Abstract
Due to resource scarcity and habitat damage, fossil fuels—especially oil and gas—are unsustainable. Recently, scientists have investigated biotechnology and microbiology as solutions for carbon-free, renewable, and alternative energy sources. A modern study shows that bacteria degrade inorganic and organic wastewater pollutants. Since they clean wastewater and generate electricity, microbial fuel cells (MFCs) are the ideal response to the concerns listed earlier. MFCs struggle with electron transport issues due to a lack of anode performance. Thus, current research focuses on the production of anode from biomass waste with minimal effort. This work developed a graphene oxide (GO) electrode using local Moringa biomass powder and found that waste-derived GO yielded 175 mV in 16 days with a power density of 1.49 mW/m2. The calculated internal resistance was 796 ῼ, while the external resistance was 1000 ῼ. It seems that electron transportation works effectively. Wastewater treatment is an additional focus for this work. Meanwhile, the inoculation source contains Pb and Hg. The removal efficiency was remarkable, such as Pb = 75.10% and Hg = 65%. Additionally, a thorough analysis of the mechanism and future prospects is also enclosed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The demand for energy has been steadily rising globally in recent years, resulting in an energy crisis on a global scale. Relying on fossil fuels—especially oil and gas—is not sustainable due to a lack of resources and the harm they do to the ecosystem. Scientists have recently focused on carbon-free, renewable, and alternative energy sources, investigating the possibility that biotechnology and microbiology may be a practical solution [1]. A viable solution to the enduring problem of global warming is the use of sustainable bioenergy. Using microorganisms, waste, and natural biomass can potentially be converted into energy. Furthermore, the research demonstrated that bacteria play a key part in the decomposition of both inorganic and organic pollutants found in wastewater. Due to their dual functionality as wastewater treatment and energy generators, microbial fuel cells (MFCs) provide a perfect solution to the problems mentioned previously [2, 3]. This has led to their rise to prominence as a research center in a number of countries [4]. The number of publications devoted to investigations of MFCs has skyrocketed since the possibility that microbial metabolism may provide electrical power became public knowledge [5,6,7]. Although the systems have a lot of promise for ecologically friendly power generation, they still need to undergo substantial development before they can be used on a large scale [6]. Although there have been significant advances in the energy sector, the high cost and poor power generation rates of MFCs have prevented their broad deployment. The electrode has a significant impact on the MFC’s performance and total cost. When it comes to constructing MFCs into a scalable and inexpensive technology, the design and material of the electrodes provide the most significant challenge [8,9,10]. The structure and material of the electrodes have recently and consistently attracted more attention in MFC research. Several electrodes have been the focus of extensive study on MFCs throughout the last 10 years [11]. Both universal and self-specific properties are present in the materials used to make the electrodes in MFCs. Fundamental materials with low prices, excellent durability, strength, steady chemical reactivity, and good conductivity are required for all electrodes, independent of type. All other things being equal, the anode electrode in particular is ultimately in charge of the electron transport. Several latest research have shown that there may be a low amount of electron transport between the electrodes [12,13,14]. Prior studies have shown that conventional materials made from carbon and metal have been previously studied [10, 15]. However, they both possess significant drawbacks. For instance, metal-based electrodes have been found to suffer from corrosion, which hampers the growth rate of bacteria. Graphite, a form of carbon, had reduced conductivity and showed decreased biocompatibility for bacterial growth [16, 17]. Biomass-derived material, often called waste material, may improve electron transportation, economics, and environmental sustainability in MFCs’ electrode development [18, 19]. Utilizing biomass resources for electricity production is essential from both an economic and environmental perspective [20, 21]. According to Ala’a et al. [22], biomass waste has the potential to make a substantial contribution to the energy production. From 2014 to 2019, there were annual quantities of roughly 1284, 10,910, and 16,094 kilotons of different waste production that can be used as biomass resources in modern technology as fruitful product. Through the removal of metal, this study produced a more sophisticated electrode material, increasing electron transportation. Recent study findings suggest that biomass graphene has earned significant attention as electrodes for MFCs [23]. However, as stated by Yaqoob et al., [24] significant alterations are required to increase the electron transfer between the bacterial cell and the anode. Waste graphene oxide (GO) materials have the capacity to function as a conducive substrate for bacterial development. The increased waste-based graphene derivatives anode has the potential to improve the interface between bacteria and the anode, which in turn improves the production of biofilms and the transfer of electrons. Several recent studies indicate that biomass-derived GO material can be an ideal option for electrode fabrication in MFCs [25,26,27]. Significant improvements are made in both the exogenous electron transport and the biocompatibility of microorganisms [28]. The modified waste materials have undergone a substantial oxygenation process, which has the ability to greatly change the interactions between Van der Waals molecules. This, in turn, may lead to a variety of solubility levels in the inoculation [29]. One of the distinctive aspects of waste GO is its distinguishing attribute. The procedures used to produce GO from waste have a substantial influence on both the material’s characteristics and its electrochemical reactivity. The initiative effectively achieved its aims by using Moringa biomass. Moringa biomass is a readily available waste material in the local area. This is also the first time that it has been reported as valuable for electrode fabrication.
The secondary application of MFCs in wastewater treatment was further taken into account. In addition, MFCs proved to be effective in bioremediating water sources for toxic metal ions while generating energy. Metal pollution is now one of among the most serious environmental problems. The three metals that provide the greatest danger to human health and safety are lead (Pb) and mercury (Hg) [30]. Therefore, it is necessary to devise a plan to filter out the very toxic metal ions without compromising the quality of the environment. The use of MFCs is much more sophisticated than earlier methods of wastewater treatment, as stated by Mathuriya et al. [31]. Advantages of MFCs over enzymatic fuel cells include higher conversion efficiency, reduced CO₂ generation compared to biological treatment methods, and the production of less harmful sludge compared to aerobic treatment procedures. Consequently, MFC technology has evolved into the most eco-friendly and promising approach to extracting toxic metals from water. Multiple prior investigations have shown that MFC technology is the most effective means of removing metal ions from wastewater [32,33,34]. There are different methods that have significant advantages for treating wastewater [35,36,37], but MFCs offer the dual advantage of producing energy while also treating wastewater, making them a better option. Removing Pb2+ and Hg2+ from wastewater has been the limited focus of the present study. The study’s first objective is to fabricate Moringa-based GO electrode.
2 Methodology
2.1 Reagent and material
Moringa-derived carbon powder was received from a friend as a gift. Mercury nitrate and lead nitrate (R & M chemicals), glucose, graphite rod, phosphate buffer pH 7, distilled water, and dichloromethane (99.6% QRec) were used.
2.2 Synthesis of Moringa-derived GO material
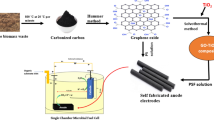
The carbon powder obtained from Moringa was used in the Hummers process to produce GO [38]. The carbon powder made from Moringa was called M-carbon. A standard reaction calls for 60 min of continuous stirring in a mixture of 5.0 g of M-carbon powder and 6.0 g of NaNO3 with 150 mL of concentrated H2SO4. Afterwards, using an ice bath maintained at a temperature range of 0 to 5 °C for 3 h, 15.0 g of KMnO4 was gradually added to the solution while stirring continuously. Without the ice bath, the oxidation process was completed by stirring the reaction for another 24 h. The mixture’s original black tint was replaced with violet and brown. A further addition to the mixture was a dropwise addition of 150 mL of distilled water. After that, the temperature was raised to 90 °C for 20 min to obtain a dark brown solution. The mixture was then given time to cool to room temperature. Two hundred milliliters of water was progressively added to the mixture in order to stop the reaction and remove the excess KMnO4 from the solution. Drop by drop, 20 mL of H2O2 solution was then added. The produced GO was rinsed with purified water, then with ethanol and diluted hydrochloric acid until the pH of the solution reached 7. It took 36 h of drying in an oven set at 40 °C for the resultant sample to be processed.
2.3 Electrode fabrication
The present electrode fabrication is in accordance with earlier literature, using similar parameters [24]. Dichloromethane, a solvent, and polysulfone, a binder, are used to produce the GO electrode. The current absorber in the GO electrode was a 2 mm graphite rod. Firstly, 20 mL of dichloromethane was mixed with 2 g of polysulfone to produce a homogenous solution. Next, the polymer mixture was mixed with 5 g of GO. To produce a paste, this was added to the binder mixture and well mixed. After the graphite rod was covered with paste, it was placed in an oven set at 50 °C for 18 h. A cylindrical rod that meets this criterion is called a GO electrode. The parameters of the GO anode are as follows: it has a height of 9.0 cm, a radius of 1.4 cm, and a surface area of 91.48 cm2.
2.4 Inoculation, setup, configuration, and process of MFCs
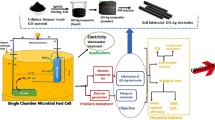
In this study, single-chamber MFCs were used as the graphic presentation shown in Figure S1. The MFCs have dimensions of 19 cm × 13 cm (h × w). The MFCs chamber contained metal-enriched effluent obtained from a local effluent management facility. Conversely, the MFCs also contained a mixture of local water and additionally a buffer solution with a pH value of 7.0. A sample of pollutant water (PW) was collected from the nearby area. Following the collection of the PW sample, it was promptly transferred to polycarbonate jars and then sent to the laboratory. Subsequently, a solution was prepared by mixing PW with lead nitrate and mercury nitrate; each has 100 mg/L. The 1.5 L of PW, which has a concentration of 200 mg/L total metals, has polluted the MFCs chamber. The pH 7 (phosphate) buffer solution is also a part of the mixture. The final inoculation pH was 7 before MFC operation. Table 1 displays the physical and chemical characteristics of both the local wastewater and the metal-added wastewater before the MFCs are put into operation. Throughout the operation of the MFCs, a daily supply of a 3.0 mL glucose solution was provided to mitigate the impact of evaporation. The glucose served as an organic substrate for bacterial nourishment. The conventional graphite rod served as the cathode in the process, while the GO electrode functioned as the anode. The electrodes were connected using copper wire and an external resistance of 1000 Ω. Throughout the experiment, an aquarium air pump was used to regularly provide oxygen to the cathodic water, ensuring that it remained in an aerobic condition. The experiment was conducted under controlled conditions with a consistent temperature maintained over a period of 25 days.
2.5 Analytical and biological characterizations of MFC operation
Electrochemical studies are conducted to assess the efficiency of the MFCs’ electron transportation application. The operational voltage was recorded using the multimeter. Ohm’s law was used to convert the voltage into current, which is expressed in amperes. Using the previously established formulas from our work, the power density (PD), current density (CD), and internal resistance (R) have been computed [24]. Furthermore, the investigation on polarization was carried out at the peak voltage. The achievement was made by changing the external resistance, ranging from 5000 to 100 ῼ, in increments of 20 s for each resistance. Cyclic v
oltammetry, also known as CV, was used to investigate the electrochemical oxidation–reduction process occurring at the electrode surface. The bioelectrochemical characteristics of the electrode’s surface were examined by gradually varying the voltage at a rate of 5 mV/s, ranging from 0.8 to 0.8 V, for the whole experiment. The MFC reactor had a platinum wire as the counter electrode and a glassy carbon as the working electrode. The reference electrode consisted of silver chloride and silver ions. A comprehensive analysis was conducted to assess the capabilities of GO anode, using the reference electrode as a standardized baseline. To analyze the biofilm formation rate, one may measure the specific capacitance, indicated as Cp (F/g). By using Eq. 1 on the collected data from the CV measurement, it becomes possible to calculate Cp.
where k is the scanning rate, A is the CV curves area, m is the loaded sample, and \((V2-V1)\) is the potential range.
Evaluation of the efficacy of Pb2+ ion and Hg2+ ion removal was conducted using an atomic adsorption spectrometer. Two mL of the chamber’s solution was removed and subjected to a metal analysis in accordance with the protocol for the sampling interval, which was set at 10 days. Utilizing Eq.2, the computation was carried out in order to ascertain the proportion of removal that was successful.
where R.E % is a removal percentage and \(C\text{intial}\) is the starting concentration while \(C\text{final}\) is the final concentration.
Furthermore, to audit the exterior sound structure of the electrodes in relation to biofilm, scanning electron microscopy (SEM) in combination with electron dispersive X-ray (EDX) was required. The elemental compositions of the electrodes in the wastewater that had metal added were ascertained using an EDX analysis. This investigation contributes to the understanding of the electrodes’ adsorption impact. Figure 1 displays a flow chart that illustrates this study while a general overview of the work is presented in Figure S2.
3 Results and discussion
3.1 GO material characterizations
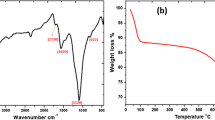
FTIR spectroscopy was used to examine the synthesized material (GO). The points seen in the GO FTIR spectra at 1150 cm−1, 1300 cm−1, 1600 cm−1, and 3398 cm−1 were identified as resulting from the distortion and stretching vibrations of OH, C–OH, C–OH, and C-O groups, respectively, as shown in Fig. 2a. It was noticed that these peaks were present in the spectra of GO. Oxygen-based functional structures were present on the border and foundation planes of the synthesized GO, which contributed to an improvement in the space between layers and the hydrophilic properties of the material. When compared to the highest that happened at 1600 cm−1, which was related to C = C skeletal vibrations, the peak that occurred at 1150 cm−1 belonged to C–OH bending with intermingled stretches with C-O vibrations [39]. In the spectrum, both peaks were discernible to viewers. It was determined that the C–OH hydroxyl stretch vibration was the cause of the significant peak that was seen at 3398 cm−1 [40]. The GO structure exhibited both C-O group stretching vibrations and OH group stretching vibrations, indicating the presence of a carboxyl group in the structure. Additionally, a comprehensive study on GO synthesis was carried out by Jahan et al. [41] and Islam et al. [42], providing a valuable framework for further research and understanding.
In Fig. 2b, the XRD patterns of GO have been presented. A peak at a wavelength of 2Ɵ = 24.1° indicated the graphite oxidation [24]. However, this peak cannot provide evidence that the graphite oxide was synthesized. At a wavelength of 2° = 9.8°, an additional peak was seen, which served as further proof that the synthesis of GO was performed successfully. Furthermore, the peak 2Ɵ = 9.8° demonstrated that there were several oxygen functional groups. This was due to the fact that the intermediate space of GO (0.84 nm) was larger than that of graphite (0.34 nm), which further confirmed the successful synthesis of GO. Further, due to noise, several irrelevant peaks are there, such as 15°, which is not important to discuss and might indicate any impurity of material.
The method of SEM was used in order to evaluate the morphological characteristics of the materials that were recently synthesized. Figure 3a depicts a scanning electron micrograph of GO. Due to the efficient exfoliation process, the GO particles were found to be homogeneous in shape and size, with some measuring up to three micrometers in diameter. This was shown by the findings, which demonstrated that the GO particles were present. The transmission electron micrographs (TEM) of the GO material revealed a significant amount of structural transparency. This was shown by the morphology of the components, which indicated that a considerable degree of oxidation had taken place. Figure 3b illustrates how the fine powder of Moringa that was used in the manufacturing of GO led to the surface of GO having an appearance that was very coherent and smooth. This sort of indication has been shown by previous study [43].
Using an EDX that was paired with a SEM allowed us to explore the material’s elemental foundation. Figure 4 displays all the elemental compositions of the produced GO material. The carbon content of the GO was determined to be 26.65%, whereas the oxygen content was 72.16%.
These results demonstrated the very high purity of the synthesized materials. The glass slide and the oxidation effect that was applied to the sample might be responsible for the presence of trace levels of S, Cl, K, and Si.
3.2 Analytical results and discussion
Figure 5a shows the events that occurred when the metal removal process included in an MFC operation started to generate electricity. During the process of removing metal, this incident was discovered. An announcement was made on the sixteenth day stating that the voltage range had reached 175 mV, the maximum level ever recorded in the experiment’s history. This marked the achievement of the highest step to date. Data showed that voltage production began at a reasonable rate and rose steadily until reaching its peak on day 16. After that, a distinct contour representing a continuous voltage pattern becomes visible for the first time. In contrast, the voltage started to decrease after a short while, decreasing all the way to 175 mV on day 16, and then it continued to fall until it reached its present level. Decreases in voltage output are signs that many bacterial species are nearing the end of their life cycles. Researching the issue for some time made it quite clear that a low voltage was still the prevalent tendency. The existence of a low voltage caused this to happen. This was the only reasonable conclusion to draw from the studies that were conducted. There is evidence that the exoelectrogens cannot get back in control of the activity, which supports the idea that the oxidation of the organic substrate is almost over or will be soon. It seems from the result of this analysis that the voltage achieved its maximum end on the 16th day of the period under consideration for the measurement. In spite of this, a number of scientific research have shown that the area of a metal that has the maximum voltage is also a good indication of metal removal [44, 45].
Furthermore, the significance of polarization was examined by dissecting the interactions among CD, PD, and V based on the level of polarization, using a wide range of environmental resistances. The CD and the V have an inverse proportional connection, as shown in Fig. 5b. The CD was 14.065 mA/m2 and the PD reached 1.49 mW/m2 at a resistance of 1000 ῼ. The experiment concluded with the following values: PD = 0.95 mW/m2, CD = 5.03 mA/m2, with external resistance = 5000 ῼ. A current density of 38.46 mA/m2 and a power density of 1.11 mW/m2 were produced with a resistance of 100 ῼ.
There is a flow of electrons that is effective; it is necessary for the internal and exterior forms of resistance to be similar to one another. This occurs as a result of the fact that both types of resistance are required for the proper transmission of electrons. As the level decreases, the severe resistance of the interior conditions shows a tendency towards lowering when evaluated by electron transit, and this course lasts as the level reduces [46]. There is still enough creation and movement of electrons for the process to be successful, even if the rate of potential maintenance is slower when the external resistance is low. The potential is nevertheless supported, even if it moves at a slower speed when external resistance is minimal [46]. Despite the reduced rate at which the potential regulates, it remains the case. Although the occurrence of potential stability is hindered when external resistance is minimal, it does not imply that it does not occur at all [46]. The voltage instability that arises from the enhanced mobility of electrons is due to their higher mobility. The cathodic reaction was augmented due to an external oxygen source. Consequently, this helped to maintain a consistent voltage output despite the presence of high resistance. Through the use of the equation for internal resistance, it was determined that the internal resistance in this particular work was 796 ῼ. This particular pattern is used in a variety of various research to provide an explanation for the results of electrochemical performance [47,48,49].
Figure 6 illustrates the progression of this event over the course of time. It also illustrates the variations in cell conductivity that the phenomenon has undergone. The procedure continued for 25 days, and data on conductivity was acquired at many different points throughout that time. From day one, when the value was 1600 mS/cm, to day zero, when it was 2900 mS/cm, these numbers have been rising consistently. This progression has occurred from the very beginning to the very end. The pace of growth has been uninterrupted up to the present day. A drop that was not only progressive but also constant began on day 15, and it continued until the very final day of the procedure. This decrease remained until the very end of the process. It is reasonable to assume that a substantial quantity of electricity was produced on day 15 due to the high conductivity; the facts corroborate this assumption. The greatest voltage was generated over days 15–17, suggesting that this was the experimental high point. This result was reached by examining the experimental pattern of voltage generation. It has been recorded in the previous literature that after a given period of time has passed, the efficacy of the system begins to begin to deteriorate as a result of a wide variety of challenges [46, 50]. Several environmental elements might be the cause of these issues, depending on the circumstances. Additionally, Rojas-Flores et al. [51], who carried out study in order to have a better understanding of the influence of conductivity, gave an analysis that was extremely similar.
By carrying out CV tests, it was possible to investigate the electrical resistance and redox potential of the operation. Over the course of the procedure, the CV was monitored on many occasions in order to get an idea of the pace at which oxidation–reduction was taking place [46]. The rate of scan that is determined to be most significant in both the forward and backward routes of movement is shown in Fig. 7a. The different current levels were discovered at different values in each of the scans (forward and reverse versions). On day 25, the value of the backward scan reached its maximum point, while the value of the forward scan reached its highest point on the same day. Specifically, the oxidation and reduction processes that occur inside the cell are represented by the forward and backward scans, respectively. A consistent increase in the rates of the oxidation–reduction activities was seen, as shown by the observation. Therefore, the maximal oxidation rate is responsible for producing the greatest quantity of electron-protons that are produced as a consequence of this. The research also demonstrates that some kinds of bacteria have the ability to oxidize glucose in a short amount of time. In addition, the Cp value was computed in order to investigate the duration of time required for the creation and solidification of an anode biofilm (Fig. 7b). The slow formation of the biofilm was evidenced by the continued increase in the Cp value over the course of time. Based on the findings, it was determined that the Cp value was highest across all of the different observation days. As an example, the first day has 0.00001 F/g, whereas the twenty-fifth day has 0.00004 F/g. The fragility of the biofilm was ultimately responsible for the drop in size. It was discovered that the biofilm had reached its full development between days 15 and 20. In addition, experts in the area explored this line of investigation in order to characterize the creation of biofilms and their continued stability [50].
3.3 Biological characterizations and discussion
The removal trend of toxic metals by MFCs is shown in Table 2, which covers a period of 25 days of operation. It turned out that the tendency of removal was steadily increasing. For the purpose of removing the toxic metal ions into insoluble forms, the bacterial colony used glucose as a fuel source. When contrasted with the findings of the earlier investigations, the current findings were quite positive. For example, Yaqoob et al. [30] showed that employing oil palm trunk sap waste as an organic substrate for a period of 90 days resulted in about 75% removal of Pb and 60% removal of Hg from MFCs. They used traditional graphite rod as an electrode. When compared to previous studies that used conventional electrodes and glucose, the current one found that glucose improved removal efficiency. In this investigation, it was found that synthesized GO greatly enhanced electron transport efficiency, which in turn accelerated removal efficiency. The extremely hazardous nature of Hg makes the efficiency of its removal a novel endeavor in the area of MFCs. In this study, the presence of glucose allowed the bacterial population to effectively remove over 65% of the Hg. Additionally, at first, since the inoculation source was new, the removal efficiency was shown to rise. In the course of the operation, the pH will rise as a result of the increased number of electrons that are moving towards the anode. This will result in a large reduction in the power of the exoelectrogens. Following the achievement of the highest possible elimination efficiency, the bacterial growth pattern exhibited a decrease in the duration of their life span. This is still another cause. A certain life pattern is followed by the bacterial population, and this pattern is to be converted into the death phase. In the event that the bacterial population entered the death phase, the capacity to decrease metals was diminished, and as a consequence, the removal efficiency was poor. There are a number of additional environmentally friendly characteristics that are involved in the functioning of MFCs, such as the pH of the solution, the external resistance, and the growth of biofilm [33, 52]. These parameters will need further investigation in the future.
In biological analysis, the SEM–EDX is used to study biofilm formation and biofilm development on the anode surface. A biofilm, often called a “city of bacterial species,” is a collection of bacteria that has congregated in one area. One factor that causes the organic substrate to be oxidized is the biofilm, which then transfers the electrons that were produced to an anode electrode [53]. The composition of the biofilm is mostly composed of bacteria to the extent of 2–5%, with a water content of 97% and an extracellular polymeric substance (EPS) content of 3–6% [54]. To add fuel to surgery, the EPS is the most essential component in the oxidation process, which is responsible for the production of electrons and protons [55]. 1–60% of EPS is made up of protein, 40–95% is made up of polysaccharide, 10% is made up of nucleic acids, and 40% is made up of lipids. The term “biofilm ageing regulator” is widely used to refer to EPS [56, 57]. As soon as the supply of organic substrate is exhausted, the amount of EPS will drop, which will lead to a reduction in the production of electrons. A SEM picture of treated anode and cathode electrodes is shown in Fig. 8. When viewed using a scanning electron microscope (SEM), the treated anode with biofilm (Fig. 8a) showed a bacterial population that had grown in a highly thick and healthy manner on the surface of the anode. The bacterial population does not experience any harmful consequences, as seen in this picture, and the biofilm continues to expand without interruption. Similar to the previous example, there are also bacterial colonies that may be observed on the surface of the cathode (Fig. 8b). Supplying glucose to the bacterial populations allowed them to consistently form biofilm on the anode surface. Both photographs displayed almost similar morphologies, according to the SEM pictures of biofilms. They are showing off a rod-or tube-shaped limb. shows that the samples contain a very low concentration of a certain kind of bacteria. Several investigations showed that scanning electron microscopy images of biofilms with rod-or tube-shaped appendages revealed the presence of pili-typed conductive bacteria. Bacteria that use their pili as a means of transmitting electrons are known as conductive pili-typed bacteria. The pili is the cell membrane of the bacterium that acts as an electrical conductor. The limited bacterial species are found in very dense conditions on the biofilm surface due to the high sugar content produced from the provided organic substrate. In order to determine if the anode surface contained any metals, an EDX examination of the anode biofilm was carried out. According to Fig. 9, there was not a single trace of metal on the anode biofilm surface. At the end of the operation, a sludge was produced, which numerous investigations suggest might be a form of metal oxide [46, 50]. When compared to the metal ions, the solid form of metal is much less hazardous to human health. For the most part, the findings of the research demonstrated that the metal ions did not exhibit any toxicity towards the bacterial population while the MFCs were operating.
3.4 Electron transportation mechanism
Microbiological catalysts play a crucial role in MFCs by oxidizing organic substrates to produce electrons and protons. This is the fundamental operating concept of MFCs [58]. In the past, a wide variety of bacterial taxa, including Proteobacteria, Actinobacteria, Firmicutes, fungi, and a great deal of other organisms, were thought to be exoelectrogens. A monosaccharide known as glucose is used in the study that is being presented here. The bioelectrogenesis process, which was carried out by the bacterial population, was responsible for converting it into the oxidation step. The following is a representation (Eqs. 3-5) of the oxidation and reduction processes that were investigated in this study [24]:
Oxidation reaction:
Reduction reaction:
Overall reaction:
where C6H12O6 represents the glucose, H2O is the water, CO2 is the carbon dioxide, H+ is the proton, and e− is the electrons.
It makes acetyl coenzyme A (acetyl CoA) when it breaks down glucose from glucose and then further breaks down glucose into pyruvate. The acetyl CoA then proceeded to complete one Kreb’s cycle, which resulted in the production of three molecules of reduced nicotinamide adenine dinucleotide (NADH) and one molecular reduction of flavin adenosine dinucleotide (FADH2) [59]. Using the electron transport chain (ETC), the generated NADH and FADH2 were transported. When everything was finished, the 34-adenine triphosphate (ATP) molecules were made. This has been the typical path taken by the exoelectrogenic species. The process of electron transport to the anode surface is universal for all exoelectrogens. The transportation routes are explained below and presented in Fig. 10.
-
1.
Direct electron transfer: Electrons are transported directly from one point to another based on two distinct subcategories. First is the transport of electrons via the conductive pili of several bacterial species. The conductive pili-type bacteria are the only bacteria that are capable of using this technique. Some of the most well-known conductive pili-typed bacterial species are Bacillus, Klebsiella pneumoniae, Acinetobacter, and Leucobacter species. There is a portion of the bacteria’s body known as the conductive pili, and it is conductive in the same way that metal is. The second method of direct electron transportation involves the use of redox-active proteins for the purpose of electron transfer. The proteins OmcZ, OmcB, OmcE, OmcT, and OmcS are the most prevalent redox-active proteins, and they are responsible for transporting electrons [60].
-
2.
Indirect electron transfer: The reduced and oxidized shuttle molecules were the means by which the procedure for the indirect transfer of electrons was carried out. Certain types of bacteria were able to manufacture their own self-reduced and oxidized shuttle molecules, which they then used in order to perform electron transport. This process was seen to be obeyed by a number of different bacterial species groups, including Geobacteraceae and Desulfuromonadacea. The following MtrF, MtrC, OmcA, MtrE, and components are the molecules that make up the self-electron shuttle mechanisms [60].
This study followed the rules of the direct electron transportation process, which involves transferring electrons via conductive pili. The biological test, more precisely the pictures obtained from the scanning electron microscope, revealed that it had a rod-shaped morphology, but the identification of the bacteria revealed that it was a conductive pili-type species. There are signs indicating that the transport of electrons was facilitated by the use of conductive pili. During the reduction process occurring on the other side, protons are transported directly to the cathode by the use of a proton exchange membrane (PEM) [30]. Through the redox routes, the metal undergo a reduction that results in their transformation into an insoluble state. Generally, the process of reduction may be written as follows (Eqs. 6-11):
-
–Conversion of Pb.2+ into Pb(s)
$$\begin{array}{ccc}{\text{Pb}}^{2+}{2\text{e}}^{-}& \to & {\text{Pb}}_{(\text{s})}\end{array}$$(6)$$\begin{array}{ccc}{2\text{Pb}}^{2+}+{2\text{H}}_{2}\text{O}& \to & 2\text{PbO}+4\text{H}\end{array}$$(7)$$\begin{array}{ccc}\text{PbO}+{2\text{e}}^{-}+{2\text{H}}^{+}& \to & {\text{Pb}}_{(\text{s})}+{\text{H}}_{2}\text{O}\end{array}$$(8) -
–Conversion of Hg.2+into Hg(s)
$$\begin{array}{ccc}{\text{Hg}}^{2+}+{2\text{e}}^{-}& \to & {\text{Hg}}_{(\text{s})}\end{array}$$(9)$$\begin{array}{ccc}{2\text{Hg}}^{2+} + {2\text{H}}_{2}\text{O}& \to & 2\text{HgO }+{4\text{H}}^{+}\end{array}$$(10)$$\begin{array}{ccc}\text{Hg O }+ {2\text{e}}^{-}+{2\text{H}}^{+}& \to & {\text{Hg}}_{(\text{s})}+ {\text{H}}_{2}\text{O}\end{array}$$(11)
4 Modern challenges and future suggestions
Biological processes allow MFC technology to transform chemical power into electricity, which makes it useful for treating a wide variety of chemical substances. Nevertheless, there have been a number of obstacles that have prevented the technique from ever being widely used in renewable energy or wastewater treatment, despite its many benefits. Electrode material stability, organic substrate stability, cathode-related problems, scaling-up concerns, and long-term bacterial stability are all part of these problems [61]. Material choices like carbon fiber paper or graphite rods provide issues with scaling due to their low cost-effectiveness, structural robustness, and durability. The stability of the electrode material, which is vital for use in industrial processes, is often neglected in favor of research concentrating on output power [62,63,64].
Research efforts moving forward should therefore concentrate on investigating conductive coatings as a way to fortify the material's structure. The anode electrodes that were developed by us (GO from waste) were put through a continuous operation for a period of 25 days in this investigation. In a remarkable turn of events, GO managed to maintain a considerable voltage output into the last days. According to these findings, anodes that have been coated with biowaste exhibit a remarkable level of stability when compared to graphite rods that have not been treated and are available for commercial use. Having declared that, it is of the utmost importance to emphasize that the stability of the anode is not adequate on its own; the maintenance of the electroactivity of microorganisms is equally crucial. Microbial electroactivity often resulted in two voltage cycles, with a decrease being seen during the second cycle, which indicated a reduction in the stability of the microorganisms on the surface. Previous studies have highlighted the relevance of choosing a suitable inoculum in order to solve issues that are associated with the stability of microorganisms [65]. When it comes to preserving microbiological stability, critical parameters such as anode surface area and biological compatibility play a vital role. On a consistent basis, the introduction of fresh microbial mixes throughout operation has the potential to improve the life cycles of microorganisms, which in turn leads to an increase in stability. The investigation of the stability of bacterial life cycles is a potentially fruitful path for the improvement of anode electrodes that exhibit constant microbial electroactivity [66]. In this particular investigation, it is essential to emphasize that every single one of the anode electrodes that were used was successful in removing contaminants from the MFCs. In addition, it is essential to investigate a wide variety of materials for biocathodes in order to maximize performance. This is because the hurdles that are presented by materials and cathode topologies in the process of developing high-performance MFCs are significant. In contrast to the results of other studies, the findings of the current investigation reveal a number of distinctive features. Nevertheless, after further inspection, it becomes clear that the energy efficiency may not be sufficient for applications that are carried out on a big scale. The most important problem is with the movement of electrons, which was negatively affected by the inefficiency in electron transport [67,68,69]. It was connected with the quality of the commercial graphite electrodes that were utilized in this experiment. This was the primary problem that was found throughout this examination. Furthermore, owing to the high oxidation potential of oxygen, the use of oxygen as an electron acceptor in the cathode chamber results in a large amount of energy consumption. It is vital to address the obstacles that are associated with oxygen consumption. These challenges include cathode-air surface contact concerns and potentially expensive catalysts [70]. Accelerating the production of MFCs is essential for the treatment of wastewater and the development of high-current density. Nevertheless, increasing the size of individual reactors leads to a decrease in power density as a consequence of increased ohmic resistance and the existence of inactive reactor volume, which ultimately results in energy production performance that is at a level that is less than ideal [71]. The performance of upscaled MFC stacks has been the subject of a great number of research; nonetheless, there are still a number of obstacles that continue to exist, the most notable of which are power density variations that are caused by reversed voltage in certain serially linked cell units [72]. Minimizing internal resistance—which includes kinetic, ohmic, and transport resistance—is crucial to maximizing energy recovery using MFCs.
5 Conclusion
The current study examines the effectiveness of MFCs anode made from Moringa and the removal of hazardous metal ions from wastewater. One of the most recent designs for bioelectrochemical fuel cells is the MFCs. This design is one of the most recent because it is more cost-effective, easier to control, and less detrimental to the environment than other options. During the research, it was found that anodes constructed of moringa material increased electron mobility, which ultimately led to higher energy performance. Furthermore, this was shown by many results acquired via the characterization of the product. The development of a substantial biofilm by GO anodes demonstrated its ability to provide long-lasting protection and chemical stability for a period of 25 days. The waste-derived GO produced an electrical potential of 175 mV over a period of 16 days, with a power density of 1.49 mW/m2. The measured internal resistance was 796 ῼ, whereas the exterior resistance was 1000 ῼ. It seems that electron transportation works effectively. In addition, there was a process of metal ion removal that occurred, and significant advancements were made rapidly. The removal efficiency was significant, with Pb being removed at a rate of 75.10% and Hg at a rate of 65%. In the future, it is advisable for MFCs to use food waste as an organic feedstock. This is an ideal circumstance. With the use of basic organic materials, a restricted number of studies have explored the capacity of MFCs to produce energy and purify wastewater. Although the organic substrate of the MFCs was not sufficiently stable at the time, the electrodes developed, resulting in noticeable improvements in electron transport. Therefore, it is imperative that future research concentrates on investigating the various organic substrate options available for use in MFCs from an engineering viewpoint. Given the fact that MFCs have convenient access to a wide array of diverse waste materials, including carbohydrates such as waste from local fruit processing, the organization may explore the feasibility of using these resources.
Data availability
The authors confirm that all data underlying the findings are fully available without restriction. Data can be obtained after submitting a request to the corresponding/first author.
References
Ramya M, Kumar PS (2022) A review on recent advancements in bioenergy production using microbial fuel cells. Chemosphere 288:132512
Logan BE (2005) Simultaneous wastewater treatment and biological electricity generation. Water Sci Technol 52(1–2):31–37
Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv 25(5):464–482
Zhou M, Chi M, Luo J, He H, Jin T (2011) An overview of electrode materials in microbial fuel cells. J Power Sources 196(10):4427–4435
Potter M (1910) On the difference of potential due to the vital activity of microorganisms. Proc Univ Durham Phil Soc 3:245–249
Franks AE, Nevin KP (2010) Microbial fuel cells, a current review. Energies 3(5):899–919
Al-Zaqri N, Alamzeb M, Hussain F, Oh S-E, Umar K (2023) Bioenergy generation and phenol degradation through microbial fuel cells energized by domestic organic waste. Molecules 28(11):4349
Rabaey K, Angenent L, Schroder U, Keller J (2009) Bioelectrochemical systems. IWA publishing
Wei J, Liang P, Huang X (2011) Recent progress in electrodes for microbial fuel cells. Biores Technol 102(20):9335–9344
Li S, Cheng C, Thomas A (2017) Carbon-based microbial-fuel-cell electrodes: from conductive supports to active catalysts. Adv Mater 29(8):1602547
Ma J, Zhang J, Zhang Y, Guo Q, Hu T, Xiao H, Lu W, Jia J (2023) Progress on anodic modification materials and future development directions in microbial fuel cells. J Power Sources 556:232486
Borja-Maldonado F, Zavala MÁL (2022) Contribution of configurations, electrode and membrane materials, electron transfer mechanisms, and cost of components on the current and future development of microbial fuel cells. Heliyon 8(7)
Schaetzle O, Barrière F, Baronian K (2008) Bacteria and yeasts as catalysts in microbial fuel cells: electron transfer from micro-organisms to electrodes for green electricity. Energy Environ Sci 1(6):607–620
Naaz T, Kumar A, Vempaty A, Singhal N, Pandit S, Gautam P, Jung SP (2023) Recent advances in biological approaches towards anode biofilm engineering for improvement of extracellular electron transfer in microbial fuel cells. Environ Eng Res 28(5)
Peera SG, Maiyalagan T, Liu C, Ashmath S, Lee TG, Jiang Z, Mao S (2021) A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells. Int J Hydrogen Energy 46(4):3056–3089
Song H-L, Zhu Y, Li J (2019) Electron transfer mechanisms, characteristics and applications of biological cathode microbial fuel cells–a mini review. Arab J Chem 12(8):2236–2243
Idris MO, Noh NAM, Hussin MH, Shukri IAM, Hamidon TS (2023) Simultaneous naphthalene degradation and electricity production in a biowaste-powered microbial fuel cell. Chemosphere 340:139985
Huggins T, Wang H, Kearns J, Jenkins P, Ren ZJ (2014) Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Biores Technol 157:114–119
Sharma M, Salama E-S, Thakur N, Alghamdi H, Jeon B-H, Li X (2023) Advances in the biomass valorization in bioelectrochemical systems: a sustainable approach for microbial-aided electricity and hydrogen production. Chem Eng J 465:142546
AaK Al-Bawwat, Jurado F, Gomaa MR, Cano A (2023) Availability and the possibility of employing wastes and biomass materials energy in Jorda. Sustainability 15(7):5879
Ala’a K, Gomaa MR, Cano A, Jurado F, Alsbou EM, (2024) Extraction and characterization of Cucumis melon seeds (Muskmelon seed oil) biodiesel and studying its blends impact on performance, combustion, and emission characteristics in an internal combustion engine. Energy Convers Manag 23:100637
AaK Al-Bawwat, Cano A, Gomaa MR, Jurado F (2023) Availability of biomass and potential of nanotechnologies for bioenergy production in Jordan. Processes 11(4):992
Aiswaria P, Mohamed SN, Singaravelu DL, Brindhadevi K, Pugazhendhi A (2022) A review on graphene/graphene oxide supported electrodes for microbial fuel cell applications: challenges and prospects. Chemosphere 296:133983
Yaqoob AA, Ibrahim MNM, Yaakop AS, Umar K, Ahmad A (2021) Modified graphene oxide anode: a bioinspired waste material for bioremediation of Pb2+ with energy generation through microbial fuel cells. Chem Eng J 417:128052
Noh NAM, Daud NNM, Hussin MH, AlObaid AA (2024) Assessment of biomass material as valuable electrode for high energy performance in microbial fuel cell with biodegradation of organic pollutant. Fuel 371:132059
Daud NNM, Hussin MH (2024) Evaluating the electrode materials to improve electricity generation with the removal of multiple pollutants through microbial fuel cells. Biomass Convers Biorefinery 1–22. https://doi.org/10.1007/s13399-023-05256-9
Yu F, Wang C, Ma J (2016) Applications of graphene-modified electrodes in microbial fuel cells. Materials 9(10):807
Mohan VB, Brown R, Jayaraman K, Bhattacharyya D (2015) Characterisation of reduced graphene oxide: effects of reduction variables on electrical conductivity. Mater Sci Eng, B 193:49–60
Dappe Y, Basanta MA, Flores F, Ortega J (2006) Weak chemical interaction and van der Waals forces between graphene layers: a combined density functional and intermolecular perturbation theory approach. Phys Rev B 74(20):205434
Asim AY, Ibrahim MNM, Yaakop AS, Ahmad A (2021) Application of microbial fuel cells energized by oil palm trunk sap (OPTS) to remove the toxic metal from synthetic wastewater with generation of electricity. Appl Nanosci 11(6):1949–1961
Mathuriya AS, Yakhmi J (2014) Microbial fuel cells to recover heavy metals. Environ Chem Lett 12(4):483–494
Fadzli F, Ibrahim M, Yaakop A (2023) Benthic microbial fuel cells: a sustainable approach for metal remediation and electricity generation from sapodilla waste. Int J Environ Sci Technol 20(4):3927–3940
Ezziat L, Elabed A, Ibnsouda S, El Abed S (2019) Challenges of microbial fuel cell architecture on heavy metal recovery and removal from wastewater. Frontiers in Energy Research 7:1
Roy H, Rahman TU, Tasnim N, Arju J, Rafid MM, Islam MR, Pervez MN, Cai Y, Naddeo V, Islam MS (2023) Microbial fuel cell construction features and application for sustainable wastewater treatment. Membranes 13(5):490
Rahman TU, Roy H, Islam MR, Tahmid M, Fariha A, Mazumder A, Tasnim N, Pervez MN, Cai Y, Naddeo V (2023) The advancement in membrane bioreactor (MBR) technology toward sustainable industrial wastewater management. Membranes 13(2):181
Kwak J-H, Islam MS, Wang S, Messele SA, Naeth MA, El-Din MG, Chang SX (2019) Biochar properties and lead (II) adsorption capacity depend on feedstock type, pyrolysis temperature, and steam activation. Chemosphere 231:393–404
I. Zinicovscaia (2016) Conventional methods of wastewater treatment, Cyanobacteria for bioremediation of wastewaters 17–25. https://doi.org/10.1007/978-3-319-26751-7_3
Cai Z, Jiang C, Xiao X, Zhang Y, Liang L 2018 Lignin-based biochar/graphene oxide composites as supercapacitor electrode materials, IOP Conference Series: Materials Science and Engineering, IOP Publishing, p 012046. https://doi.org/10.1088/1757-899X/359/1/012046
Arampatzidou AC, Deliyanni EA (2016) Comparison of activation media and pyrolysis temperature for activated carbons development by pyrolysis of potato peels for effective adsorption of endocrine disruptor bisphenol-A. J Colloid Interface Sci 466:101–112
Durmus Z, Kurt BZ, Durmus A (2019) Synthesis and characterization of graphene oxide/zinc oxide (GO/ZnO) nanocomposite and its utilization for photocatalytic degradation of basic Fuchsin dye. ChemistrySelect 4(1):271–278
Jahan N, Roy H, Reaz AH, Arshi S, Rahman E, Firoz SH, Islam MS (2022) A comparative study on sorption behavior of graphene oxide and reduced graphene oxide towards methylene blue. Case Studies in Chemical and Environmental Engineering 6:100239
Islam MS, Roy H, Ahmed T, Firoz SH, Chang SX (2023) Surface-modified graphene oxide-based composites for advanced sequestration of basic blue 41 from aqueous solution. Chemosphere 340:139827
Ealias AM, Saravanakumar M 2017 A review on the classification, characterisation, synthesis of nanoparticles and their application, IOP Conf Ser Mater Sci Eng, p. 032019.https://doi.org/10.1088/1757-899X/263/3/032019
Fadzli FS, Rashid M, Yaqoob AA, Ibrahim MNM (2021) Electricity generation and heavy metal remediation by utilizing yam (Dioscorea alata) waste in benthic microbial fuel cells (BMFCs). Biochem Eng J 172:108067
Chen Z, Higgins D, Yu A, Zhang L, Zhang J (2011) A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ Sci 4(9):3167–3192
Aleid GM, Alshammari AS, Alomari AD, Almukhlifi HA, Ahmad A (2023) Dual role of sugarcane waste in benthic microbial fuel to produce energy with degradation of metals and chemical oxygen demand. Processes 11(4):1060
Sajana T, Ghangrekar M, Mitra A (2014) Effect of presence of cellulose in the freshwater sediment on the performance of sediment microbial fuel cell. Biores Technol 155:84–90
Sajana T, Ghangrekar M, Mitra A (2013) Application of sediment microbial fuel cell for in situ reclamation of aquaculture pond water quality. Aquacult Eng 57:101–107
Azari MAG, Gheshlaghi R, Mahdavi MA, Abazarian E (2017) Electricity generation from river sediments using a partitioned open channel sediment microbial fuel cell. Int J Hydrogen Energy 42(8):5252–5260
Aleid GM, Alshammari AS, Alomari AD, Ahmad A, Alaysuy O, Ibrahim MNM (2023) Biomass and domestic waste: a potential resource combination for bioenergy generation and water treatment via benthic microbial fuel cell, Environ Sci Pollut Res 1–14 >https://doi.org/10.1007/s11356-023-29430-8
Rojas-Flores S, Benites SM, La Cruz-Noriega D, Cabanillas-Chirinos L, Valdiviezo-Dominguez F, Quezada Álvarez MA, Vega-Ybañez V, Angelats-Silva L (2021) Bioelectricity production from blueberry waste. Processes 9(8):1301
Mathuriya AS, Yakhmi J (2014) Microbial fuel cells to recover heavy metals. Environ Chem Lett 12:483–494
Rusyn I (2021) Role of microbial community and plant species in performance of plant microbial fuel cells. Renew Sustain Energy Rev 152:111697
Singh S, Songera DS (2012) A review on microbial fuel cell using organic waste as feed. CIBTech Journal of Biotechnology 2(1):17–27
Feng Q, Xu L, Liu C, Wang H, Jiang Z, Xie Z, Liu Y, Yang Z, Qin Y (2020) Treatment of shale gas fracturing wastewater using microbial fuel cells: mixture of aging landfill leachate and traditional aerobic sludge as catholyte. J Clean Prod 269:121776
Di Martino P (2018) Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS microbiology 4(2):274
Kumar MA, Anandapandian KTK, Parthiban K (2011) Production and characterization of exopolysaccharides (EPS) from biofilm forming marine bacterium. Braz Arch Biol Technol 54(2):259–265
Nevin KP, Kim B-C, Glaven RH, Johnson JP, Woodard TL, Methé BA, DiDonato RJ Jr, Covalla SF, Franks AE, Liu A (2009) Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS ONE 4(5):e5628
Mailloux R (2015) Still at the center of it all; novel functions of the oxidative Krebs cycle. Bioenergetics 4(1):1–14
Parveen T, Ahmad A, Lokhat D, Setapar SHM (2021) A glimpse into the microbial fuel cells for wastewater treatment with energy generation, Desalin. Water Treat 214:379–389
Choudhury P, Prasad Uday US, Bandyopadhyay TK, Ray RN, Bhunia B (2017) Performance improvement of microbial fuel cell (MFC) using suitable electrode and Bioengineered organisms: A review. Bioengineered 8(5):471–487
You J, Ye L, Kong X, Duan Y, Zhao J, Chen J, Chen D (2023) Efficient biodechlorination at the Fe3O4-based silicone powder modified chlorobenzene-affinity anode. J Hazard Mater 457:131794. https://doi.org/10.1016/j.jhazmat.2023.131794
Do M, Ngo H, Guo W, Liu Y, Chang S, Nguyen D, Nghiem L, Ni B (2018) Challenges in the application of microbial fuel cells to wastewater treatment and energy production: a mini review. Sci Total Environ 639:910–920. https://doi.org/10.1016/j.scitotenv.2018.05.136
Daud NNM, Hussin MH, Yong C, AlObaid AA (2024) Optimizing microbial fuel cells performance: an innovative approach integrating anode materials, dual-pollutant treatment, and long-term operation. Fuel 364:131160
Mathuriya AS (2013) Inoculum selection to enhance performance of a microbial fuel cell for electricity generation during wastewater treatment. Environ Technol 34(13–14):1957–1964. https://doi.org/10.1080/09593330.2013.808674
Koók L, Nemestóthy N, Bélafi-Bakó K, Bakonyi P (2020) Investigating the specific role of external load on the performance versus stability trade-off in microbial fuel cells. Biores Technol 309:123313
Zhang X, Li X, Zhao X, Li Y (2019) Factors affecting the efficiency of a bioelectrochemical system: a review. RSC Adv 9(34):19748–19761
Kumar R, Singh L, Zularisam AW (2016) Exoelectrogens: recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew Sustain Energy Rev 56:1322–1336
Huang L, Regan JM, Quan X (2011) Electron transfer mechanisms, new applications, and performance of biocathode microbial fuel cells. Biores Technol 102(1):316–323
Shi X, Feng Y, Wang X, Lee H, Liu J, Qu Y, He W, Kumar SS, Ren N (2012) Application of nitrogen-doped carbon powders as low-cost and durable cathodic catalyst to air–cathode microbial fuel cells. Biores Technol 108:89–93
You J, Ji Z, Zhao J, Sun H, Ye J, Cheng Z, Kong X, Chen J, Chen D (2023) Configurations of bioelectrochemical reactor for environmental remediation: a review. Chem Eng J 471:144325. https://doi.org/10.1016/j.cej.2023.144325
Lee SH, Ahn Y (2019) Upscaling of microfluidic fuel cell using planar single stacks. Int J Energy Res 43(9):5027–5037
Funding
The author extends his appreciation to the Prince Sattam bin Abdulaziz University for funding this research work through the project number (PSAU/ 2024/01/28434).
Author information
Authors and Affiliations
Contributions
Akil Ahmad: conceptualization, methodology, writing—original draft preparation, visualization, electrochemical tests, biological investigation, reviewing and editing, supervision, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, A. Exploitation of moringa biomass to fabricate graphene electrode for electricity generation with wastewater treatment through microbial fuel cells. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-06134-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-06134-8