Abstract
Metastasis to regional lymph nodes constitutes the main route toward progression and dissemination of head and neck carcinoma; at the same time it is the most significant adverse prognostic indicator for this disease. In recent years, significant focus has been given on the molecular mechanisms behind lymph node metastasis of head and neck cancer. The aim of this study is to assess the role of growth factor expression and function in association with lymph node metastasis and overall prognosis of head and neck cancer. Current literature, searching for experimental data regarding the molecular pathways of lymph node dissemination of head and neck cancer, is reviewed giving special emphasis on the expression and prognostic significance of specific growth factors. Members of the vascular endothelial growth factor (VEGF), mostly VEGF-C and VEGF-D, with their action through the receptors VEGFR-3 and VEGFR-2, constitute the most extensively studied growth factors associated with lymphangiogenesis so far. High expression of these as well as other molecules, including angiopoietins, insulin-like growth factor, and fibroblast growth factor, has been associated with lymph node metastasis and poor prognosis in head and neck squamous cell carcinoma. Numerous growth factors seem to play an important role regarding the lymph node metastatic potential of head and neck cancer. Further research is necessary in order to further clarify the molecular pathways and introduce novel therapeutic options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinoma (HNSCC) constitutes the fifth most common type of cancer worldwide. Although treatment strategies have been altered significantly with the extensive use of laser microsurgery and organ preservation modalities [1, 2], HNSCC continues to demonstrate low overall survival rates. Prognosis of this disease has been shown to be affected by numerous factors [2]. Among these, nodal status seems to be of utmost importance. In fact, lymph node metastasis is considered as the most significant independent adverse prognostic indicator for patients with HNSCC [2, 3]. Recent studies have implicated the role of lymphangiogenesis in disease progression of HNSCC. Dissemination of tumor cells to regional lymph nodes seems to be an early and crucial event in the metastatic process, and newly formed lymphatic vessels serve as the primary conduit for this purpose [4]. In fact, there is increasing evidence that several growth factors are involved in the molecular signaling pathways that lead to the development of intratumoral and peritumoral lymphatic vessels and promote the dissemination of malignant disease to regional lymph nodes and beyond [5, 6].
Herein, the current knowledge on the role of tumor lymphangiogenesis and growth factors in the lymphatic spread of HNSCC is reviewed, highlighting their significance for the assessment of prognosis as well as for the design of new targeted therapies.
The lymphatic system and lymphangiogenesis
The lymphatic system generally consists of thin-walled, low-pressure vessels, nodes that occur along the course of these vessels, and aggregations of lymphoid tissue, such as the spleen and the thymus [7]. The origin and development of the lymphatic vasculature is still a controversial issue. At least two relevant theories have been proposed so far: the centripetal and the centrifugal. According to the centripetal theory, lymph vessels originate from cells in perivenous mesenchymal tissue spaces that become flattened and form small lumens [8]. On the other hand, the centrifugal theory states that the sprouting of the venous endothelium is responsible for the development of lymphatic sacs that further extend into the surrounding tissues and organs [8]. The expression of two transcriptional factors, Sox18 and Prox1, in a subgroup of venous endothelial cells is an important step for the initiation of this process [9]. Further studies are necessary in order to clearly define the origin of the lymphatic network.
The lymphatic vasculature consists of smaller blind-ended capillaries and larger collecting lymphatic vessels. The lymphatic capillaries are composed of a single layer of endothelial cells that overlap and lack a continuous basement membrane and pericytes. These capillaries demonstrate a micromorphology that varies in different regions of the head and neck. They have the appearance of a three-dimensional, finely meshed polygonal network in the skin and the galea layer; a tree-like network in the mucous membrane of the nasal cavity; finally, a coral-like structure in the mucous membrane of the oropharyngeal, laryngeal, and esophageal walls [10]. The cytoplasm of the endothelium of the lymphatic vessels is highly attenuated. Fewer tight junctions are found in the endothelium of lymphatic vessels than that of blood vessels, and this may be the cause of their greater permeability [7]. When interstitial fluid and pressure increase, the connecting tissue fibers become stretched, thereby opening the lymphatic lumen. As the lumen widens, the endothelial cells, which overlap under normal conditions, move apart, effectively opening intercellular channels to aid fluid and macromolecular uptake into the lymphatic vessel [11].
Lymphangiogenesis, the process of new lymphatic capillary proliferation, occurs, except from embryonic state, in healing tissues, inflammation, and neoplasms [12]. It is believed to be closely associated with lymph node metastasis [13], which in turn is a significant indicator of tumor aggressiveness and a prognostic factor for HNSCC. The recent identification of several lymphatic endothelial markers has provided an important aid in the study of the lymphatic vasculature development in human cancer. One of the most reliable markers of the lymphatic endothelium is podoplanin, which is recognized by the D2-40 monoclonal antibody [14]. Other markers employed in the detection of lymphatic vessels include lymphatic vascular endothelial cell hyaluronan receptor-1 (LYVE-1) [15], Prox-1[16], and sialomucin CD34 [17]. A higher degree of lymphangiogenesis and lymphatic vessel density is associated with increasing frequency of nodal metastases and poor prognosis in several types of solid tumors [18, 19]. This association has been repeatedly confirmed for HNSCC [14, 20, 21]. Both intratumoral and peritumoral newly formed lymphatic vessels have been identified in HNSCC. Intratumoral lymphatics with ill-defined lumina have been found within sheets of tumor cells in carcinomas with a pushing margin and in areas containing leukocyte infiltration in carcinomas with an invasive margin [5]. Furthermore, peritumoral lymphatics were shown to have more dilated open lumina than did the intratumoral lymphatics. Although studies in some animal models have shown that intratumoral lymphatic vessels are compressed and nonfunctional, a significant correlation was found between intratumoral lymphatics and the spread of HNSCC to regional lymph nodes, particularly in oropharyngeal [22] and laryngeal cancer [14, 20]. Regarding peritumoral lymphatics, an association has been found with shorter disease-free survival [6], although opposite findings have also been reported [23]. These controversial results could arise owing to the different methods used to quantify lymphatic vessel density, and could also reflect subjective factors and the intrinsic difficulties regarding lymphatic vessel identification when studying single tissue sections taken at a single time point, which might lead to misleading vessel classification.
Metastatic spread of tumor cells to lymph nodes has occurred in animal models in the absence of lymphangiogenesis, suggesting that the importance of lymphangiogenesis in metastasis may vary and depend on parameters, such as tumor type or proximity of the primary tumor site to the lymphatic network [24]. In the mean time, the presence of tumor cells has been shown to also induce, apart from lymphangiogenesis, additional changes in the morphology and function of other vessels inside the lymph nodes. In this way, a site of rearrangement of the vessel structures inside the node is consequently provided resulting in abnormal connections between the vascular and lymphatic networks. These changes may occur before or after the arrival of tumor cells inside the node and may eventually facilitate the spread to more distant lymph nodes or major organs, such as the lungs, brain, or bone. Dissemination of tumor cells from lymph nodes to distant organs may be achieved either via blood vessels directly associated with the nodes or after entering the venous system via the major lymphatic ducts [25].
VEGF and lymph node metastasis
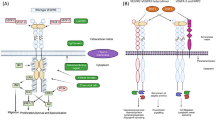
The vascular endothelial growth factor (VEGF) family consists of hypoxia-inducible angiogenic factors that are found on the vascular endothelium [26]. This group of angiogenic factors belongs to the platelet-derived growth factor family and includes VEGF-A (or vascular permeability factor), VEGF-B (or VEGF-related factor, VRF), VEGF-C (or VEGF-related protein, VRP), VEGF-D (or c-fos-induced growth factor), VEGF-E, and placenta growth factor (PlGF). Three VEGF tyrosine kinase receptors have been identified: VEGFR-1 (Flt-1), VEGFR-2 (Flk-1, KDR), and VEGFR-3. Neuropilins 1 and 2 form another class of high-affinity non-tyrosine kinase receptors for VEGFs on endothelial and neuronal cell surfaces [27]. The role of the VEGF family in angiogenesis and tumor growth has been well established [28, 29]. It is particularly evident in the case of VEGF-A, which demonstrates potent angiogenetic properties in response to hypoxia and has been found to overexpress in most solid tumor types [28]. In addition, it has been shown that VEGF-C and VEGF-D also possess angiogenic properties in vitro and in vivo [30, 31].
In the process of lymphangiogenesis, VEGF-C and VEGF-D are the most extensively studied molecules up to date. Both factors exert their lymphangiogenic activity by binding to the VEGFR-3 receptor, whose expression in adults is mainly restricted to lymphatic endothelial cells and hematopoietic cells of monocytic lineage [32]. In cancer, however, this receptor may also be expressed in blood vessels [33]. Two isoforms of the VEGFR-3 receptor have been described, VEGFR-3s (short) and VEGFR-3l (long), which differ as a result of alternative splicing. The long one among these isoforms is the predominant form in most tissues [34]. After stimulation of the VEGFR-3 receptor by VEGF-C and VEGF-D, a cascade of signals leads to growth and migration of the lymphatic endothelial cells, which protects them from apoptosis. These signals are, in part, transduced via a protein kinase C-dependent activation of the p42/p44 MAPK signaling cascade [35]. Proteolytic processing of VEGF-C and VEGF-D by plasmin and proprotein convertases [36, 37] is an important step for their action, since it regulates their biologic activity and receptor specificity, increasing affinity to VEGFR-3 and facilitating binding to the VEGFR-2 receptor, which is also expressed in lymphatic endothelial cells [38]. In addition, current research has focused on the potential role of nitric oxide synthase in VEGF-C induced lymphangiogenesis, providing evidence that VEGFR-2 and VEGFR-3 activate nitric oxide synthase in lymphatic endothelial cells, and nitric oxide donors induce proliferation and survival of cultured lymphatic endothelial cells in a dose-dependent manner [39]. Moreover, activation of the VEGFR-2 receptor by VEGF-A can also lead to lymphatic vessel development, and this has been demonstrated in a tumor xenograft model utilizing fibrosarcoma cells [40], although not confirmed by other studies [41].
Beyond the observations in experimental models, the relationship of the VEGF family and lymphatic spread of malignant disease has been studied in some of the most prevalent tumor types, such as colorectal, lung, and breast cancer [42–44]. In HNSCC, however, only a few studies have demonstrated the association of these lymphangiogenic factors with lymphatic metastasis and overall prognosis. Increased expression of VEGF-C and VEGF-D in oral squamous cell carcinoma has been reported to be significantly associated with lymphatic vessel density, lymph node metastasis, and poor prognosis (Table 1) [45, 46]. Among these two factors, VEGF-C was found to be more significantly correlated to lymph node metastasis than VEGF-D. VEGF-C was also shown to be superior as a predictive factor for lymph node metastasis in comparison to lymphatic vessel density. The invasive potential of tumor cells, which has been correlated in vivo with metastatic potential in mouse models [47] was also shown to correlate directly with VEGF-C levels in UM-SCC-1 and SCC116 HNSCC cell lines, further implicating VEGF-C in the early promotion of metastasis in head and neck cancer (Table 1) [48]. In another study, VEGF expression was correlated with prognosis in patients with early stage (T1–T2) laryngeal cancer treated with primary radiotherapy. A trend toward higher local relapse was demonstrated in the VEGF positive group, whereas increased VEGF expression was correlated with higher clinical local stage and decreased overall survival [49]. Moreover, VEGF-A expression in another study was reported to be associated with advanced T stage for oral, pharyngeal, and laryngeal squamous cell carcinoma. However, the association with nodal status was significant only for oral and pharyngeal carcinoma (Table 1) [50]. In the same study, the combined overexpression of VEGF-A and the proliferation marker Ki-67 in oral and oropharyngeal squamous cell carcinoma was documented to demonstrate a significantly higher likelihood ratio for positive lymph nodes. This ratio represented an index calculated by dividing the percentage of positive nodes by the percentage of negative nodes. Again in this study it was suggested that the combined expression of VEGF-A and Ki-67 was more effective in predicting positive nodal status in early local tumors compared to more advanced disease. In addition, patients with early oral and oropharyngeal carcinoma could be divided into high or low risk groups for occult metastasis on the basis of biological expression of these markers [50]. So far, it remains uncertain whether VEGF-A acts directly as a lymphangiogenic factor or via the induction of other associated molecules. Supporting the latter, recent studies on tumors with positive lymph nodes have demonstrated co-expression of VEGF-A and VEGF-C [51, 52]. In any case, it is obvious that further studies are necessary in order to better clarify the role of VEGF in the lymphatic spread of head and neck cancer, as well as any implications in overall prognosis and treatment.
Other factors implicated in lymphangiogenesis
Apart from VEGF, a number of other growth factors have been the target of research studies in an effort to identify the molecular pathways of tumor lymphangiogenesis and lymph node metastasis. Angiopoietins, a family of four growth factors which have opposing actions in blood vascular endothelial cells, have been shown to function as ligands for the endothelial-specific tyrosine kinase receptor Tie 2 [53]. Angiopoietin-1 (Ang-1) stimulates the endothelial cell migration in vitro and increases angiogenesis in transgenic mouse models [54], as well as lymphangiogenesis in the mouse cornea [55]. Indirect action of Ang-1 via the VEGFR-3 pathway was demonstrated in a study in which the effects of Ang-1 were inhibited in mice treated with a soluble form of VEGFR-3 [56]. Moreover, VEGFR-3 expression on lymphatic endothelial cells after treatment with Ang-1 indicated that Ang-1 induces higher VEGFR-3 levels in these cells, providing evidence that Ang-1 may promote lymphangiogenesis by rendering lymphatic vessels more responsive to VEGF-C or VEGF-D emanating from the surrounding microenvironment [56]. Another member of the angiopoietin family, angiopoietin-2 (Ang-2), has been significantly correlated with lymph node metastasis and decreased survival in gastric and colorectal cancer [57, 58]. In oral squamous cell carcinoma, overexpression of Ang-1 and Ang-2 has been reported and associated with worse prognosis, although high expression of Ang-1 only could be correlated with lymph node metastasis (Table 1) [59].
The insulin-like growth factor (IGF) system consists of two ligands, IGF-1 and IGF-2; three cell-membrane receptors, IGF-1 receptor (IGF-1R), insulin receptor (IR), and IGF-2 receptor (IGF-2R); and six high-affinity IGF-binding proteins, IGFBP-1 through -6 [60]. These binding proteins modulate the biological activity of IGFs [61]. In vitro and in vivo studies have demonstrated the angiogenic action of IGF-1 and IGF-2 [62, 63], as well as the increased expression of members of the IGF family in several solid tumors [64–66]. Recent research has highlighted the direct lymphangiogenic activity of IGF-1 and IGF-2 factors, which stimulate a signaling pathway independent from that triggered by the VEGF-C/VEGF-D/VEGFR-3 system. Both factors induce proliferation and migration of lymphatic endothelial cells, stimulating phosphorylation of intracellular signaling components of these cells, such as extracellular signal-regulated kinase (ERK), Akt, and Src [67]. This lymphangiogenic activity may promote lymphatic spread, metastasis, and recurrence of malignant tumors. Increased expression of IGF-1R has been detected immunohistochemically in primary undifferentiated oropharyngeal and nasopharyngeal tumors as well as metastatic lymph nodes [68], and has been associated with poor prognosis, increased metastatic potential, and high recurrence rates (Table 1) [69]. On the other hand, down-regulation of IGFBP-3 has been found in patients with tongue carcinoma and significantly associated with shorter disease-specific survival [70]. In addition, patients with head and neck cancer have been shown to have increased plasma levels of IGFBP-1 and IGFBP-2 in contrast to the levels of IGF-1 and IGFBP-3, which were found to be low [71]. However, no significant association was demonstrated between these findings and the tumor size or nodal status.
The fibroblast growth factor (FGF) family consists of about 23 different FGFs and 4 FGF-receptors (FGFR-1 through FGFR-4) that have been identified and characterized in vertebrates [72]. FGF-2 was one of the first angiogenic factors identified for its potent activity on vascular endothelial cell proliferation [73]. In addition, FGF-2 was reported to induce lymphatic vessel growth in a mouse cornea assay by promoting the secretion of VEGF-C in blood vascular endothelial cells [74], whereas systemic treatment with a blocking antibody against VEGFR-3 inhibited the FGF-2-induced corneal lymphangiogenesis [75]. Several studies have demonstrated overexpression of the members of the FGF family in malignant tumors and an association with metastasis and decreased survival (Table 1) [76–79]. However, studies on the potential role of the FGF family in the lymphatic spread of HNSCC are lacking. This is also true regarding a number of other growth factors, such as hepatocyte growth factor and platelet-derived growth factor-BB, whose potential lymphangiogenic activity has only recently been identified [80, 81].
Therapeutic implications and future steps
Tumor metastasis to regional lymph nodes often represents the first step of tumor dissemination and serves as a major prognostic indicator for the progression of HNSCC [25]. Studying the molecular pathways of lymphangiogenesis and lymph node metastasis in HNSCC may eventually allow for early detection of cases with high metastatic potential. This would allow for better prognosis assessment and even affect treatment strategies, limiting, for example, elective neck dissection only to high risk cases. In addition, studying the molecular mechanisms behind lymphangiogenesis may lead to the development of new chemotherapeutic agents. In fact, various agents targeting tumor lymphangiogenesis have already been used in animal models. These include inhibitors of the VEGF signaling pathway, such as a soluble version of VEGFR-3 [82], and neutralizing antibodies against VEGF-C, VEGF-D, VEGF-A, and VEGFR-3 [83–85]. Combined inhibition of both VEGFR-2 and VEGFR-3 might result in a more potent blockade of tumor-associated lymphatic vessel development. In addition, the effects of inhibition of other growth factors and their receptors on lymphangiogenesis and lymph node metastasis need to be studied further. It becomes obvious that this is an area of great scientific interest and hopefully future studies will lead to the development of better prognostic tools as well as novel therapeutic options.
References
Karatzanis AD, Waldfahrer F, Psychogios G, Hornung J, Zenk J, Velegrakis GA, Iro H (2010) Effect of repeated laser microsurgical operations on laryngeal cancer prognosis. Head Neck 32(7):921–928
Karatzanis AD, Waldfahrer F, Psychogios G, Hornung J, Zenk J, Velegrakis GA, Iro H (2010) Resection margins and other prognostic factors regarding surgically treated glottic carcinomas. J Surg Oncol 101(2):131–136
Takes RP (2010) Staging of the neck in patients with head and neck squamous cell cancer: imaging techniques and biomarkers. Oral Oncol 40(7):656–667
Wissmann C, Detmar M (2006) Pathways targeting tumor lymphangiogenesis. Clin Cancer Res 12(23):6865–6868
Zhang Z, Helman JI, Li LJ (2010) Lymphangiogenesis, lymphatic endothelial cells and lymphatic metastasis in head and neck cancer—a review of mechanisms. Int J Oral Sci 2(1):5–14
Franchi A, Gallo O, Massi D, Baroni G, Santucci M (2004) Tumor lymphangiogenesis in head and neck squamous cell carcinoma: a morphometric study with clinical correlations. Cancer 101(5):973–978
Leak LV, Burke JF (1966) Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am J Anat 118(3):785–809
Kato S, Shimoda H, Ji RC, Miura M (2006) Lymphangiogenesis and expression of specific molecules as lymphatic endothelial cell markers. Anat Sci Int 81(2):71–83
Adams RH, Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8(6):464–478
Pan WR, le Roux CM, Levy SM, Briggs CA (2010) The morphology of the human lymphatic vessels in the head and neck. Clin Anat 23(6):654–661
Witte MH, Bernas MJ, Martin CP, Witte CL (2001) Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech 55(2):122–145
Alitalo K, Carmeliet P (2002) Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1(3):219–227
Swartz MA, Skobe M (2001) Lymphatic function, lymphangiogenesis and cancer metastasis. Microsc Res Tech 55(2):92–99
Garcia-Carracedo D, Rodrigo JP, Astudillo A, Nieto CS, Gonzalez MV (2010) Prognostic significance of lymphangiogenesis in pharyngolaryngeal carcinoma patients. BMC Cancer 10:416. doi:10.1186/1471-2407-10-416
Jackson DG (2004) Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS 112(7–8):526–538
Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G (2002) An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 21(7):1505–1513
Fiedler U, Christian S, Koidl S, Kerjaschki D, Emmett MS, Bates DO, Christofori G, Augustin HG (2006) The sialomucin CD34 is a marker of lymphatic endothelial cells in human tumors. Am J Pathol 168(3):1045–1053
Lee SK, Cho EY, Kim WW, Kim SH, Hur SM, Kim S, Choe JH, Kim JH, Kim JS, Lee JE, Nam SJ, Yang JH (2010) The prediction of lymph node metastasis in ductal carcinoma in situ with microinvasion by assessing lymphangiogenesis. J Surg Oncol 102(3):225–229
Coşkun U, Akyürek N, Dursun A, Yamaç D (2010) Peritumoral lymphatic microvessel density associated with tumor progression and poor prognosis in gastric carcinoma. J Surg Res 164(1):110–115
Audet N, Beasley NJ, MacMillan C, Jackson DG, Gullane PJ, Kamel-Reid S (2005) Lymphatic vessel density, nodal metastases and prognosis in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 131(12):1065–1070
Miyahara M, Tanuma J, Sugihara K, Semba I (2007) Tumor lymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer 110(6):1287–1294
Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG (2002) Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 62(5):1315–1320
Maula SM, Luukkaa M, Grénman R, Jackson D, Jalkanen S, Ristamäki R (2003) Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res 63(8):1920–1926
Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO (2005) Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res 65(21):9789–9798
Tobler NE, Detmar M (2006) Tumor and lymph node lymphangiogenesis—impact on cancer metastasis. J Leukoc Biol 80(4):691–696
Roy H, Bhardwaj S, Ylä-Herttuala S (2006) Biology of vascular endothelial growth factors. FEBS Lett 580(12):2879–2887
Petrova TV, Makinen T, Alitalo K (1999) Signaling via vascular endothelial growth factor receptors. Exp Cell Res 253(1):117–130
Ferrara N, Alitalo K (1999) Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 5(12):1359–1364
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25(4):581–611
Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 159(2):290–298
Marconcini L, Marchio S, Morbidelli L, Cartocci E, Albini A, Ziche M, Bussolino F, Oliviero S (1999) C-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc Natl Acad Sci USA 96(17):9671–9676
Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K (1995) Expression of the fms-like tyrosine kinase FLT4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 92(8):3566–3570
Kubo H, Fujiwara T, Jussila L, Hashi H, Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, Yamaoka Y, Nishikawa SI (2000) Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood 96(2):546–553
Hughes DC (2001) Alternative splicing of the human VEGFGR-3/FLT4 gene as a consequence of an integrated human endogenous retrovirus. J Mol Evol 53(2):77–79
Makinen T, Veikkola T, Mustioki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K (2001) Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J 20(17):4762–4773
McColl BK, Baldwin ME, Roufail S, Freeman C, Moritz RL, Simpson RJ, Alitalo K, Stacker SA, Achen MG (2003) Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J Exp Med 198(6):863–868
Siegfried G, Basak A, Cromlish JA, Benjannet S, Marcinkiewicz J, Chrétien M, Seidah NG, Khatib AM (2003) The secretory proprotein convertases furin, PC5 and PC7 activate VEGF-C to induce tumorigenesis. J Clin Invest 111(11):1723–1732
Saaristo A, Veikkola T, Enholm B, Hytönen M, Arola J, Pajusola K, Turunen P, Jeltsch M, Karkkainen MJ, Kerjaschki D, Bueler H, Ylä-Herttuala S, Alitalo K (2002) Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity and leakiness but no sprouting angiogenesis in the skin or mucous membranes. FASEB J 16(9):1041–1049
Lahdenranta J, Hagendoorn J, Padera T, Hoshida T, Nelson G, Kashiwagi S, Jain RK, Fukumura D (2009) Endothelial nitric oxide synthase mediates lymphangiogenesis and lymphatic metastasis. Cancer Res 69(7):2801–2808
Björndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D, Wu L, Cao Y (2005) Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res 65(20):9261–9268
Gannon G, Mandriota SJ, Cui L, Baetens D, Pepper MS, Christofori G (2002) Overexpression of vascular endothelial growth factor-A165 enhances tumor angiogenesis but not metastasis during beta-cell carcinogenesis. Cancer Res 62(2):603–608
Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y, Sugimachi K (2000) Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer 83(7):887–891
Kajita T, Ohta Y, Kimura K, Tamura M, Tanaka Y, Tsunezuka Y, Oda M, Sasaki T, Watanabe G (2001) The expression of vascular endothelial growth factor C and its receptors in non-small cell lung cancer. Br J Cancer 85(2):255–260
Salven P, Lymboussaki A, Heikkilä P, Jääskela-Saari H, Enholm B, Aase K, von Euler G, Eriksson U, Alitalo K, Joensuu H (1998) Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am J Pathol 153(1):103–108
Shintani S, Li C, Ishikawa T, Mihara M, Nakashiro K, Hamakawa H (2004) Expression of vascular endothelial growth factor A, B, C, and D in oral squamous cell carcinoma. Oral Oncol 40(1):13–20
Sugiura T, Inoue Y, Matsuki R, Ishii K, Takahashi M, Abe M, Shirasuna K (2009) VEGF-C and VEGF-D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: Implications for use as a prognostic marker. Int J Oncol 34(3):673–680
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25(1):9–34
Bock J, Sinclair L, Bedford N, Jackson RE, Lee JH, Trask DK (2008) Modulation of cellular invasion by VEGF-C expression in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 134(4):355–362
Parikh RR, Yang Q, Haffty BG (2007) Prognostic significance of vascular endothelial growth factor protein levels in T1-2 N0 laryngeal cancer treated with primary radiation therapy. Cancer 109(3):566–573
Boonkitticharoen V, Kulapaditharom B, Leopairut J, Kraiphibul P, Larbcharoensub N, Cheewaruangroj W, Chintrakarn C, Pochanukul L (2008) Vascular endothelial growth factor A and proliferation marker in prediction of lymph node metastasis in oral and pharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 134(12):1305–1311
Mohammed RA, Green A, El-Shikh S, Paish EC, Ellis IO, Martin SG (2007) Prognostic significance of vascular endothelial growth factor-A, -C and -D in breast cancer and their relationships with angio- and lymphangiogenesis. Br J Cancer 96(7):1092–1100
Kondo K, Kaneko T, Baba M, Konno H (2007) VEGF-C and VEGF-A synergistically enhance lymph node metastasis of gastric cancer. Biol Pharm Bull 30(4):633–637
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87(7):1161–1169
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87(7):1171–1180
Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, Maekawa H, Kimura Y, Ohmura M, Miyamoto T, Nozawa S, Koh GY, Alitalo K, Suda T (2005) Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood 105(12):4649–4656
Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmén C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K (2005) Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 105(12):4642–4648
Wang J, Wu K, Zhang D, Tang H, Xie H, Hong L, Pan Y, Lan M, Hu S, Ning X, Fan D (2005) Expressions and clinical significances of angiopoietin-1, -2 and Tie2 in human gastric cancer. Biochem Biophys Res Commun 337(1):386–393
Chung YC, Hou YC, Chang CN, Hseu TH (2006) Expression and prognostic significance of angiopoietin in colorectal carcinoma. J Surg Oncol 94(7):631–638
Chien CY, Su CY, Chuang HC, Fang FM, Huang HY, Chen CM, Chen CH, Huang CC (2008) Angiopoietin-1 and -2 expression in recurrent squamous cell carcinoma of the oral cavity. J Surg Oncol 97(3):273–277
Samani AA, Yakar S, LeRoith D, Brodt P (2007) The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 28(1):20–47
Clemmons DR (1997) Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev 8(1):45–62
Lee OH, Bae SK, Bae MH, Lee YM, Moon EJ, Cha HJ, Kwon YG, Kim KW (2000) Identification of angiogenic properties of insulin-like growth factor II in in vitro angiogenesis models. Br J Cancer 82(2):385–391
Rabinovsky ED, Draghia-Akli R (2004) Insulin-like growth factor I plasmid therapy promotes in vivo angiogenesis. Mol Ther 9(1):46–55
Reinmuth N, Fan F, Liu W, Parikh AA, Stoeltzing O, Jung YD, Bucana CD, Radinsky R, Gallick GE, Ellis LM (2002) Impact of insulin-like growth factor receptor-I function on angiogenesis, growth and metastasis of colon cancer. Lab Invest 82(10):1377–1389
Mohanraj L, Oh Y (2011) Targeting IGF-I, IGFBPs and IGF-I receptor system in cancer: the current and future in breast cancer therapy. Recent Pat Anticancer Drug Discov 6(2):166–177
Chang MH, Lee J, Han J, Park YH, Ahn JS, Park K, Ahn MJ (2009) Prognostic role of insulin-like growth factor receptor-1 expression in small cell lung cancer. APMIS 117(12):861–869
Björndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y (2005) Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA 102(43):15593–15598
Friedrich RE, Hagel C, Bartel-Friedrich S (2010) Insulin-like growth factor-1 receptor (IGF-1R) in primary and metastatic undifferentiated carcinoma of the head and neck: a possible target of immunotherapy. Anticancer Res 30(5):1641–1643
Yuan Y, Zhou X, Song J, Qiu X, Li J, Ye L, Meng X, Xia D (2008) Expression and clinical significance of epidermal growth factor receptor and type 1 insulin-like growth factor receptor in nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol 117(3):192–200
Papadimitrakopoulou VA, Brown EN, Liu DD, El-Naggar AK, Jack Lee J, Hong WK, Lee HY (2006) The prognostic role of loss of insulin-like growth factor-binding protein-3 expression in head and neck carcinogenesis. Cancer Lett 239(1):136–143
Brady G, O’Regan E, Miller I, Ogungbowale A, Kapas S, Crean SJ (2007) Serum levels of insulin-like growth factors (IGFs) and their binding proteins (IGFBPs), -1, -2, -3, in oral cancer. Int J Oral Maxillofac Surg 36(3):259–262
Ornitz DM, Itoh N (2001) Fibroblast growth factors. Genome Biol 2(3):reviews3005–reviews3005.12. doi:10.1186/gb-2001-2-3-reviews3005
Shing Y, Folkman J, Sullivan R, Butterfield C, Murray J, Klagsbrun M (1984) Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science 223(4642):1296–1299
Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J, Kaipainen A (2004) Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci USA 101(32):11658–11663
Kubo H, Cao R, Brakenhielm E, Mäkinen T, Cao Y, Alitalo K (2002) Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA 99(13):8868–8873
Donnem T, Al-Shibli K, Al-Saad S, Busund LT, Bremnes RM (2009) Prognostic impact of fibroblast growth factor 2 in non-small cell lung cancer: coexpression with VEGFR-3 and PDGF-B predicts poor survival. J Thorac Oncol 4(5):578–585
Wang J, Yu W, Cai Y, Ren C, Ittmann MM (2008) Altered fibroblast growth factor receptor 4 stability promotes prostate cancer progression. Neoplasia 10(8):847–856
de la Torre NG, Buley I, Wass JA, Turner HE (2006) Angiogenesis and lymphangiogenesis in thyroid proliferative lesions: relationship to type and tumour behaviour. Endocr Relat Cancer 13(3):931–944
Drugan CS, Paterson IC, Prime SS (1998) Fibroblast growth factor receptor expression reflects cellular differentiation in human oral squamous carcinoma cell lines. Carcinogenesis 19(6):1153–1156
Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M (2005) Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J 24(16):2885–2895
Cao R, Björndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y (2004) PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 6(4):333–345
Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, Alitalo K, Jooss K (2005) Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res 65(15):6901–6909
Achen MG, Roufail S, Domagala T, Catimel B, Nice EC, Geleick DM, Murphy R, Scott AM, Caesar C, Makinen T, Alitalo K, Stacker SA (2000) Monoclonal antibodies to vascular endothelial growth factor-D block interactions with both VEGF receptor-2 and VEGF receptor-3. Eur J Biochem 267(9):2505–2515
Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3(5):391–400
Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA (2005) Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst 97(1):14–21
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karatzanis, A.D., Koudounarakis, E., Papadakis, I. et al. Molecular pathways of lymphangiogenesis and lymph node metastasis in head and neck cancer. Eur Arch Otorhinolaryngol 269, 731–737 (2012). https://doi.org/10.1007/s00405-011-1809-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-011-1809-2