Abstract
In the present study, the performance of dual chamber microbial fuel cells (MFC) was investigated employing sludges collected from drains, a textile effluent treatment plant, and the Buriganga river as the sources of microbes, while a mixed liquor prepared from dustbin waste and gruel was used as substrates for the growth of bacteria. Six experiments were carried out under aerobic conditions throughout the study and inspected for seven days. Agar salt bridge was used instead of the typical membrane for proton exchange. For analyzing the electrical parameters of the MFCs such as voltage, current density and power density were recorded for each experiment. Reduction of Biological Oxygen Demands (BOD5) of the sludges was also observed in this study. Regarding electrical performance, the Buriganga sludge afforded the best results demonstrating maximum voltage, current density, and power density as 244.88 mV, 35.16 mA/m2, and 8.61 mW/m2, respectively. However, the BOD5 reduction was found to be 50.15%, 43.64%, 47.32%, and 35.20% for sludges collected from a drain of a residential area, a textile effluent treatment plant, a tannery, and the Buriganga river, respectively. Counts of aerobic Escherichia coli and Bacillus subtilis in different sludges were also documented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
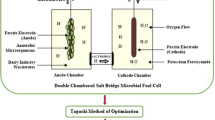
The necessity to reduce carbon emissions globally has gained considerable attention in recent times, leading to the implementation of stricter policies and regulations [1]. As conventional fuel sources are of confined origins and a number of environmental issues are being brought on by carbon emissions, which pose a major threat to human existence and health, it is high time for the exploration for renewable energy sources [2, 3]. Biofuels can be the alternate sources to meet up the energy demand [4]. Microbial fuel cell (MFC) technology has found immense interest in recent years as a promising approach to mitigate energy scarcity globally [5]. MFC is a unique bio-electrochemical system, which can utilize different substances of organic origins as energy source [6], is able to generate electricity from wastes and reducing organic contaminants from wastewater, and is representing a sustainable technology for the future [7]. Exoelectrogens, a specific species of bacteria possessing the catalytic activity, are used in MFC for producing electricity from biomass. This type of bacteria is capable of transferring electrons also [8,9,10]. In a typical MFC, an anode chamber is fed with microbes and substrate; anode and cathode chambers are separated by proton exchangeable membrane (PEM) (Fig. 1). Available bacteria in the anode chamber usually function as catalysts [11,12,13,14]. Electrons generated due to the oxidation of organic substrates are transported from the anode to cathode through an external wire, while the liberated protons transfer through PEM [10]. Nowadays, ionogels are used in PEMs. Ionic liquids possess special characteristics composed of organic cations, and inorganic/organic anions are enclosed in a solid structure within hybrid materials called ionogels. Through the creation of nanochannels and the creation of extensive hydrogen bonding networks with matrix materials and other proton conductors, ionogels in PEMs provide increased conductivity [15, 16]. Thus, in MFC, biodegradation of organic matters and production of electricity take place by the microbes [17, 18]. For example, fermentation of glucose in the presence of Clostridium butyricum produces CO2, electron, and protons as shown in Eq. 1 [19].
A conventional model of MFC [10]
In the cathode compartment, electrons coming through an external load are recaptured by oxygen molecules, which combine with protons to form water [19]. The potential difference coupled to electron flow generates electricity in the fuel cell. Anaerobic MFC is also possible where electrons are generated by bacteria through the anaerobic oxidation of organic matter (by non-fermentative reactions) and passed to the respiratory enzymes in the inner cell membrane of the bacteria [17, 21].
The PEM is employed to separate the substrate molecules in the anode from electron capturing molecules (O2) in the cathode while permitting transfer of H+ from the anode to the cathode compartment. This allows the maintenance of charge balance and eliminates cross-contaminating reactions [20]. The PEM plays vital role in MFC construction. In recent years, many research groups are devoted in developing artificial PEM. Aromatic polymers such as poly (arylene ether ether ketone) (PEEK), poly (arylene ether sulfone), and their derivatives are generally used as high-performance PEMs [22]. Salt bridge has been used as an alternative to PEM by different research groups [23,24,25,26,27,28]. Salt bridges are generally made up of a mixture of agar (C14H24O9), salt, and water which are less expensive than Nafion membranes and have a higher amount of self-resistance to proton transportation [28]. The effect of mediators likeanthraquinone-2,6-disulfonate (AQDS), neutral red, and humic acids to shift electrons to an external electrode from inside the cell was also studied [17, 21]. The laboratory-scale version of an MFC has been still lower than what is shown in an ideal case. The efficiency of an MFC is affected by several parameters [29]. Electrodes are important components of an MFC that help to improve its efficiency [30]. Carbon materials containing graphite fiber brush, carbon cloth, graphite rod, carbon paper, reticulated vitreous carbon (RVC), and carbon felt are the most commonly used materials in anodes because of their stability in microbial cultures, high electric conductivity, and large surface area [10, 31,32,33,34,35]. Furthermore, heterogeneous fabrication methods and modification methods incorporating nano materials have been tested for increasing the power density and expanding the electron accepting capability [36]. As cathode electrodes, carbon paper, carbon felt, carbon brush, carbon fiber, graphite of various types, Pt (Pt is commonly used as a cathode catalyst, while alternative polymer binders such as perfluoro sulfonic, Cu, Cu–Au, tungsten carbide, granular graphite, reticulated vitreous carbon (RVC) have also been tested [37,38,39]. A number of factors which control the release of electrons from substrate molecules in solution, their shifting through an external load, and their recapture by oxygen molecules. Fluctuations of temperature affect device kinetics, mass transfer (activation energy, mass transfer coefficient, and solution conductivity) [40], thermodynamics (free Gibbs energy and electrode potential), and microbial community existence and dispersion [40, 41]. Temperature has been shown to be a significant parameter for electricity generation [42, 43]. Temperature increases power generation of MFC exponentially owing to anodic biofilm growth, which impacts bio catalytic activity and therefore efficiency [44,45,46]. An MFC can operate at temperatures ranging from 5 to 40 °C, with an optimal working range of 18 to 35 °C [47,48,49].
As a process for both wastewater treatment and electricity production, MFCs are being studied in recent years [50]. Different types of wastewaters, which include wastewater from food production, swine wastewater, and sanitary waste, comprise numerous biodegradable natural materials that could be used to run MFCs [50]. MFCs have been first used to purify wastewater in 1991 [52].
In this study, two types of MFC were constructed, one using PET bottles and the other of acrylic box, and in both cases, agar salt bridge was used instead of PEM, and sludges from drains, textile effluent treatment plant, and Buriganga river were used under aerobic condition. Mixed liquor prepared from dustbin waste and starch of boiled rice was used as source of carbohydrates (substrate) for the growth of bacteria. The Biological Oxygen Demand (BOD5) of the sludges was also recorded before and after conducting each experiment. The counts of Escherichia coli and Bacillus subtilis (CFU/ml) in different sludges were also made.
The novelty of the current study is the use of cheap agar salt bridge instead of expensive nafion membrane, dustbin waste as substrate, and different types of sludges as the source of microorganisms. Moreover, MFCs were operated at ambient temperature and without agitation in the cathode chamber, so there was no external power consumption.
2 Materials and methods
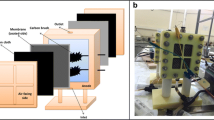
Agar (C14H24O9) and sodium chloride (NaCl) were used for constructing salt bridge, and Sorbitol MacConkey agar (SMAC) and NaCl-Glycine-Kim-Goepfect (NGKG) were used for microbiological study. All analytical grade chemicals were purchased from Merck, Germany, and used as received. Copper mesh and brass mesh (shown in Fig. 2) were collected from local market (Dhaka, Bangladesh) and used as cathode and anode, respectively. An air compressor (Model no. SEBO Air Pump-348A) was used in experiment 6 to provide external air supply in the anode chamber.
Figure 3 illustrates the configuration of MFC that was used during this study. Two types of MFCs were constructed; one was H-shaped and the other was box type. Plastic (PET) bottles were utilized to construct anode and cathode chambers of H-shaped MFCs (experiments 1 to 4) shown in Fig. 4. For other types, acrylic boxes were used to construct anode and cathode compartments (experiments 5 and 6) as shown in Fig. 5. A cylindrical salt bridge having surface areas of 0.00025 m2 was used for H-type MFC while in the box-type MFCs the salt bridge had surface area of 0.006 m2 and 38.1-mm-thick membrane of agar placed between anode and cathode compartments. The dimensions of different components of MFCs are mentioned in Table 1.
2.1 Preparation of substrate
The non-degradable components (e.g., plastic products) were segregated manually from the waste collected from a dustbin and used without further processing. Gruel, a waste produces during cooking rice (1 l) was mixed with dustbin waste and blended for 10 min. The same proportions of starch and dustbin waste were used for the preparation of the substrate for all the studies.
2.2 Preparation of salt bridge
For H-shaped MFCs, agar salt bridges were prepared following a procedure reported previously elsewhere [23]. In brief, agar (4 g), sodium chloride (22 g), and water (180 ml) were mixed in a conical flask, and the mixture was heated with stirring at 95 °C for 10–12 min, and the hot solution was poured into a PVC pipe (4 inches, 0.1016 m long and 0.5 inches, and 0.0127 m diameter). Then it was cooled to room temperature. For box-type MFCs, the same procedure was followed to prepare the solution using 15 g agar, 90 g sodium chloride, and 450 ml water. The hot solution was poured into the empty area created in between the boundaries of the anode and the cathode chamber.
2.3 Amounts of ingredients
Four types of sludges and same organic substrate were fed in an anode chamber, and water was taken as an electron acceptor in the cathode chamber. Different amounts of sludge, substrate and electron acceptor in different experiments of this study is mentioned in Table 2.
2.4 Measurement of electrical parameters
A multimeter (Model no. DT-9205A) was used the measurement of electrical parameters. All the cells were kept in aerobic condition, and readings were taken for seven days across 101 Ω, 261 Ω, 472 Ω, 991 Ω, 1950 Ω, 4910 Ω, and 9850 Ω external loads [23]. The current density was obtained from the current that was calculated using the Ohm’s law (Eq. 2).
where I = current, V = measured voltage, and R = resistance.
2.5 BOD measurement
Biological Oxygen Demand (BOD5) values of sludges before and after the experiment were recorded using Lovibond BD 600 measuring system.
2.6 Microbiological study
The amount of aerobic Escherichia coli and Bacillus subtilis was also inspected in this study. Sorbitol MacConkey Agar (SMAC) and NaCl-Glycine-Kim-Goepfect (NGKG) medium were applied for determination of the presence of aerobic Escherichia coli and Bacillus subtilis in the sludges.
2.6.1 Escherichia coli count
Sorbitol MacConkey Agar (SMAC) (51.5 g) was mixed with distilled water (1 l), and the solution was sterilized by autoclaving at 121 °C for 15 min. After that, the medium was cooled down to 50 °C and poured on Petri dishes in a laminar flow. After drying, the samples of the sludge were dispersed on the surface of the plate, and the plates were incubated at 30 °C for 24 h. After 24 h, the colonies of Escherichia coli were visually inspected and counted manually.
2.6.2 Bacillus subtilis count
NaCl-Glycine-Kim-Goepfect (NGKG) (13.3 g) was mixed with distilled water (450 ml), and the solution was sterilized by autoclaving at 121 °C for 15 min. After that, the medium was cooled down to 50 °C followed by the addition of 100 ml 20% egg yolk with the solution. 20% egg yolk was prepared adding egg yolk (20 ml) with sterilized saline solution (80 ml) aseptically. Next it was poured on Petri dishes in a laminar flow. After drying, the samples of the sludge were dispersed on the surface of the plate, and the plates were incubated at 30 °C for 24 h. After 24 h, the colonies of Bacillus subtilis were visually inspected and counted manually.
3 Results and discussion
3.1 Performance of MFC
Total six experiments were carried out in this study; experiments 1–4 were conducted in H-shaped MFCs) whereas experiments 5–6 in box-type MFCs (Fig. 4). In experiment 1, experiment 2, experiment 3, and experiment 4, sludges collected from drain, textile effluent treatment plant, drain adjacent to tannery, and the Buriganga river were used as the source of microorganisms respectively. In experiments 5 and 6, drain sludge was used and external air supply was provided with experiment 6.
For each experiment, the open-circuit voltage (OCV) and voltages across different loads (e.g., 101 Ω, 261 Ω, 472 Ω, 991 Ω, 1950 Ω, 4910 Ω, and 9850 Ω) were measured for seven days. First reading of open-circuit voltage (OCV) was taken after 4 h of starting the MFC and data were recorded at a fixed interval of 24 h (Fig. 5). At the beginning OCV for all the experiments were close to 100–200 mV. For experiment 1 (drain sludge), OCV decreased steadily from days 1 to 7, while for experiments 2 (textile effluent treatment plant sludge) and 4 (Buriganga sludge) OCV slowly increased to a maximum of 500 mV on day 3 and then reduced steadily to 204 mV. For experiment 3 (tannery drain sludge), OCV was almost constant at 119 mV from day 1 to day 7. For box-type MFCs, OCV reached a maximum of 922 mV on day 5 then started to decay (experiment 5). For experiment 6, OCV increased until day 6 and reached at 1496 mV and then reduced. This phenomenon could be explained with the fact that under additional air supply and nutrients contents microbial growth was enhanced and after 6 days nutrients were not sufficient for the growth although air supply was continued. Another interesting finding was that tannery drain sludge (experiment 3) demonstrated the lowest output which may be due to the presence of different types of fungicides used in tanning hindered the growth of microorganisms.
Polarization curve is also widely used for analyzing the performance of MFC, which is the representation of the cell voltage and power density in terms of current (or current density) [9]. In the present study, after 48 h (2 days) of operation MFCs, the output voltage was almost constant for the following 72 h (day 3, day 4, and day 5). Figures 6, 7, and 8 represent the polarization curve on day 3, day 4, and day 5, respectively. Polarization curve was propagated by varying loads as 101 Ω, 261 Ω, 472 Ω, 991 Ω, 1950 Ω, 4910 Ω, and 9850 Ω after obtaining stable voltage. Among the H-shaped MFCs (expt. 1–4), the highest output was provided by the Buriganga sludge on day 3 which produced maximum current density and power density of 35.16 mA/m2 and 8.61 mW/m2, respectively (Fig. 6). This may be attributed to the presence of some bacterial strains in Buriganga sludge, which are more effective in bioenergy production. In case of box-type MFCs, experiment 6 with external air supply gave better results compared to experiment 5 providing 16.4 mA/m2 and 6.74 mW/m2 as peak current density and power density, respectively, at day 5 (Fig. 8). The value of electrical parameters (voltage, current density, and power density) started to decrease after a certain period of time for each experiment where there was no air supply, while experiment 6 with air supply demonstrated upward trend (Table 3). The reason behind is that the amount of oxygen and inside the chamber started to reduce from day one and consequently hindered the growth of aerobic bacteria in the sludges.
Table 3 represents the comparison for electrical parameters obtained from different MFCs constructed in this study with previous research works.
3.2 Reduction of BOD5 of the sludges
Figure 9 illustrates the change in BOD5 of the sludges before and after conducting the experiments. In H-type MFCs, the BOD5values for drain sludge, textile ETP sludge, tannery drain sludge, and the Buriganga sludge had been reduced by 48.47%, 43.64%, 47.32%, and 35.20%, respectively (experiments 1, 2, 3, and 4), while for box-type MFCs, the reduction of BOD5 value for drain sludge was found to be 46.88% and 50.15% (experiments 5 and 6) respectively. The maximum BOD5 reduction in this study was found for experiment 6 where additional air was pumped into the anode chamber. The organic substances were decomposed during the bacterial growth and thus BOD5 of each sludge reduced significantly. When there was an additional air supply in experiment 6, the BOD5 removal was highest (50.15%) which further supports the aerobic decomposition of the substrates.
3.3 Microbiological studies
The colonies of bacteria were visually inspected. The measuring unit was colony-forming unit per milliliter (CFU/ml). Figure 10 shows the microscopic view (using 40 × lens) of Escherichia coli and Bacillus subtilis in the drain sludge. Figure 11 represents count of Escherichia coli [log(CFU/ml)] and Bacillus subtilis [log(CFU/ml)] in different types of sludges, respectively.
Significant counts of Escherichia coli and Bacillus subtilis were found in all sludges except the one collected from a drain close to a tannery. Although the tannery drain sludge contained a good number of Bacillus subtilis, it contained a negligible number of Escherichia coli. The maximum electrical output from the Buriganga sludge (voltage 244.88 mV) and the minimum output from the tannery drain sludge (84.44 mV) indicated that both Escherichia coli and Bacillus subtilis played a significant role in energy production of the MFCs studied in the present investigation, and the results were in good agreement with previous study [57]. However, the best result for the Buriganga sludge may be attributed to the presence of some more effective microorganism which were not identified in the current investigation.
4 Conclusion
A total number of six experiments were investigated throughout the project, and all of them were maintained under aerobic conditions and observed for seven days. Among six experiments, only experiment 6 was provided with external air supply, which facilitated the bacterial growth and consequently stable outputs were attained. Besides this, two types of bacteria were detected and counted in the sludges which were Escherichia coli and Bacillus subtilis. The reduction of BOD5was also measured, and the maximum reduction was 50.15% for drain sludge (expt. 6) where additional air played a vital role to reduce BOD5 due to ease of oxidation of the substrates. The result in the current investigation revealed that organic waste can be used as a substrate and different sludges can be used as the sources of microorganisms in MFC. The reduction of Biological Oxygen Demand (BOD5) of the sludge implied that in MFC both power generation and effluent treatment can be done simultaneously.
However, due to lack of laboratory facilities and financial support, it was not possible to identify the proportion of particular carbohydrate (e.g., starch, glucose, and sucrose) in organic substrate, and biochemical test of the sludge was not done to identify the composition of the sludge.
References
Liu L, Peng Y, Zhang W, Ma X (2023) Concept of rapid and controllable combustion for high power-density diesel engines. Energy Convers Manage 276:116529. https://doi.org/10.1016/j.enconman.2022.116529
Ng F-L, Jaafar MM, Phang SM, Chan Z, SallehNA Azmi SZ, Yunus K, Fisher AC, Periasamy V (2014) Reduced graphene oxide anodes for potential application in algae biophotovoltaic platforms. Sci Rep 4:7562. https://doi.org/10.1038/srep07562
Liu L, Wu Y, Wang Y, Wu J, Fu S (2022) Exploration of environmentally friendly marine power technology-ammonia/diesel stratified injection. J Clean Prod 380:135014. https://doi.org/10.1016/j.jclepro.2022.135014
Ahmad QA, Qazi JI (2014) Int J Energy Environ Eng 5:94. https://doi.org/10.1007/s40095-014-0094-4
Nasirahmadi S, Akbari-adergani B (2018) Int J Energy Environ Eng 9:249
Sirinutsomboon B (2014) Modeling of a membraneless single-chamber microbial fuel cell with molasses as an energy source. Int J Energy Environ Eng 5:1–9
Gatti MN, Milocco RH (2017) Int J Energy Environ Eng 8:303. https://doi.org/10.1007/s40095-017-0249-1
Ren H, Lee H-S (2012) JChae, Miniaturizing microbial fuel cells for potential portable power sources: promises and challenges. Microfluid Nanofluid 13(3):353–381
Lovley DR (2008) Extracellular electron transfer: wires, capacitors, iron lungs, and more. Geobiology 6(3):225–231
Logan BE, Hamelers B, Rozendal R, Schroder U, Keller S, Freguia J, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Virdis B, Freguia S, Rozendal RA, Rabaey K, Yuan Z, Keller J (2011) Microbial fuel cells. Elsevier Science publisher, Amsterdam
Rahimnejad M, Ghasemi M, Najafpour GD, Ghoreyshi A, Bakeri G, Hassaninejad K, Talebnia F (2012) Acetone removal and bioelectricity generation in dual chamber Microbial Fuel Cell. Am J Biochem Biotech 8:304–310
Rahimnejad M, Najafpour G, Ghoreyshi AA (2011) Effect of mass transfer on performance of microbial fuel cell. Mass Trans Chem Eng Proc 5:233–250
Hassan SH, El-Rab S, Rahimnejad M, Ghasemi M, Joo J, Ok Y, Kim I, Oh S (2014) Electricity generation from rice straw using a microbial fuel cell. Int J Hydro Eng 39:9490–9496
Zhou Y, Wang B, Ling Z, Liu Q, Fu X, Zhang Y, Zhang R, Hu S, Zhao F, Li X, Bao X, Yang J (2024) Advances in ionogels for proton-exchange membranes. Sci Total Environ 921:171099. https://doi.org/10.1016/j.scitotenv.2024.171099
Zhang Y, Wu MX, Zhou G, Wang XH, Liu X (2021) A rising star from two worlds: collaboration of COFs and ILs. Adv Funct Mater 31(40):2104996. https://doi.org/10.1002/adfm.202104996
Park DH, Zeikus JG (2000) Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl Environ Microb 66:1292–1297
Oh SE, Logan BE (2005) Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res 39:4673–4682
Suzuki S, Karube I (1984) Energy production with immobilized. Cells Appl Biochem Bioeng 4:281–310
Higgins SR, Lau C, Plamen A, Minteer SD, Cooney MJ (2011) Hybrid biofuel cell: microbial fuel cell with an enzymatic air-breathing cathode. ACS Catal 1(9):994–997. https://doi.org/10.1021/cs2003142
Bond DR, Holmes DE, Tender LM, Lovley DR (2002) Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483–485
Wang S, McGrath JE (2003) Synthesis of poly(arylene ether)s.Synthetic Methods in Step-Growth Polymers. Wiley: New York, pp 327
Uddin SS, Mahmood ZH, Nurnabi M (2018) Performance analysis of a dual chamber microbial fuel cell using kitchen wastes as substrate. 2018 International Conference on Innovation in Engineering and Technology (ICIET). https://doi.org/10.1109/CIET.2018.8660915
Sarker S, Chowdhury MB, Riyadh SS, Hossain T, Uddin HS, Syfuddin HM (2017) A mediator-less double chambered microbial fuel cell using sewerage sludge with output of relatively higher voltage and higher efficiency. Biores Commun 3(2):420–427
Min B, Cheng S, Logan BE (2005) Electricity generation using membrane and salt bridge microbial fuel cells. Water Res 39(9):1675–1686. https://doi.org/10.1016/j.watres.2005.02.002
Khan MR, Bhattacharjee R, Amin MSA (2012) Performance of the salt bridge based microbial fuel cell. Int J Eng Technol 1(2):115–123
Sivakumar D (2021) Wastewater treatment and bioelectricity production in microbial fuel cell: salt bridge configurations. Int J Environ Sci Technol 18(6):1379–1394. https://doi.org/10.1007/s13762-020-02864-0
Bose D, Bose A, Kundani D, Gupta D, Jain H (2018) Comparative analysis of carbon cloth and aluminum electrodes using agar salt-bridge based microbial fuel cell for bioelectricity generation from effluent derived wastewater. 17(4):1201–1205
Zhang Y, Liu M, Zhou M et al (2019) Microbial fuel cell hybridsystems for wastewater treatment and bioenergy production: synergistic effects, mechanisms and challenges. Renew Sust Energy Rev 103:13–29
Huggins T, Wang H, Kearns J, Jenkins P, Ren ZJ (2014) Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresource Technol 157:114–119
Bettin C (2006) Applicability and feasibility of incorporating microbial fuel cell technology into implantable biomedical devices. Dissertation, The Ohio State University
Jung S, Regan JM (2007) Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl Microbiol Biot 77:393–402
Rahimnejad M, Mokhtarian N, Najafpour G, Daud W, Ghoreyshi A (2009) Low voltage power generation in a biofuel cell using anaerobic cultures. World Appl Sci J 6:1585–1588
Nevin KP, Richter H, Covalla SF, Johnson JP, Woodard TL, Orloff AL, Jia H, Zhang M, Lovley DR (2008) Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ Microbiol 10:2505–2514
Park DH, Zeikus JD (2002) Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol Bioeng 81:348–355
Zhou M, Chi M, Luo J, He H, Jin T (2011) An overview of electrode materials in microbial fuel cells. J Power Sources 196:4427–4435
Chen GW, Choi SJ, Lee TH, Lee GY, Cha JH, Kim CW (2008) Application of biocathode in microbial fuel cells: cell performance and microbial community. Appl Microbiol Biot 79:379–388
Ghasemi M, Daud WRW, Rahimnejad M, Rezayi M, Fatemi A, Jafari Y, Somalu M, Manzour A (2013) Copper phthalocyanine and nickel nanoparticles as novel cathode catalysts in microbial fuel cells. Int J Hydrog Energy 38:9533–9540
Park D, Zeikus J (2002) Impact of electrode composition on electricity generation in a single-compartment fuel cell using Shewanellaputrefaciens. Appl Microbiol Biot 59:58–61
Ren H, Jiang C, Chae J (2017) Effect of temperature on a miniaturized microbial fuel cell (MFC). Micro Nano Syst Lett 5:13
Wang XL, Wu C, Zhang JQ et al (2011) Acclimation stage on the performance of microbial fuel cells subjected to variation in COD, temperature, and electron acceptor. In: Advanced Materials Research, pp 2346–2350
Santoro C, Arbizzani C, Erable B, Ieropoulos I (2017) Microbialfuel cells: from fundamentals to applications. A review. J Power Sources 356:225–244
Gadkari S, Fontmorin JM, Yu E, Sadhukhan J (2020) Influence of temperature and other system parameters on microbial fuel cell performance: numerical and experimental investigation. Chem Eng J 388:124–176
Gonzalez del Campo A, Lobato J, Cañizares P et al (2013) Short term effects of temperature and COD in a microbial fuel cell. Appl Energy 101:213–217
Commault AS, Barrière F, Lapinsonnière L et al (2015) Influence of inoculum and anode surface properties on the selection of Geobacter-dominated biofilms. Bioresour Technol 195:265–272
Patil SA, Harnisch F, Kapadnis B, Schröder U (2010) Electroactive mixed culture biofilms in microbial bioelectrochemical systems: the role of temperature for biofilm formation and performance. Biosens Bioelectron 26:803–808
Li J (2013) An experimental study of microbial fuel cells for electricity generating: performance characterization and capacity improvement. J Sustain Bioenergy Syst 3:171–178
Zhang Y, Sun J, Hu Y, Wang Z, Li S (2014) Effects of periodically alternating temperatures on performance of single-chamber microbial fuel cells. Int J Hydrogen Energy 39(15):8048–8054
Wei L, Han H, Shen J (2013) Effects of temperature and ferrous sulfate concentrations on the performance of microbial fuel cell. Int J Hydrogen Energy 38(25):11110–11116
Almatouq A, Babatunde AO (2016) Concurrent phosphorus recovery and energy generation in mediator-less dual chamber microbial fuel cells: mechanisms and influencing factors. Int J Environ Res Public Health 13:375. https://doi.org/10.3390/ijerph13040375
Rahimnejad M, Adhami A, Darvari S et al (2015) Microbial fuel cell as new technology for bioelectricity generation: a review. Alexandria Eng J 54:745–756
Habermann W, Pommer EH (1991) Biological fuel cells with sulphide storage capacity. Appl Microbiol Biotechnol 35:128–133
Deng Q, Li X, Zuo J, Ling A, Logan BE (2009) Power generation using an activated carbon fiber felt cathode in an upflow microbial fuel cell. J Power Sources 195:1130–1135
TerHeijne A, Hamelers HVM, De Wilde V, Rozendal RA, Buisman CJN (2006) A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cell. Environ Sci Technol 40:5200–5205
Tang X, Guo K, Du Z, Tian J (2010) Microfiltration membrane performance in two-chamber microbial fuel cells. Biochem Eng J 52:194–198
Sun J, Hu Y, Bi Z, Cao Y (2009) Improved performance of air-cathode single-chamber microbial fuel cell for wastewater treatment using microfiltration membranes and multiple sludge inoculation. J Power Sources 187:471–479
Ren J, Li N, Du M, Zhang Y, Hao C, Hu R (2021) Study on the effect of synergy effect between the mixed cultures on the power generation of microbial fuel cells. Bioengineered 12(1):844–854. https://doi.org/10.1080/21655979.2021.1883280
Acknowledgements
The authors want to convey gratitude towards the Department of Applied Chemistry and Chemical Engineering, Semiconductor Technology Research Centre (STRC), and Department of Botany and Centre for Advanced Research for Sciences (CARS) of the University of Dhaka for laboratory supports.
Author information
Authors and Affiliations
Contributions
Sheikh Shehab Uddin: conducting experimental studies, formal analysis, and writing draft manuscript. Md. Mahidul Haque Prodhan: manuscript proofreading and editing draft manuscript. Mohammad Nurnabi: conceptualization, formal analysis, editing draft manuscript, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uddin, S.S., Prodhan, M.M.H. & Nurnabi, M. Studies on agar salt bridge based dual chamber microbial fuel cells using sludge and dustbin waste. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05718-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05718-8