density (MLD) and contributed to poorer prognosis of ESCC patients. Mechanistically, ZNF468 enhanced lymphangiogenesis and lymphatic metastasis in ESCC via forming a complex with PRMT1 and HAT1 on the VEGF-C promoter, eliciting epigenetic histone H4R3me2a/H3K9ac-mediated transactivation of VEGF-C. Furthermore, we found that ZNF468-mediated lymphangiogenesis and lymphatic metastasis dependent on the PI3K/AKT and ERK1/2 signaling (Fig. 8D). Therefore, this study unveils a novel mechanism underlying lymphatic metastasis in tumors and inhibition of VEGF-C or PRMT1 emerges as a promising treatment strategy for patients with ESCC.
It has been well established that VEGF-C/VEGFR-3 signal pathway plays an important role in lymphangiogenesis and involved in many biological processes, such as cell proliferation and invasion [41, 42]. Overexpression of VEGFR-3 can destroy the adhesion junctions between lymphatic endothelial cells, which leads to the loss of the barrier function of lymphatic endothelial cells, subsequently provides the possibility for tumor cells to infiltrate and escape lymphatic ducts [43]. There are several small-molecule that targeting VEGFR-3, such as mF431C1, a monoclonal antibody targets VEGFR-3 and is able to specifically inhibit the activity of VEGFR-3 [44]. In ovarian cancer tissue sections, peptide III was found to co-localize with VEGFR-3 in lymphatic endothelial cells, suggesting that polypeptide III may be a potential peptide small molecule targeting VEGFR-3 for the treatment of ovarian cancer [45]. Fucoidan, discovered in 2016, can inhibit lymphangiogenesis in xenograft mice in vivo by degrading VEGFR-3 and PROX1, subsequently preventing tumor metastasis [46]. Sunitinib, a drug targeting VEGFR2, also has the same inhibitory effect on VEGFR-3, which can obviously inhibit the formation and growth of lymphatic vessels and inhibited the growth of transplanted tumors formed by VEGFR-C-overexpressing tumor cells in mice [47]. In addition, other anti-angiogenesis tyrosine-kinase inhibitor such as sorafenib and Vatalanib can also target VEGFR-3 and inhibit its activity [48]. However, blocking VEGFR-3 signal pathway by using sunitinib or sorafenib have potential side effects such as arterial hypertension, thyroid dysfunction and metabolic disorders [49]. Therefore, it is of urgent need to identify more effective therapeutic targets that regulate VEGFR-3 in an appropriate manner. At present, we found that ZNF468 overexpressing enhanced the capacity of lymphangiogenesis and lymphatic metastasis in ESCC both in vitro and in vivo via epigenetic upregulating VEGF-C expression in ESCC and subsequently activating the PI3K/Akt and ERK1/2 signaling in HLEC. Inhibited the expression of VEGF-C or PRMT1 significantly repressed the capacity of lymphangiogenesis and lymphatic metastasis induced by ZNF468, suggesting that ZNF468 could contribute to VEGF-C/VEGFR-3 signaling activation and thereby represent a novel target for ESCC treatment.
Although great progress has been made in the etiology research of gastrointestinal malignancies and the comprehensive treatment based on surgical resection, chemotherapy and radiotherapy, the treatment of tumors still faces great challenges, due to the significant differences in treatment sensitivity and adverse reactions among different individuals [50]. For example, the effectiveness of commonly used anti-tumor chemotherapy drugs for patient treatment is not only less than 70%, but due to the lack of genetic analysis of individualized chemotherapy drugs, about 20- 35% of patients may have received inappropriate drug treatment [51, 52]. Due to the lack of predictive biomarkers for early diagnosis, the survival prognosis and quality of life of gastrointestinal cancer patients remain poor, emphasizing the necessity of early detection and intervention. Therefore, searching for diagnostic biomarkers related to cancer and comparing genetic differences between individuals may provide new directions for the development of clinical treatment of tumors, ultimately guiding individualized clinical treatment of tumors. In our study we reported that ZNF468 was overexpressed in ECSS and associates with lymphangiogenesis and lymph node metastasis with poor prognosis in ESCC patient. Interestingly, we also found that ZNF468 was upregulated in multiple cancer types, and high level of ZNF468 was associated with shorter overall survival and disease-free survival not only in ESCC but also in multiple cancers such as PAAD, LIHC, LGG, LUSC, BRCA, Sarcoma in TCGA public dataset. More importantly, we found that the expression of ZNF468 and VEGF-C was in closely relation in esophageal cancer and many other tumors. This suggests the potential clinical implications of ZNF468 in prognosis evaluation and personalized treatment of various types of cancers. Therefore, the molecular mechanism and clinical significance underlying ZNF468 regulated lymph node metastasis in others type of cancer is worthy of further investigation.
Data availability
No datasets were generated or analysed during the current study.
References
H. Sung, J. Ferlay, R.L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal et al., Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021)
A.P. Thrift, Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat. Rev. Gastroenterol. Hepatol. 18, 432–443 (2021)
X.C. Li, M.Y. Wang, M. Yang, H.J. Dai, B.F. Zhang, W. Wang et al., A mutational signature associated with alcohol consumption and prognostically significantly mutated driver genes in esophageal squamous cell carcinoma. Ann. Oncol. 29, 938–944 (2018)
J. Chang, W. Tan, Z. Ling, R. Xi, M. Shao, M. Chen et al., Genomic analysis of oesophageal squamous-cell carcinoma identifies alcohol drinking-related mutation signature and genomic alterations. Nat. Commun. 8, 15290 (2017)
M.A. Shah, E.B. Kennedy, D.V. Catenacci, D.C. Deighton, K.A. Goodman, N.K. Malhotra et al., Treatment of locally Advanced Esophageal Carcinoma: ASCO Guideline. J. Clin. Oncol. 38, 2677–2694 (2020)
S.F. Schoppmann, B. Jesch, J. Zacherl, M.F. Riegler, J. Friedrich, P. Birner, Lymphangiogenesis and lymphovascular invasion diminishes prognosis in esophageal cancer. Surgery. 153, 526–534 (2013)
Z. Lin, W. Chen, Y. Chen, X. Peng, K. Zhu, Y. Lin et al., A new classification of lymph node metastases according to the lymph node stations for predicting prognosis in surgical patients with esophageal squamous cell carcinoma. Oncotarget. 7, 76261–76273 (2016)
J. Yang, Z. Lu, L. Li, Y. Li, Y. Tan, D. Zhang et al., Relationship of lymphovascular invasion with lymph node metastasis and prognosis in superficial esophageal carcinoma: systematic review and meta-analysis. BMC Cancer. 20, 176 (2020)
K. Vaahtomeri, K. Alitalo, Lymphatic vessels in Tumor Dissemination versus Immunotherapy. Cancer Res. 80, 3463–3465 (2020)
M.A. Swartz, The physiology of the lymphatic system. Adv. Drug Deliv Rev. 50, 3–20 (2001)
S.J. Mandriota, L. Jussila, M. Jeltsch, A. Compagni, D. Baetens, R. Prevo et al., Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 20, 672–682 (2001)
Q. Ma, L.C. Dieterich, K. Ikenberg, S.B. Bachmann, J. Mangana, S.T. Proulx et al., Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread. Sci. Adv. 4, eaat4758 (2018)
R.F. Tang, J. Itakura, T. Aikawa, K. Matsuda, H. Fujii, M. Korc et al., Overexpression of lymphangiogenic growth factor VEGF-C in human pancreatic cancer. Pancreas. 22, 285–292 (2001)
A. Furudoi, S. Tanaka, K. Haruma, Y. Kitadai, M. Yoshihara, K. Chayama et al., Clinical significance of vascular endothelial growth factor C expression and angiogenesis at the deepest invasive site of advanced colorectal carcinoma. Oncology. 62, 157–166 (2002)
I. Omoto, M. Matsumoto, H. Okumura, Y. Uchikado, T. Setoyama, Y. Kita et al., Expression of vascular endothelial growth factor-C and vascular endothelial growth factor receptor-3 in esophageal squamous cell carcinoma. Oncol. Lett. 7, 1027–1032 (2014)
Y.Z. Zeng, Y.Q. Zhang, X.Q. Lin, J.Y. Chen, F. Zhang, J.L. Zhu et al., Co-expression of VEGF-C and survivin predicts poor prognosis in esophageal squamous cell carcinoma. Transl Cancer Res. 10, 210–222 (2021)
S. Zaib, N. Rana, I. Khan, Histone modifications and their role in Epigenetics of Cancer. Curr. Med. Chem. 29, 2399–2411 (2022)
G. Millán-Zambrano, A. Burton, A.J. Bannister, R. Schneider, Histone post-translational modifications - cause and consequence of genome function. Nat. Rev. Genet. 23, 563–580 (2022)
E.M. Michalak, M.L. Burr, A.J. Bannister, M.A. Dawson, The roles of DNA, RNA and histone methylation in ageing and cancer. Nat. Rev. Mol. Cell. Bio. 20, 573–589 (2019)
E.L. Greer, Y. Shi, Histone methylation: a dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 13, 343–357 (2012)
P. Hublitz, M. Albert, A.H.F.M. Peters, Mechanisms of transcriptional repression by histone lysine methylation. Int. J. Dev. Biol. 53, 335–354 (2009)
J. Liu, X. Bu, C. Chu, X.M. Dai, J.M. Asara, P. Sicinski et al., PRMT1 mediated methylation of cGAS suppresses anti-tumor immunity. Nat. Commun. 14 (2023)
A. Verma, A. Singh, M.P. Singh, M.A. Nengroo, K.K. Saini, S.R. Satrusal et al., EZH2-H3K27me3 mediated KRT14 upregulation promotes TNBC peritoneal metastasis. Nat. Commun. 13 (2022)
X. Li, M. Han, H. Zhang, F. Liu, Y. Pan, J. Zhu et al., Structures and biological functions of zinc finger proteins and their roles in hepatocellular carcinoma. Biomark. Res. 10, 2 (2022)
J. Jen, Y.C. Wang, Zinc finger proteins in cancer progression. J. Biomed. Sci. 23, 53 (2016)
J.H. Laity, B.M. Lee, P.E. Wright, Zinc finger proteins: new insights into structural and functional diversity. Curr. Opin. Struct. Biol. 11, 39–46 (2001)
J. Zhang, X. Wen, N. Liu, Y.Q. Li, X.R. Tang, Y.Q. Wang et al., Correction to: epigenetic mediated zinc finger protein 671 downregulation promotes cell proliferation and tumorigenicity in nasopharyngeal carcinoma by inhibiting cell cycle arrest. J. Exp. Clin. Cancer Res. 40, 394 (2021)
N. An, H. Peng, M. Hou, D. Su, L. Wang, X. Shen et al., The zinc figure protein ZNF575 impairs colorectal cancer growth via promoting p53 transcription. Oncol. Res. 31, 307–316 (2023)
R. Hu, G. Peng, H. Dai, E.K. Breuer, K. Stemke-Hale, K. Li et al., ZNF668 functions as a tumor suppressor by regulating p53 stability and function in breast cancer. Cancer Res. 71, 6524–6534 (2011)
W. He, S. Lin, Y. Guo, Y. Wu, L.L. Zhang, Q. Deng et al., Targeted demethylation at ZNF154 promotor upregulates ZNF154 expression and inhibits the proliferation and migration of Esophageal Squamous Carcinoma cells. Oncogene. 41, 4537–4546 (2022)
Y. Shimada, M. Imamura, T. Wagata, N. Yamaguchi, T. Tobe, Characterization of 21 newly established esophageal cancer cell lines. Cancer. 69, 277–284 (1992)
J. Zhu, Y. Wu, Y. Yu, Y. Li, J. Shen, R. Zhang, MYBL1 induces transcriptional activation of ANGPT2 to promote tumor angiogenesis and confer sorafenib resistance in human hepatocellular carcinoma. Cell. Death Dis. 13, 727 (2022)
C. Lin, A. Liu, J. Zhu, X. Zhang, G. Wu, P. Ren et al., miR-508 sustains phosphoinositide signalling and promotes aggressive phenotype of oesophageal squamous cell carcinoma. Nat. Commun. 5, 4620 (2014)
J. Zhu, G. Wu, L. Song, L. Cao, Z. Tan, M. Tang et al., NKX2-8 deletion-induced reprogramming of fatty acid metabolism confers chemoresistance in epithelial ovarian cancer. EBioMedicine. 43, 238–252 (2019)
C. Huang, Y. Chen, Lymphangiogenesis and colorectal cancer. Saudi Med. J. 38, 237–244 (2017)
S.E. Duff, C. Li, M. Jeziorska, S. Kumar, M.P. Saunders, D. Sherlock et al., Vascular endothelial growth factors C and D and lymphangiogenesis in gastrointestinal tract malignancy. Br. J. Cancer. 89, 426–430 (2003)
X.G. Li, X. Hu, B. Patel, Z. Zhou, S. Liang, R. Ybarra et al., H4R3 methylation facilitates β- transcription by regulating histone acetyltransferase binding and H3 acetylation. Blood. 115, 2028–2037 (2010)
A. Wasik, K. Ratajczak-Wielgomas, A. Badzinski, P. Dziegiel, M. Podhorska-Okolow, The role of Periostin in Angiogenesis and Lymphangiogenesis in Tumors. Cancers (Basel) 14 (2022)
R. Jr Roskoski, Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol. Res. 120, 116–132 (2017)
A.W. Lund, Lymph node metastasis: an immunological burden. J. Exp. Med. 220 (2023)
A.D. Karatzanis, E. Koudounarakis, I. Papadakis, G. Velegrakis, Molecular pathways of lymphangiogenesis and lymph node metastasis in head and neck cancer. Eur. Arch. Otorhinolaryngol. 269, 731–737 (2012)
G. Oliver, J. Kipnis, G.J. Randolph, N.L. Harvey, The lymphatic vasculature in the 21(St) Century: Novel Functional roles in Homeostasis and Disease. Cell. 182, 270–296 (2020)
S. Vimalraj, K.N.G. Hariprabu, M. Rahaman, P. Govindasami, K. Perumal, S. Sekaran et al., Vascular endothelial growth factor-C and its receptor-3 signaling in tumorigenesis. 3 Biotech. 13, 326 (2023)
B. Pytowski, J. Goldman, K. Persaud, Y. Wu, L. Witte, D.J. Hicklin et al., Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J. Natl. Cancer Inst. 97, 14–21 (2005)
L.F. Shi, Y. Wu, C.Y. Li, Identification of high-affinity VEGFR3-binding peptides through a phage-displayed random peptide library. J. Gynecol. Oncol. 26, 327–335 (2015)
Y. Yang, Z. Gao, Y. Ma, H. Teng, Z. Liu, H. Wei et al., Fucoidan inhibits lymphangiogenesis by downregulating the expression of VEGFR3 and PROX1 in human lymphatic endothelial cells. Oncotarget. 7, 38025–38035 (2016)
Y. Kodera, Y. Katanasaka, Y. Kitamura, H. Tsuda, K. Nishio, T. Tamura et al., Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res. 13, R66 (2011)
T. Schomber, A. Zumsteg, K. Strittmatter, I. Crnic, H. Antoniadis, A. Littlewood-Evans et al., Differential effects of the vascular endothelial growth factor receptor inhibitor PTK787/ZK222584 on tumor angiogenesis and tumor lymphangiogenesis. Mol. Cancer Ther. 8, 55–63 (2009)
C. Kollmannsberger, Sunitinib side effects as surrogate biomarkers of efficacy. Cuaj-Can Urol. Assoc. 10, S245–S247 (2016)
Y. Wu, Y. Cheng, X. Wang, J. Fan, Q. Gao, Spatial omics: navigating to the golden era of cancer research. Clin. Transl Med. 12, e696 (2022)
C. Zhang, C. Xu, X. Gao, Q. Yao, Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 12, 2115–2132 (2022)
G. Gong, Y.Y. Guan, Z.L. Zhang, K. Rahman, S.J. Wang, S. Zhou et al., Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 128, 110301 (2020)
Funding
This work was supported by National Natural Science Foundation of China (No. 82103637, 82003128). Guangzhou Basic and Applied Basic Research Foundation (No.SL2023A04J00425). Medical Science and Technology Research Foundation of Guangdong Province (No. A2024066). Guangdong Provincial Student Innovation and Entrepreneurship Training Program Project (G202410573045).
Author information
Authors and Affiliations
Contributions
J. Zhu, G. Wu, J. Shen and R. Zhang supervise the project, review and editing the manuscript. X. Qiu performed all the in vitro experiments. S. Ou conducted the molecular cloning. X. Jin and X. Nie performed IHC assay. J Shen provided patient tissue samples and analyzed clinical data. J. Zhu and X Qiu performed in vivo experiments. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All experimental procedures in studies involving animals were in accordance with the ethical standards of the Institutions at which the studies were conducted and were approved by Institutional Animal Care and Use Committee of Guangdong Pharmaceutical University. Prior patient consent and approval from the Institutional Research Ethics Committee were obtained for the use of these clinical materials for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, J., Qiu, X., Jin, X. et al. ZNF468-mediated epigenetic upregulation of VEGF-C facilitates lymphangiogenesis and lymphatic metastasis in ESCC via PI3K/Akt and ERK1/2 signaling pathways. Cell Oncol. (2024). https://doi.org/10.1007/s13402-024-00976-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s13402-024-00976-0