Abstract
The chemical profiles of essential oils (EOs) of 7 Cinnamomum species (C. sulphuratum, C. perotettii, C. verum, C. wightii, C. camphora, C. glanduliferum, and C. malabatrum) composed from assorted parts of Western Ghats were analyzed. The antioxidant, antibacterial, and larvicidal potentialities of 7 essential oils were also investigated. Cinnamomum verum was observed to have the maximum yield (1.96%) of EO followed by C. malabatrum (1.71%). GC-MS analysis of EOs reported the highest amount of eugenol (38.04%), shyobunol (13.31%) in C. verum, whereas C. perrottetii identified 14–42 phytocompounds including terpenes, sesquiterpenes, monoterpenes, diterpenes, and phenylpropanoids, esters, aldehydes, and fatty acids. In the antibacterial activity, the higher zone of inhibition of C. malabatrum showed against S. pyogenes (28 ± 2.5 mm) and E. faecalis (27 ± 1.24 mm) followed by C. verum has shown notable inhibition zone against K. pneumoniae (26 ± 1.15 mm) and S. flexneri (24.33 ± 0.88 mm), with MIC value against C. verum, showed MIC value (1.17 ± 0.34 μg/mL) against S. flexneri, S. pyogenes, and K. pneumonia. All the Cinnamomum oil samples exhibited appreciable antioxidant radical scavenging activities with IC50 values ranged 10.83–15.06 μg/mL. All the oil samples showed promising larvicidal activity against the late-third instar larvae of Aedes aegypti. Extending the present study with in vivo and in vitro animal studies will confirm the potentiality of Cinnamomum oils in the field of biomedicine to detect novel medicines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cinnamomum is reported to have higher medicinal value and larvicidal properties which can be considered a commercial cultivable species for the rural economy [1]. EOs are volatile lipophilic compounds secreted through aromatic plant cells with diverse morphology, synthesized through numerous plant organs such as buds, branches, stems, roots, berries, seeds, flowers, bark, and wood, and stored in secretory cells, channels, cavities, trichomes, or epidermal cells [2]. The secretory cells range from highly specialized to non-specialized cells, osmophores, ducts, and cavities reported in dissimilar plant parts are the repositories of Eos [3]. EOs is short-molecular-weight compound mixtures with a strong odor obtained from aromatic plants [4, 5]. They are the mixtures of a large number of compounds having differentiated structures mainly of terpenes or phenylpropanoid derivatives. EOs is significant derived metabolites reported in aromatic and medicinal plants used in fields and industries such as pharmaceuticals, cosmetics, food, and aromatherapy [6]. EOs obtained from plant sources are the best alternative to synthetic oils used in human health care. They are also reported as the best food additives to retain the quality and value of nutrition [7]. Moreover, spice and aromatic plants are reported to be prosperous sources of volatile compounds. Many of these secondary metabolites are approved for human consumption through the United States Food and Drug Administration, widely used in flavors and fragrances, food preservation, and pharmaceutical industries [8].
The different compounds in EO samples are recognized to have acquired an assortment of biological significance, which capacity has been accountable for therapeutic consequences such as insecticidal, anti-allergic, anti-inflammatory, antioxidant, and anticancer properties, along with their antimicrobial characteristics [9,10,11]. Multidrug resistance and side effects of antibiotics increased the interest and need for developing new antimicrobial agents from plants [12].
The antimicrobial activity of EO is also proposed to the morphological changes produced in bacteria by the volatile compounds, which modulate bacterial growth and are capable of trapping the free radicals and preventing oxidation [8]. The essential oils from cinnamon improve the activity of antioxidant enzymes by reducing the reactive oxygen-free radical [4]. Many studies on EO reported that the antioxidant activity is unpaid to the existence of several monoterpenes and sesquiterpene compounds [13, 14]. By the action of an antibacterial representative on bacteria, their ROS content increases. When it exceeds the antioxidant defense ability, the protein and further intracellular substances of the bacteria get injured and hence affect the metabolic process of the bacteria [15]. Also, the EOs of several plant species have been documented to exhibit toxicity effects against a few species of mosquitoes. Only substantial numbers of studies have been attempted on the usage of EOs of cinnamon as food additives and medicines. The comparative analysis of volatile composition among the species helps to understand the medicinal values of the EO-yielding species of a group [16].
The genus Cinnamomum contains shrubs and evergreen trees belonging to the Lauraceae family consisting of about species are 250 in subtropical and tropical regions, mainly in Asia, and a few in central and South America and Australia [17]. The bark of Cinnamomum is widely employed as a spice and denoted as a highly valued oldest spice which ranks second next to pepper and is commonly used for flavoring, preservation, cosmetics, and perfumery industries and in preparation of various ayurvedic and allopathic medicines [18]. The EOs from the species of Cinnamomum are reported as an important source of chemicals with pharmaceutical uses and hence, current investigate works were alerted on Cinnamomum ([19, 20]. The plants of this genus are reported with numerous pharmacological properties; antioxidant, antibacterial, and anti-inflammatory properties [21, 22]. The occurrence of bulky amounts of phytoconstituents such as camphor, α-terpineol, linalool, eucalyptol, and borneol in the EO is accountable for their biological activities [20]. The EOs of Cinnamomum are reported to be carminative and expectorant; the bark oil is an anthelmintic, aphrodisiac, and tonic and also useful in treating bronchitis, parched mouth, itching, diarrhoea, piles, flatulence, headache, heart, urinary diseases, etc. [23].
The current study of seven Cinnamomum species C. verum, C. sulphuratum, C. perrottetii, C. camphora, C. wightii, C. glanduliferum, and C. malabatrum selected and focused for the identification of secondary metabolites in essential oils by gas chromatography mass spectroscopy (GC-MS), extracted essential oils targeted pharmacological applications like free radical scavenging assay by DPPH method, antibacterial activity against two gram-positive bacteria, such as Streptococcus pyogenes and Enterococcus faecalis and four gram-negative bacteria, Pseudomonas aeruginosa, Enterobacter aerogenes, Klebsiella pneumoniae, and Shigella flexneri human pathogenic bacteria and larvicidal activity against Aedes aegypti.
2 Materials and methods
2.1 Plant materials
Fresh leaves of seven species of Cinnamomum, Cinnamomum verum J. Presl., Cinnamomum sulphuratum Nees., Cinnamomum perrottetii Meisn., Cinnamomum camphora (L.) J. Presl., Cinnamomum wightii Meisn., Cinnamomum glanduliferum (Wall.) Meisn., and Cinnamomum malabatrum (Burm.f.) Presl. were composed of dissimilar forest areas of the Western Ghats. The collected leaf specimens were properly identified with standard journalism and authenticated with official voucher specimens (Fig. 1). The receipt specimens of all the studied species (Cinnamomum verum J. Presl.: GUD 942, C. sulphuratum Nees.: GUD 943, C. perrottetii, Meisn.: GUD 944, C. camphora (L.) J. Presl.: GUD 945, C.wightii Meisn.: GUD 946, C. glanduliferum (Wall.) Meisn.: GUD 947, and C. malabatrum (Burm.f.) Presl.): GUD 948) were placed in the herbarium (GUD) of the Gandhigram Rural Institute (Deemed to be University), Department of Biology, Gandhigram, Dindigul (DT), Tamil Nadu.
2.2 Isolation of essential oils
The fresh leaves (200–500 g) of all the species of Cinnamomum were cut into small pieces (2–3 cm) individually and subjected to hydro-distillation with the Clevenger-type equipment (Borosilicate Glass) for about 4–6 h at ambient temperature (60–80 °C), the yield of EOs was calculated. The EOs collected from each species were dried over anhydrous Na2SO4 and then stored at 4 °C in a dark place for further analysis and further work [12, 13].
2.3 Analysis using gas chromatography and mass spectrometry (GC-MS)
The essential oil samples were diluted in n-hexane (10 μL/300 μL), and 10 μL of each solution was added to a split-splitless injector attached to an HP-5 column (30 m × 0.32 mm, film thickness 0.25 μm), which was used in a spit-mode gas chromatography experiment on an Agilent Technologies 7890 apparatus. As a carrier gas, helium (1 mL/min/210 °C) was employed. The column temperature was linearly programmed from 40 to 260 °C at 4 °C/min, while the injector and detector temperatures were set at 250 and 280 °C, respectively. Without using any correction variables, the percentage composition was calculated from the peak areas. The HPG 1800 C Series II GCD analytical instrument with an HP-5MS column was used to perform the GC-MS. Re-recorded mass spectra in the m/z 40–450 range were done so in EI mode (70 eV). By calculating the oils’ Kovats retention index (RI) and comparing their mass spectra to reference compounds from the Nirst and Willey libraries, the volatile components of the oils were located [12].
2.4 Antibacterial activities of essential oils
Two gram-positive bacteria, such as Streptococcus pyogenes (MTCC 442) and Enterococcus faecalis (MTCC 439), and four gram-negative bacteria, Pseudomonas aeruginosa (ATCC 27853), Enterobacter aerogenes (MTCC 111), Klebsiella pneumoniae (MTCC 432), and Shigella flexneri (MTCC 1457) were used. The microbial cultures were obtained from the Laboratory of the Department of Biology, The Gandhigram Rural Institute, Dindigul, India. The diameter of the zone of inhibition of EOs was evaluated based on the qualitative determination of the antibacterial activity of essential oils [14]. Different concentrations (50–150 μg/mL) of sample solutions were added (50–90 μL) to each well, and the plates were incubated at respective temperatures of 37 °C for 24 h. Gentamicin (Laborate Pharmaceuticals India Ltd.) (10 μg/mL) and 1% DMSO (SRL chemical) were used as the experimental +ve and −ve controls. The triplicates were maintained, and the inhibition zone diameter (IZD) was observed and calculated for every organism and expressed the value as mean ± S.E. [14].
2.5 MIC (minimal inhibitory concentration) of EOs determination
The testing of MIC, seven essential oil samples selected, and the bacterial strains was determined through the method of microdilution as prearranged through the CLSI method by using 96-well microtiter plates (Tarsons trust delivered, Cat. No. 980040) with some modifications [24,25,26], minimal concentrations of essential oil samples that inhibit or kill the microorganisms were resoluted through the dilution of broth method. Each EO was subjected to dilution to 1 mg/mL for testing, followed by a series of two-fold dilutions from 150 to 0.29 μg/mL. Gentamicin (Laborate Pharmaceuticals India Ltd.) (1 mg/mL) and DMSO (SRL chemical) (100%) were taken as positive and negative controls respectively. The early bacterial inoculums were attuned to 5 × 106 CFU/mL and in a sterile 96-well microplate, nutrient broth (95 μL) as well as respective pathogenic culture (1 × 105 cells/ mL), were added into each well. The oil samples 100 μL were added to consecutive wells and incubated at 37 °C for 18 h. For MIC determination, resazurin (10 μL, 0.02% (w/v)) was transferred to each well followed by 2–4 h of further incubation. Incubated then wells were subjected to illustration evaluation of color changes that imply bacterial growth. The color changes indicated resazurin (HiMedia) reduction (blue/purple) to resorufin (pink). The 570 nm absorbance was read by Robonik Readwell Touch Automatic ELISA plate analyzer. The low concentration at which absolute inhibition of MIC values was recorded; all the experimental trials were performed in triplicates [16].
2.6 Antioxidant activities of Eos
The capability of the DPPH radical scavenging assay of EO was also analyzed spectrophotometrically. DPPH (0.1 mM; 1 mL) solution was added to different concentrations (10–50 μg/mL) of sample solution and then incubated for about 30 min. The reaction mixtures were read by a spectrophotometer (GENESYS 180 UV – Visible spectrophotometer) at 517 nm. The standard ascorbic acid (Sigma-Aldrich) in various concentrations (10–50 μg/mL), the DPPH radical scavenging activity was calculated against sample concentrations, and IC50 values were obtained from the graph [24, 27,28,29].
2.7 Larvicidal bioassay
The larvicidal assay was experimented on the 3rd instar larvae of Aedes aegypti as per the methodology of WHO with minor modifications [30]. The larval colonies of A. aegypti were collected from ICMR, Madurai, CRME (Centre for Research in Medical Entomology). Dissimilar test concentrations (50–250 μg/ml) from the stock essential oils were prepared with methanol (0.1 mM) (HiMedia). The appropriate volume of methanol dissolved in distilled water has served as a negative control. Ten healthy 3rd instar larvae were released into every glass beaker containing the respective examination solution. The controls tested were also done in a similar with each duplicate. The death rate of larvae was intended after one hour of the revelation period with an interval of 1 h up to 10 h. The motionless and unreactive larvae were considered dead. The departed larvae were noted and counted, and the regular % of transience was considered using Abbott’s procedure.
where X is the survival % in the larvae population control, and Y is the % of survival in the larvae population treated.
2.8 Statistical analysis
The mortality of the larvae at various concentrations was analyzed by the probit analysis method described by Raymond in 1985. The attentiveness that caused 90% and 50% death (LC50) have resulted in assurance intervals for mathematically important comparisons. The significance of the differences between the treatments versus concentrations was established by Oneway ANOVA and p values < 0.05, were measured as noteworthy. The data were analyzed statistically and completed using IBM SPSS (Statistics version 21), and the graphs were plotted using ggplot 2 version 3.3.6. in R version 4.1.1 [24].
3 Results and discussion
3.1 Chemical composition of essential oils
The present study confirmed that species of Cinnamomum were rich repositories of terpene compounds and oxygenated derivatives. A comparison between the chemical compositions of oils with the previous reports has indicated the compositional differences of essential oils according to the circulation and components accumulation in the same plant parts. The EOs isolated from Cinnamomum leaves were characterized by a diverse group of sesquiterpenes with varying areas of distribution. Few previous studies attempted on essential oils have shown variation in the chemical constituents from the same plant part, this variation was due to the influence of ecological factors such as climate and soil [31,32,33] genetic and developmental factors [33, 34] and also depends on the geographical region where the plant grow [35,36,37].
The yield of EOs from Cinnamomum species mottled from 0.142 to 1.96% v/w. The greatest surrender was obtained from C. verum (1.96% v/w) followed by C. malabatrum (1.71%), C. sulphuratum (1.17% v/w), C. wightii (1.014% v/w), C. camphora (0.32% v/w), and C. glanduliferum (0.171% v/w) whereas it was minimum yield from C. perrottetii (0.142% v/w). The higher quantity was obtained from C. verum (1.96% v/w) and C.sulphuratum (1.18% v/w) making these species a new natural potential source of volatile aromatic compounds. The GC-MS results demonstrated that sesquiterpenes were the principal class of compound in C. sulphuratum, C. perrottetii, C. wightii, C. camphora, and C. glanduliferum. In contrast to that, monoterpenes were predominantly reported in C. verum and esters in C. malabatrum. The predominant volatile components detected from the species of Cinnamomum were eugenol (38.04%), shyobunol (13.31%), isocaryophyllene (10.36%) along with germacrene D (7.89%), β-caryophyllene (11.33%), α-cadinol (10.7%), and Isoeugenol acetate (27.95%) in C. verum, C. perrottetii, C. camphora, C. sulphuratum, C. wightii, C. glanduliferum, and C. malabatrum, respectively (Table 1).
A total of 42 phytoconstituents were detected from C. sulphuratum including 31 sesquiterpenes, 7 sesquiterpenoids, and 4 monoterpenes. Germacrene D was established as the chief compound eluted at a retention time of 17.95 with a 7.89 % distribution. Furthermore, β-selinene (5.66%) and α-selinene (5.58%) were detected at the elution time of 18.128 and 18.266, respectively. Among the identified phytocompounds, the minor bioactive compounds of the essential oil were allo-ocimene (0.34%) and α-cubebene (0.32%) which were detected at the elution times 12.33 and 23.12 (Table 2 and Fig. 2a). The C. sulphuratum EO chemical composition from Kerala were rich with benzyl benzoate and another accession from Kerala has reported with (E)-cinnamyl acetate, linalool and benzyl benzoate as main constituents. C. sulphuratum from Kodagu district and Gudalur, Nilgiri region of Tamil Nadu showed dissimilarities of chemical constituents, in which α-phellandrene, (E)-caryophyllene, linalool, and benzyl benzoate were the major compounds from Kodaku [102] and (E)-cinnamyl acetate, linalool, α-cadinol, benzyl benzoate, α-cadinene, and α-muurolol from Gudulur. The Northeast India leaf has also shown disparity with a significant presence of geranial, neral, and geraniol [103].
a GC-MS Chromatogram of C. sulphuratum leaf essential oil. b GC-MS Chromatogram of C. perrottetti leaf essential oil. c GC-MS Chromatogram of C. verum leaf essential oil. d GC-MS Chromatogram of C. wightii leaf essential oil. e GC-MS Chromatogram of C. camphora leaf essential oil. f GC-MS Chromatogram of C. glanduliferum leaf essential oil. g GC-MS Chromatogram of C. malabatrum leaf essential oil
Out of 35 compounds reported from the E of C. perrottetii, 17 sesquiterpenes contributed to the main division of the EOs, along with 8 sesquiterpenoids, 7 monoterpenes, 2 monoterpenoids, and 1 diterpene. Shyobunol (13.31%) was detected as the dominant compound recorded at the elution time of 22.27 followed by caryophyllene (10.91%), α-Cadinol (10.86%), germacrene-D (8.9%), viridiflorol (5.96%), and germacrene D-4-Ol (4.43%) at the retention time of 16.7, 21.49, 17.98, 20.30, and 19.91, respectively (Table 2 and Fig. 2b). Schmidt et al. [104] and Boniface et al. [105] reported eugenol (74.9%) as the basic constituent of the C. perrottetii oil from Srilanka. Although, (E)-cinnamaldehyde (37.6%), cinnamyl benzoate (16.4%), and cinnamyl acetate (23.7%), as major constituents of the fresh leaves of C. perrottetii. The studies carried out by Unlu et al. [106] found that the major compounds C. perrottetii oil were (E) (E)-cinnamyl acetate (7.44%), cinnamaldehyde (68.95%), and benzaldehyde (9.94%). When compared to data observed from the current study with prior reports, the major components of C. perrottetii, have indicated that there was no similarity recorded in the principle component, shyobunol (13.31%) from the EOs.
Volatile phytocompounds 29 were identified from the EO of C. verum including 9 monoterpenes, 7 sesquiterpenes, 5 esters, 4 phenylpropanoids, 3 sesquiterpenoids, and 1 monoterpenoid. Eugenol (38.04%), one of the important phenylpropanoids was prominently detected at the elution time of 15.593. Cinnamyl acetate (22.36%), benzyl alcohol (8.13%), and cis-cinnamaldehyde (4.81%) were the other compounds subsequently eluted at 17.42, 11.344, and 13.603 retention times (Table 2 and Fig. 2c).
The EO chemical combination of C. verum collected from various geographical regions confirmed the dissimilarities of phytocompounds, in which (E)-linalool (2.30%), eugenol (86.02%), and caryophyllene (5.70%) recorded from the leaf samples of Fiji islands [107]. By the present study, C.verum essential oil from Palni hills, Tamil Nadu has also detected eugenol as a major compound (3.9%) [108]. C. verum essential oil from Brazil recorded linalool (5.4%), E-cinnamaldehyde (4.0%), β-phellandrene (3.4%), eugenol (3.4%), α-pinene (3.9%), and benzaldehyde (2.7%) as major constituents [109] and the Vietnam samples reported linalool (22%), caryophyllene oxide (5.6%), methyl eugenol (0.7%), β-bisabolene (7.7%), eugenol (0.1%), bicyclogermacrene (11.2%), (E)-nerolidol (1.3%), and γ-cadinene (4.0%) as significant compounds [110]. The present study has reported eugenol and benzyl benzoate compared to the previous studies [23, 109, 111,112,113].
GC-MS chromatogram of essential oil of C. wightii has detected 45 compounds, of which, 25 were sesquiterpenes and the rest of them were categorized into 6 sesquiterpenoids, 5 monoterpenes, 2 monoterpenoids, together with 2 esters, 2 fatty acids, 2 aldehydes, and 1 diterpene(Table 2 and Fig. 2d). β-caryophyllene was reported with an area of 11.33% which has eluted at 16.73 along with α cadinol. 𝛿-cadinene (5.14%), γ-muurolene (4.49%), globulol (4.46%), and allo-ocimene (4.11%) were further recognized based on the elution time. The oil leaf EO from seven different accessions of the Western Ghats showed a disparity in the chemical composition compared to the present study with the presence of monoterpenes such as β-phellandrene, α-pinene, linalool, limonene, p-cymene, methyl eugenol, (E)-caryophyllene, elemicin, α-phellandrene, and spathulenol [114].
Among 41 phytocompounds reported from the essential oil of C. camphora, 24 sesquiterpenes 5 sesquiterpenoids, 4 monoterpenes, 3 monoterpenoids, 2 diterpenes, 2 aldehydes, and 1 fatty acid were reported. Isocaryophyllene showed a major peak at a maintenance time of 16.65 with a relation profusion of 10.36%. The caryophyllene oxide (8.74%) and α-cardinal (8.71%) were reported as leading compounds eluted at 20.03 and 21.43 (Table 2 and Fig. 2e). By analyzing the result of GC-MS data of C. camphora, phytone (0.19%), piperitone (0.16%), and benzaldehyde (0.14%) were the meagre compounds eluted at 24.62, 12.98, and 12.72 retention times respectively. The C. camphora compound composition of oil has differed considerably from the previous reports by having isocaryophyllene (10.36%), β-caryophyllene oxide (8.74%), and α-cadinol (8.71) as the major constituents. However, all the phytocomponents reported by this study were similar to the previous study reports at different geographical regions [43, 115,116,117,118,119]. Comparatively, a different compound composition of C. camphora essential oil from China was reported with borneol as the major constituent, and another study reported a dissimilar composition with eucalyptol (16.8%), isoborneol (8.1%), camphor (5.0%), linalool (26.6%), and α-terpineol (8.7%), and β-phellandrene (5.1%) as major compounds [120].
Furthermore, 32 compounds were reported from C. glanduliferum, with 18 sesquiterpenes and 7 sesquiterpenoids, 2 monoterpenoids, 4 monoterpenes, 1 diterpene, and 1 fatty acid, α-cadinol has been observed from the large area of distribution with an area percentage of 10.7% followed by β-caryophyllene (9.35%), β-elemene (6.31%), 𝛿-muurolene (5.11%), and α-cadinene (5.9%) at the retention time of 16.65, 15.95, 21.14, respectively (Table 2 and Fig. 2f). The C. glanduliferum EO composed in various geographical regions showed different composition in which, (E)-nerolidol (52.2 %) and caryophyllene oxide (6.0%) was recorded in samples from the Chessa area of Arunachal Pradesh [121], α-terpineol (9.40 %), α-pinene (20.28 %), 1, 8-cineole (41.42 %), and germacrene D-4-ol (6.10 %) from Uttarakhand [122], eucalyptol (59.44%), sabinene (14.99%), a-pinene (5.27%), a-terpineol (6.44%), β-elemene, and β-pinene (3.75%) from Al-Zohria Garden, Cairo, Egypt [123], and a new benzyl benzoate affluent chemotype was reported from Southern Western Ghats, India [36].
C. malabatrum leaf essential oil was reported with a total of fourteen compounds consisting of esters, sesquiterpenes, phenyl propanoids, monoterpene, and sulfone compounds. Isoeugenol acetate (27.95% at retention time 15.14), (E)-cinnamyl acetate (4.5% at retention time 17.075), cinnamaldehyde (4.28% at retention time 13.304), and benzyl acetate (1.02% retention time 10.68) were the major compounds (Table 2. and Fig. 2g). A previous study in C. malabatrum essential oil reported cinnamyl acetate and benzyl benzoate which were also detected in the present study [41].
By comparing the present attempt with the previous studies, the principle components of EO of species of Cinnamomum have varied due to geographical distribution and season of procurement. Nevertheless, the dissimilarity in the yield of EO and its chemical composition among the plants comes to analogous taxonomic groups anticipated to vary across agro-climatic and geographical conditions. It is as well as influenced by numerous external and internal environmental factors [2, 124, 125].
3.2 Antibacterial activity of EOs
The antibacterial potentialities of EOs from seven species of Cinnamomum were evaluated against 6 selected bacterial strains through the agar well method (diffusion). The results indicated that there has been a significant increase in the inhibition of bacterial growth with an increase in oil concentrations. The growth of microorganisms was inhibited and a maximum zone of inhibition (32 ± 0.88 mm) in Gentamicin. The leaf oils of Cinnamomum spp. were shown a synergistic effect with an inhibition zone alongside all experienced microorganisms (Fig. 3).
C. verum has shown notable inhibition zone against E. faecalis (19 ± 0.57 mm), S. flexneri (24.33 ± 0.88 mm), S. pyogenes (23.33 ± 0.66 mm), K. pneumoniae (26 ± 1.15 mm), P. aeruginosa (21.33 ± 1.33 mm), and E. aerogenes (21.66 ± 1.0 mm) at higher concentration (150 μg/ml). Among the screened bacteria, K. pneumoniae exhibited higher antibacterial activity with the highest zone of inhibition (26 ± 1.15 mm) (Table 3). S. pyogenes showed higher susceptibility to the essential oils isolated from C. sulphuratum with a higher zone of inhibition (23.33 ± 1 mm). E. aerogenes exhibited a moderate inhibition zone (21.66 ± 0.88 mm) followed by S. flexneri (18.33 ± 0.33 mm) and P. aeruginosa (18.33 ± 1.2 mm) (Figs. 4 and 5).
Cinnamomum perrottetii exhibited very similar trends of antibacterial activities and S. pyogenes (19.66 ± 1.33 mm) showed the prevailing zone of inhibition. E. faecalis and E. aerogenes were recorded with an almost similar zone of inhibition (18 ± 0.57 mm and 18.33 ± 0.88 mm) followed by P. aeruginosa and S. flexneri (16.33 ± 1.2 mm and 16 ± 1 mm). Among the tested oil samples, C. wightii and C. camphora exhibited less susceptibility to selected pathogens. C. wightii displayed a moderate zone of inhibition for all screened bacteria and the highest resistance was recorded in E. aerogenes (24.33 ± 0.33 mm). The essential oil of C. camphora showed higher inhibitory activity against S. flexneri (19 ± 1 mm) and P. aeruginosa (16.66 ± 0.66 mm), S. pyogenes and K. pneumoniae with an equal value of inhibition at higher concentrations. Among the screened strains, E. faecalis and E. aerogenes exhibited 100% resistance against essential oil isolated from C. camphora (Figs. 6, 7, and 8).
C. malabatrum EO has shown a minimum inhibition zone of about 11 ± 0.58 mm, 11 ± 0.47 mm, and 20 ± 1.15 mm against S. flexneri, K. pneumoniae, and S. pyogenes at initial concentration (50 μg/mL). By increasing the oil concentration to 100 μg/mL, there was an increased antibacterial activity with an inhibition zone. Maximum zone of inhibition was noticed at higher concentration (150 μg/mL) against all microbial species such as K. pneumoniae (18 ± 2.08 mm), S. flexneri (20 ± 1.18 mm), S. pyogenes (28 ± 2.5 mm), E. faecalis (27 ± 1.24 mm) P. aeruginosa, (16 ± 1.56 mm), and E. aerogenes (16 ± 2.1 mm) (Figs. 9 and 10).
The data observed by the agar well dispersion assay of the essential oil suggested that the samples have synergistic antibacterial potentialities alongside gram +ve and −ve bacteria. The results suggested that S. flexneri, K. pneumoniae, and E. aerogenes were found to be more susceptible when compared with other selected strains [107]. E. faecalis and E. aerogenes showed higher resistance against all essential oils of Cinnamomum [108]. Nonetheless, all the oils exhibited antibacterial activity against screened bacteria and the inhibition zones were more eminent in C. verum followed by C. malabatrum, C. sulphuratum, C. glanduliferum, C. perrottetii, C. wightii, and C. camphora. Essential oils are a rich repository of volatile constituents such as phenol-derived aromatic and aliphatic compounds, terpenes, and terpenoids responsible for bactericidal activities [109]. Several studies have revealed that cinnamon has a strapping and reliable inhibitory effect against human pathogens [67, 126,127,128,129,130,131,132]. Previous studies have also confirmed that the phytocomponents, sesquiterpenes, cinnamaldehyde, and eugenol are accountable for the antibacterial activity of cinnamon oil [132,133,134,135]. Also, the bactericidal action mechanisms of cinnamaldehyde and eugenol of essential oils were recorded [136].
Gram -ve organisms are supposed to be less responsive to EOs than gram-positive bacteria [10, 14]. However, the present study has revealed equal inhibitory action of all oil samples with clear zones of inhibition that portray higher antibacterial efficacy. The essential oils are considered antimicrobials due to the hydrophobic character compounds are cause the destruction of cell structures, leading to the escape of the cell membrane and increasing the membrane permeability [132, 137] and thereby in a cascade type of action that affects other cellular structures. The essential oils damage lipids and proteins by getting coagulated in the cytoplasm of the bacterial cell [138, 139].
3.3 Minimal inhibitory concentration (MIC) determination of EOs
C. verum showed a similar MIC value (1.17 ± 0.34 μg/mL) against S. flexneri, S. pyogenes, and K. pneumonia and it was highest against E. faecalis (9.375 ± 0.95 μg/ml). Klebsiella sp. membrane was destroyed by inducing oxidative damage by C. verum bark essential oil [140]. Apart from bark, EOs obtained from flowers and leaves also act as strong antibacterial agents against both gram +ve and −ve bacteria [141]. C. glanduliferum exhibited the least MIC value of 2.34 ± 0.67 μg/mL against E. aerogenes. E. faecalis and S. pyogenes exhibited the MIC value of 18.75 ± 0.63 μg/mL and 18.75 ± 0.46 μg/mL. The MIC value (3.43 μg/mL) was observed against P. aeruginosa and C. glanduliferum. C. sulphuratum showed minimum inhibition at concentrations ranging from 1.17 to 18.75 μg/mL against S. pyogenes and E. aerogenes, S. flexneri, K. pneumonia, and P. aeruginosa. The essential oil of C. wightii exhibited MIC values of 9.375 ± 0.87, 1.17 ± 0.97, 9.375 ± 1.23, etc. μg/mL against E. faecalis, S. flexneri, S. pyogenes, etc. Marasini et al. [142] reported MIC value of 49 μg/mL against S. pyogenes by C. camphora extracts however, the present study showed more inhibitory effect compared to previous studies. C. camphora essential oil showed less inhibitory activity against E. faecalis and Enterobacter aerogenes compared to the other seven essential oils at higher concentrations (150 μg/mL). C. perrottetii exhibited varying minimum inhibitory concentrations against bacterial species with higher efficiency against S. pyogenes (2.34 μg/mL) and low efficiency against K. pneumonia with MIC value of 18.75 μg/mL. The most effective microbial inhibitory activity was observed in C. malabatrum leaf essential oil against E. faecalis and S. pyogenes with MIC value of about 1.17 μg/mL.
3.4 Free radical scavenging (antioxidant) activity of EOs
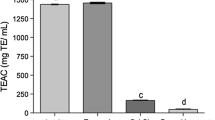
The antioxidant properties of the EOs were found promising, highly comparable with Standard (Ascorbic acid), and found to be significantly augmented with the augment in attentiveness. The antioxidant activity of EOs was recorded in terms of IC50 value and the minimum IC50 value corresponds to a greater potency [143]. All the EOs exhibited appreciable inhibition with IC50 values ranging 10.83–15.06 μg/mL which was secure to IC50 value (18.33 μg/mL) of the standard. C. verum was observed as a prospective foundation of antioxidants and recorded with the lowest IC50 value (10.83 μg/mL) followed by C. perrottetii (11.63 μg/mL), C. glanduliferum (13.02 μg/mL), C. camphora (13.04 μg/mL), C. sulphuratum(13.58 μg/ml), and C. wightii (15.06 μg/mL) (Fig. 11). However, C. malabatrum has recorded a higher IC50 value of about 15.86 μg/mL, since it was lower than standard (ascorbic acid).
The magnitude of antioxidant activity of volatile oils is generally attributed to the effects caused through the interaction of all phytoconstituents of the oil and also by the number of compounds that have been involved. Among all the oil samples studied, C. verum exhibited maximum antioxidant activity. Terpenes are considered to be a potential natural antioxidant and the main components of the aromatic plants Eos [144]. Thus, this movement could be accredited to the terpene oil content which is in agreement with the reports of Palozza and Krinsky [145]. GC-MS results of oils have also confirmed that sesquiterpenes were the principal compound of all the species studied, whereas, monoterpenes and phenylpropanoids were predominantly reported in C. verum.
DPPH scavenging activity was observed to be increased with an augment in the phenolic components such as flavonoids, phenolic diterpenes, and phenolic acids. Essential oils contain relatively higher amounts of phenolic compounds, which play a significant responsibility in antioxidant activity by acting as H donors [146, 147] In vitro studies have confirmed the antioxidant effects of EOs. However, previous reports suggested that synergistic interaction between major and minor compounds attributed to the hydrogen-donating capacity which results in the antioxidant activity of monoterpenes [148, 149] sesquiterpenes [150], phenolic compound, Eugenol [74, 151,152,153,154] reported with significant concentrations. Several studies have proven the potency of the phenolic derivative in which eugenol has the most powerful antioxidant activity and radical-scavenging activity [155, 156].
3.5 Larvicidal activity of EOs of Cinnamomum spp.
The toxicity effects of EOs of seven Cinnamomum species were analyzed beside fourth instar larvae of Aedes aegypti. All the oil samples exhibited promising larvicidal activity and the results were recorded with statistical data regarding LC50, LC90, and confidence limits by probit analysis using PROBIT software SPSS. The mortality percentages of third instar larvae of A. aegypti tested against essential oils were recorded as dose-dependent by increased with increasing concentrations. The lethal efficiency of oils in the concentration with 90% mortality (LC90), 50% death (LC50), and 95% confidence period values were calculated. The positive control temephos showed LC50 and LC90 values at 0.021 μg/mL (0.020–0.023) and 0.042 μg/mL (0.035–0.05). Negative control (DMSO) has not shown mortality and is active at ≤ 10 h exposure. The strong larvicidal activity was observed in C. verum with the most effective LC50 and LC90 values of 101.87 μg/mL (56.21–147.54 μg/mL) and 266.34 μg/mL (181–351.78 μg/mL) respectively. C. sulphuratum exhibited 104.579 μg/mL (66.39–141.05 μg/mL) and 242.61 μg/mL (159.544–325.687 μg/mL) LC90 and LC50 values respectively (Table 4).
The oils of C. perrottetii and C. camphora showed an almost similar susceptibility rate against the larvae of A. aegypti. C. perrottetii has shown moderate activity whereas C. wightii showed mild larvicidal activity with LC50 and LC90 values. Among the evaluated samples, A. aegypti exhibited more susceptibility to essential oils of C. glanduliferum with higher LC50 and LC90 values. The oil of C. verum has shown more sensitive to larvicidal activity with a high percentage of mortality. The consequence of the differentiations between the control and test studies was evaluated through one-way ANOVA, and p-value < 0.05 which were considered highly important. C. malabatrum oil had an LC50 value of 105.54 μg/mL with a lower assurance boundary (LCC) of 81.945 μg/mL and an higher assurance limit (UPL) of 128.545 μg/mL concentrations. LC90 value of 253.977 μg/mL (180.196–631.575) were obtained (Table 4).
There were only a few studies conducted on the larvicidal efficiency of EO in selected Cinnamomum species [141, 157, 158]. The effectiveness of these oils with a larvicidal property must be emphasized by oils the chemical compositions. In most of the oils of Cinnamomum, the dominant constituents were found as terpene derivatives and phenylpropanoids, which are responsible for larvicidal potentiality. Similar results were reported in essential oils with a higher content of phenylpropanoids [159], eugenol [160,161,162,163] and sesquiterpenes [164]. The larvicidal activity of a few species of Cinnamomum was corroborated with the present findings [158, 165,166,167,168,169,170,171,172,173].
Efficient larvicidal activity of larval and adult individuals of A. aegypti essential oil-based terpene compounds. Moreover, the structural characteristics of essential oils also contributed considerably to the consideration of larvicidal activity. The structural and activity associations of monoterpenes [174] and eugenol [162] derivatives alongside A. aegypti were supported by present findings. The results of our current study have demonstrated the potential alternative sources of mosquito larvicides, therefore, the plant-based essential oil compounds are competent when compared to existing synthetic chemicals which can be used to control the population of Aedes aegypti. Primarily essential oils affect the midgut epithelium and secondarily the malpighian tubules and gastric caeca in the mosquito larvae [175, 176] and also through physiology paralyzing and osmoregulation systems of the organisms [177].
4 Conclusion
The current study recorded the list of phytocompounds from the leaf EOs of seven species of Cinnamomum, C. sulphuratum, C. perotettii, C. verum, C. wightii, C. camphora, C. glanduliferum, and C. malabatrum. The GC-MS analysis of Eos indicated that the highest amount of eugenol and shyobunol was recorded in C. verum and C. perrottetii. In antibacterial activity, the higher zone of inhibition of Eos of C. malabatrum showed against S. pyogenes and E. faecalis followed by C. verum has shown a notable inhibition zone against K. pneumoniae . The total antioxidant assessed by IC50 values and larvicidal activities against Aedes aegypti indicated the biomedical efficiency of all seven species studied. Due to its higher efficiency, the multipurpose tree C. verum can be cultivated as a commercial crop instead of utilizing natural resources for the pharmaceutical industry for the development of drugs. Further, this research can be extended with in vivo and in vitro animal studies to confirm the potentiality of seven species of Cinnamomum in the field of biomedicine to detect novel medicines.
References
Bozin B, Mimica-Dukic N, Simin N, Anackov G (2006) Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem 54:1822–1828. https://doi.org/10.1021/jf051922u
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils–a review. Food Chem Toxicol 46:446–475. https://doi.org/10.1016/j.fct.2007.09.106
Shaaban HAE, El-Ghorab AH, Shibamoto T (2012) Bioactivity of essential oils and their volatile aroma components: Review. J Essen Oil Res 24:203–212. https://doi.org/10.1080/10412905.2012.659528
Liu Z, Li H, Zhu Z, Huang D, Qi Y, Ma C, Zou Z, Ni H (2022) Cinnamomum camphora fruit peel as a source of essential oil extracted using the solvent-free microwave-assisted method compared with conventional hydrodistillation. LWT 153:112549. https://doi.org/10.1016/j.lwt.2021.112549
Meziane IAA, Maizi N, Abatzoglou N, Benyoussef EH (2020) Modelling and optimization of energy consumption in essential oil extraction processes. Food and Bioprod Process 119:373–389. https://doi.org/10.1016/j.fbp.2019.11.018
Shahrivari S, Alizadeh S, Ghassemi-Golezani K, Aryakia E (2022) A comprehensive study on essential oil compositions, antioxidant, anticholinesterase and antityrosinase activities of three Iranian Artemisia species. Sci Rep 12:1–12. https://doi.org/10.1038/s41598-022-11375-6
Bhavaniramya S, Vishnupriya S, Al-Aboody MS, Vijayakumar R, Baskaran D (2019) Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci Technol 2:49–55. https://doi.org/10.1016/j.gaost.2019.03.001
Al-Zereini WA, Al-Trawneh IN, Al-Qudah MA, Tum Allah HM, Al Rawashdeh HA, Abudayeh ZH (2022) Essential oils from Elettaria cardamomum (L.) Maton grains and Cinnamomum verum J. Presl barks: Chemical examination and bioactivity studies. J Pharm Pharmacogn Res 10:173–185
Lee R, Balick MJ (2005) Sweet wood-Cinnamon and its importance as a spice and medicine. Explore: J Sci Heal 1:61–64. https://doi.org/10.1046/j.1365-2672.2001.01428.x
Seow YX, Yeo CR, Chung HL, Yuk HG (2013) Plant essential oils as active antimicrobial agents. Crit Rev Food Sci Nutr 54:625–644. https://doi.org/10.1080/10408398.2011.599504
Mabberley D (2008) Mabberley’s plant-book: a portable dictionary of plants, their classifications and uses. Cambridge, UK: Cambridge University Press 3:188–496
Manikandan G, Ramasubbu R (2020) Antimicrobial activity of leaf extracts of Memecylon heyneanum Benth. ex Wight & Arn.: An Endemic Tree Species of Southern Western Ghats. Adv Zoo & Bot 8(3):258–268. http://www.hrpub.org. https://doi.org/10.13189/azb.2020.080323
Satyal P, Chhetri BK, Dosoky NS, Poudel A, Setzer WN (2015) Chemical composition of Nardostachys grandiflora rhizome oil from Nepal–a contribution to the chemotaxonomy and bioactivity of nardostachys. Nat Prod Commun 10(6):1067–1070
Yu H, Ren X, Yang F, Xie Y, Guo Y, Cheng Y, Yao W (2022) Antimicrobial and anti-dust mite efficacy of Cinnamomum camphora Chvar. Borneol essential oil using pilot-plant neutral cellulase-assisted steam distillation. Lett Appl Microbiol 74:258–267. https://doi.org/10.1111/lam.13610
Bhattacharya P, Dey A, Neogi S (2021) An insight into the mechanism of antibacterial activity by magnesium oxide nanoparticles. J Mater Chem B 9:5329–5339. https://doi.org/10.1039/D1TB00875G
Singh G, Maurya S, de Lampasona MP, CAN C (2007) A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem Toxicol 45:1650–1661. https://doi.org/10.1016/j.fct.2007.02.031
Kumar S, Kumari R, Mishra S (2019) Pharmacological properties and their medicinal uses of Cinnamomum: a review. J Pharm Pharmacol 3:13–16. https://doi.org/10.1111/jphp.13173
Kirtikar KR, Basu BD (1984) Indian Medicinal Plants. Vol. III, Bishen Singh and Mahendra Pal Singh, Dehradun, Allahabad. 1664-1666
Dai J, Zhang X, Luo Z, Wang R, Liu Z, He X, Rao Z, Guan H (2020) Variation of the stable isotopes of water in the soil-plant-atmosphere continuum of a Cinnamomum camphora woodland in the East Asian monsoon region. J Hydrol 589:125–199
Wan N, Li Y, Huang XY, Li YH, Zheng Q, Wu ZF (2022) A comparative evaluation of chemical composition and antimicrobial activities of essential oils extracted from different chemotypes of Cinnamomum camphora (L.) Presl. Grasas y Aceites 73:e441–e441
Bouyahya A, Lagrouh F, El Omari N, Bourais I, El Jemli M, Marmouzi I, Salhi N, Faouzi MA, Belmehdi O, Dekka N, Bakri Y (2020) Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocat Agric Biotechnol 23:101471. https://doi.org/10.1016/j.bcab.2019.101471
Ali SS, Abd Elnabi MK, Alkherkhisy MM, Hasan A, Li F, Khalil M, Sun J, El-Zawawy N (2022) Exploring the potential of Cinnamomum zeylanicum oil against drug resistant Helicobacter pylori-producing cytotoxic genes. J Appl Biomed 20:22–36
Wang R, Wang R, Yang B (2009) Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov Food Sci Emerg Technol 10:289–292. https://doi.org/10.1016/j.ifset.2008.12.002
Sasikala N, Ramasubbu R (2022) Chemical composition, antimicrobial and antioxidant properties of essential oils of Trichopus zeylanicus ssp. travancoricus. Ind J Natur Prod Resour 12:570–577. https://doi.org/10.56042/ijnpr.v12i4.35929
Singh C, Singh S, Pande C, Tewari G, Pande V, Sharma P (2013) Exploration of antimicrobial potential of essential oils of Cinnamomum glanduliferum, Feronia elephantum, Bupleurum hamiltonii and Cyclospermum leptophyllum against foodborne pathogens. Pharma Biol 51:1607–1610. https://doi.org/10.3109/13880209.2013.805234
Elyemni M, El Ouadrhiri F, Lahkimi A, Elkamli T, Bouia A, Eloutassi N (2022) Chemical composition and antimicrobial activity of essential oil of wild and cultivated Rosmarinus officinalis from two Moroccan localities. Ecol Eng:23
Das S, Diyali S, Vinothini G, Perumalsamy B, Balakrishnan G, Ramasamy T, Dharumadurai D, Biswas B (2020) Synthesis, morphological analysis, antibacterial activity of iron oxide nanoparticles and the cytotoxic effect on lung cancer cell line. Heliyon 6(9):e04953. https://doi.org/10.1016/j.heliyon.2020.e04953e04953
Soler-Rivas C, Esp NJC, Wichers HJ (2000) An easy and fast to test to compare total free radical scavenger capacity of foodstuffs. Phytochem Anal 11:330–338. https://doi.org/10.1021/jf9908188
Molyneux P (2004) The use of the stable radical Diphenyl picryl hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 26:211–219
World Health Organization (1981) Instruction for determining the susceptibility or resistance of mosquito larvae to insecticide. 1981 WHO/VBC/81.80
Bailer J, Aichinger T, Hackl G, de Hueber K, Dachler M (2001) Essential oil content and composition in commercially available dill cultivars in comparison to caraway. Ind Crop Prod 14:229–239. https://doi.org/10.1016/S0926-6690(01)00088-7
Cannon JB, Cantrell CL, Astatkie T, Zheljazkov VD (2013) Modification of yield and composition of essential oils by distillation time. Ind Crop Prod 41:214–220. https://doi.org/10.1016/j.indcrop.2012.04.021
Mugao LG, Gichimu BM, Muturi PW, Mukono ST (2020) Characterization of the volatile components of essential oils of selected plants in Kenya. Biochem Res Int 2020: 8861798. https://doi.org/10.1155/2020/8861798
Murarikova A, Tazky A, Neugebauerova J, Plankova A, Jampílek J, Mucaji P, Mikus P (2017) Characterization of essential oil composition in different basil species and pot cultures by a GC-MS method. Molecules 22:1221. https://doi.org/10.3390/molecules22071221
Zandi-Sohani N, Hojjati M, Carbonell-Barrachinan AA (2012) Bioactivity of Lantana camara L. essential oil against Callosobruchus maculatus (Fabricius). Chil J AgricRes 72:502
Rameshkumar KB, George V (2006) Cinnamomum sulphuratum Nees-a Benzyl Benzoate - Rich New Chemotype from Southern Western Ghats, India. J Essent Oil Res 18:521–522. https://doi.org/10.1080/10412905.2006.9699159
Sriramavaratharajan V, Murugan R (2020) Chemical profiling of the Leaf essential oils of Cinnamomum species used as a spice in Southern India. JBAPN 10:317–324. https://doi.org/10.1080/22311866.2020.1806730
Saranya M, Arun T, Iyappan P (2012) In vitro antibacterial activity and preliminary phytochemical analysis of leaf extracts of Argemone Mexicana Linn–A medicinal plant. Int J Curr Pharm Res 4:85–87
Joshi RK (2013) Chemical composition of the essential oil of Chromolaena odorata (L.) RM King & H. Rob. roots from India. J Chem 2013:195057. https://doi.org/10.1155/2013/195057
Bos R, Woerdenbag HJ, Hendriks H, Smit HF, Wikstrm HV, Scheffer JJC (1997) Composition of the essential oil from roots and rhizomes of Valeriana wallichii DC. Flavour Fragr J 12:123–131
Leela NK, Prasath D, Venugopal MN (2008) Essential oil composition of selected cardamom genotypes at different maturity levels. Indian J Hortic 65:366–369
Ananthakrishnan R, Santhosh Kumar ES, Rameshkumar KB (2018) Comparative chemical profiles of essential oil constituents of eight wild Cinnamomum species from the Western Ghats of India. Nat Prod Commun 13:1934578X1801300525. https://doi.org/10.1177/1934578X1801300525
Su J (2012) Composition and biological activities of the essential oil extracted from a novel plant of Cinnamomum camphora Chvar Borneol J Med Plants Res:6. https://doi.org/10.5897/JMPR12.157
Adedeji J, Hartman TG, Rosen RT, Ho CT (1991) Free and glycosidically bound aroma compounds in hog plum (Spondias mombins L.). J Agric Food Chem 39:1494–1497
Eri S, Khoo BK, Lech J, Hartman TG (2000) Direct thermal desorption-gas chromatography and gas chromatography-mass spectrometry profiling of hop (Humulus lupulus L.) essential oils in support of varietal characterization. J Agric Food Chem 48:1140–1149
Chung TY, Eiserich JP, Shibamoto T (1993) Volatile compounds isolated from edible Korean chamchwi (Aster scaber Thunb). J Agric Food Chem 41:1693–1697. https://doi.org/10.1021/jf00034a033
Chang R, de Morais SA, Napolitano DR, Duarte KC, Guzman VB, Nascimento EAD (2011) A new approach for quantifying furanodiene and curzerene: a case study on the essential oils of Eugenia uniflora L., Myrtaceae (Pitangueira) leaves. Rev Brasi Farmacogn 21:392–396. https://doi.org/10.1590/S0102-695X2011005000042
Hung NH, Huong LT, Chung NT, Thuong NTH, Satyal P, Dung NA, Tai TA, Setzer WN (2020) Callicarpa species from central Vietnam: essential oil compositions and mosquito larvicidal activities. Plants 9:113. https://doi.org/10.3390/plants9010113
Prasad W, Khamrui K, Mandal S, Badola R (2018) Effect of combination of essential oils on physicochemical and sensorial attributes of burfi in comparison with individual essential oil and BHA. Int J Dairy Technol 71:810–819. https://doi.org/10.1111/1471-0307.12512
Sitarek P, Rijo P, Garcia C, Skała E, Kalemba D, Białas AJ, Szemraj J, Pytel D, Toma M, Wysokińska H, Śliwiński T (2017) Antibacterial, anti-inflammatory, antioxidant, and antiproliferative properties of essential oils from hairy and normal roots of Leonurus sibiricus L. and their chemical composition. Oxid Med Cell Long 2017:7384061. https://doi.org/10.1155/2017/7384061
Son LC, Dai DN, Thang TD, Huyen DD, Ogunwande IA (2014) Analysis of the essential oils from five Vietnamese Litsea species (Lauraceae). J Essen Oil-Bear Plants 17:960–971. https://doi.org/10.1080/0972060X2014.935068
Kurkçuoglu M, Yildiz G, Kose YB (2019) Essential oil composition of two Scutellaria species from Tokat, Turkey. J Turkish Chem Soc Sect 6:115–118. https://doi.org/10.18596/jotcsa.466906
Szafranek B, Chrapkowska K, Pawińska M, Szafranek J (2005) Analysis of leaf surface sesquiterpenes in potato varieties. J Agric Food Chem 53:2817–2822. https://doi.org/10.1021/jf040437g
Wesołowska A, Grzeszczuk M, Kulpa D (2015) GC-MS analysis of the essential oil from flowers of Chrysanthemum coronarium L. propagated conventionally and derived from in vitro cultures. Acta Chromatogr 27:525–539. https://doi.org/10.1556/achrom.27.2015.3.9
Formisano C, Rigano D, Senatore F, Raimondo FM, Maggio A, Bruno M (2012) Essential oil composition and antibacterial activity of Anthemis mixta and A. tomentosa (Asteraceae). Nat Prod Commun 7:1934578X1200701035
Hedin PA, Thompson AC, Gueldner RC (1972) Application of a sequential reduction regimen to fractionation of essential oils. Anal Chem 44(1254):1257. https://doi.org/10.1021/ac60315a030
Leela NK, Vipin TM, Shafeekh KM, Priyanka V, Rema J (2009) Chemical composition of essential oils from aerial parts of Cinnamomum malabatrum (Burman f.) Bercht & Presl. Flavr Fragr J 24:13–16. https://doi.org/10.1002/ffj.1910
Duquesnoy E, Dinh NH, Castola V, Casanova J (2006) Composition of a pyrolytic oil from cupressus funebris Endl. of Vietnamese origin. Flavour Fragr J 21:453–457
Davies NW (1990) Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J Chromatogr A 503:1–24
Custers Y (2009) GC volatile components analysis of different parts of Litchi chinensis. Thesis Uni. Gent.:1–58
Politeo O, Jukic M (2007) Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem 101:379–385. https://doi.org/10.1016/j.foodchem.2006.01.045
Yagi S, Babiker R, Tanova T, Schohn H (2016) Chemical composition, antiproliferative, antioxidant and antibacterial activities of essential oils from aromatic plants growing in Sudan. Asian Pac J Trop Med 9:763–770. https://doi.org/10.1016/j.apjtm.2016.06.009
Couladis M, Tsortanidou V, Francisci-Ortego J, Santos-Guerra A, Harvala C (2001) Composition of the essential oils of Argyranthemum species growing in the Canary Islands. Flavr Fragr J 16:103–106. https://doi.org/10.1002/ffj.954
Jirovetz L, Buchbauer G, Stoyanova AS, Georgiev EV, Damianova ST (2003) Composition, quality control, and antimicrobial activity of the essential oil of long-time stored dill (Anethum graveolens L.) seeds from Bulgaria. J Agric Food Chem 51:3854–3857
Karioti A, Skaltsa H, Demetzos C, Perdetzoglou D, Economakis CD, Salem AB (2003) Effect of nitrogen concentration of the nutrient solution on the volatile constituents of leaves of Salvia fruticosa Mill. in solution culture. J Agric Food Chem 51:6505–6508
Aissaoui M, Chalard P, Figuérédo G, Marchioni E, Zao M, Benayache F, Benayache S (2014) Chemical composition of the essential oil of Salvia verbenaca (L.) Briq. ssp. pseudo-jaminiana (Chev.) M. Res J Pharm Biol Chem Sci 5:368–372
Kim YG, Lee JH, Kim SI, Baek KH, Lee J (2015) Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int J Food Microbiol 195:30–39. https://doi.org/10.1016/j.ijfoodmicro.2014.11.028
Miyazawa M, Nomura M, Marumoto S, Mori K (2013) Characteristic odor components of essential oil from Scutellaria laeteviolacea. J Oleo Sci 62:51–56. https://doi.org/10.5650/jos.62.51
Pino JA, Mesa J, Muñoz Y, Martí MP, Marbot R (2005) Volatile components from mango (Mangifera indica L.) cultivars. J Agric Food Chem 53:2213–2223
Wangchuk P, Keller PA, Pyne SG, Taweechotipatr M, Kamchonwongpaisan S (2013) GC/GC-MS analysis, isolation and identification of bioactive essential oil components from the Bhutanese medicinal plant, Pleurospermum amabile. Nat Prod Commun 8:1934578X1300800930. https://doi.org/10.1177/1934578X1300800930
Vidic D, Ćavar S, Šolić ME, Maksimović M (2010) Volatile constituents of two rare subspecies of Thymus praecox. Nat Prod Comm 5(7):1123–1126. https://doi.org/10.1177/1934578X1000500730
Wu PS, Kuo YT, Chen SM, Li Y, Lou BS (2014) Gas chromatography-mass spectrometry analysis of photosensitive characteristics in citrus and herb essential oils. J Chromatogr Sep Tech 6:1–9. https://doi.org/10.4172/2157-7064.1000261
Choi HS (2003) Character impact odorants of Citrus hallabong [(C. unshiu Marcov x C.sinensis Osbeck) x C. reticulata Blanco] cold- pressed peel oil. J Agric Food Chem 51:2687–2692. https://doi.org/10.1021/jf021069o
Raina AP, Negi KS (2015) Essential oil composition of Valeriana jatamansi Jones from Himalayan regions of India. Indian J Pharm Sci 77:218
Cullere L, Escudero A, Cacho J, Ferreira V (2004) Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J Agric Food Chem 52:1653–1660. https://doi.org/10.1021/jf0350820
Cavalli JF, Tomi F, Bernardini AF, Casanova J (2003) Composition and chemical variability of the bark oil of Cedrelopsis grevei H. Baillon from Madagascar. Flavour Fragr J 18:532–538. https://doi.org/10.1002/ffj.1263
Galindo-Cuspinera V, Lubran MB, Rankin SA (2002) Comparison of volatile compounds in water-and oil-soluble annatto (Bixa orellana L.) extracts. J Agric Food Chem 50:2010–2015. https://doi.org/10.1021/jf011325h
Zhao MP, Liu QZ, Liu Q, Liu ZL (2017) Identification of larvicidal constituents of the essential oil of Echinopsgrijsii roots against the three species of mosquitoes. Mol 22:205
Sadashiva CT, Sharanappa P, Naidoo Y, Balachandran I (2013) Chemical composition of essential oil from the leaves of Premnacoriacea Clarke. Afr J Biotech 12
Lesueur D, Ban NK, Bighelli A, Muselli A, Casanova J (2006) Analysis of the root oil of Fokienia hodginsii (Dunn) Henry et Thomas (Cupressaceae) by GC, GC–MS and 13C-NMR. Flavr Fragr J 21:171–174. https://doi.org/10.1002/ffj.1557
Mastelic J, Jerkovic I, Mesic M (2006) Volatile constituents from flowers, leaves, bark and wood of Prunus mahaleb L. Flavour Frag J 21:306–313
Salvatore MM, Giambra S, Naviglio D, DellaGreca M, Salvatore F, Burruano S, Andolfi A (2018) Fatty acids produced by Neofusicoccum vitifusiforme and N. parvum, fungi associated with grapevine Botryosphaeria dieback. Agric 8:189. https://doi.org/10.3390/agriculture8120189
Nadaf M, Halimi M, Mortazavi M (2012) Identification of nonpolar chemical composition Spartium junceum flower growing in Iran by GC-MS. Middle-East J Sci Res 11:221–224
Valim MF, Rouseff RL, Lin J (2003) Gas chromatographic− olfactometric characterization of aroma compounds in two types of cashew apple nectar. J Agric Food Chem 51:1010–1015. https://doi.org/10.1021/jf025738+
Kobaisy M, Tellez MR, Dayan FE, Duke SO (2002) Phytotoxicity and volatile constituents from leaves of Callicarpa japonica. Thunb Phytochem 61:37–40
Jeribi C, Karoui IJ, Benhassine D, Abderrabba M (2016) Chemical composition of Cardopatium corymbosum leaves essential oil. J Essen Oil-Bear Plants 19:1471–1477. https://doi.org/10.1080/0972060X.2016.1224685
Tellez MR, Canel C, Rimando AM, Duke SO (1999) Differential accumulation of isoprenoids in glanded and glandless Artemisia annua L. Phytochem 52:1035–1040. https://doi.org/10.1016/S0031-9422(99)00308-8
De Morais SR, Oliveira TLS, Bara MTF, Conceicao ECD, Rezende MH, Ferri PH, Paula JRD (2012) Chemical constituents of essential oil from Lippiasidoides Cham. (Verbenaceae) leaves cultivated in Hidrolândia, Goiás, Brazil. Int J Anal Chem. https://doi.org/10.1155/2012/363919
Kim KR, Kim H (2000) Gas chromatographic profiling and screening for phenols as isobutoxycarbonyl derivatives in aqueous samples. J Chromatogr A 866:87–96. https://doi.org/10.1016/S0021-9673(99)01068-7
Balogun OS, Ajayi OS, Adeleke AJ (2017) Hexahydrofarnesyl acetone-rich extractives from Hildegardia barteri. J. Herbs Spices Med Plants 23:393–400. https://doi.org/10.1080/10496475.2017.1350614
Aminkhah M, Asgarpanah J (2017) GC-MS Analysis of the essential oil from Artemisia aucheri Boiss. Fruits. J Chil Chem Soc 62:3581–3582
Kandpal V, Joshi PK, Joshi N (2016) GC-MS analysis of seed essential oil of Chenopodiumambrosioides L. collected from Himalayan region. J Essen Oil-Bear Plants 19:258–261. https://doi.org/10.1080/0972060X.2015.1113891
Baharum SN, Bunawan H, Ghani MAA, Mustapha WAW, Noor NM (2010) Analysis of the chemical composition of the essential oil of Polygonum minus Huds. using two-dimensional gas chromatography-time of-flight mass spectrometry (GC-TOF MS). Mol 15:7006–7015. https://doi.org/10.3390/molecules15107006
Gerasimenko VA, Kirilenko AV, Nabivach VM (1981) Capillary gas chromatography of aromatic compounds found in coal tar fractions. J Chromatogr A 208:9–16. https://doi.org/10.1016/S0021-9673(00)87953-4
Mazimba O, Masesane IB, Majinda RR, Muzila A (2012) GC-MS analysis and antimicrobial activities of the non-polar extracts of Mundulea sericea. South Afr J Chem 65:50–52
El-Sayed AM, Heppelthwaite VJ, Manning LM, Gibb AR, Suckling DM (2005) Volatile constituents of fermented sugar baits and their attraction to lepidopteran species. J Agric Food Chem 53:953–958
Sotomayor JA, Martínez RM, García AJ, Jordán MJ (2004) Thymus zygis subsp. gracilis: watering level effect on phytomass production and essential oil quality. J Agric Food Chem 52:5418–5424
Vichi S, Pizzale L, Conte LS, Buxaderas S, López-Tamames E (2003) Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: characterization of virgin olive oils from two distinct geographical areas of northern Italy. J Agric Food Chem 51:6572–6577
Mondello L, Dugo P, Basile A, Dugo G, Bartle KD (1995) Interactive use of linear retention indices, on polar and apolar columns, with a MS-library for reliable identification of complex mixtures. J Microcolumn 7:581–591
King MF, Matthews MA, Rule DC, Field RA (1995) Effect of beef packaging method on volatile compounds developed by oven roasting or microwave cooking. J Agric Food Chem 43:773–778
Ramsey JD, Lee TD, Osselton MD, Moffat AC (1980) Gas-liquid chromatographic retention indices of 296 non-drug substances on SE-30 or OV-1 likely to be encountered in toxicological analyses. J Chromatogr A 184:185–206
Sunil Kumar KN, Rajalekshmi M, Sangeetha B, Ravishankar B, Muralidhar R, Yashovarma B (2013) Chemical Fingerprint of Leaves of Cinnamomum sulphuratum Nees Growing in Kodagu, Karnataka. J Pharmacog Phytochem 2:164–169
Baruah A, Nath SC, Leclercq PA (1999) Leaf and stem bark oils of Cinnamomum sulphuratum Nees from Northeast India. J Essent Oil Res 11:194–196. https://doi.org/10.1080/10412905.1999.9701108
Schmidt Co E, Kitzing K, Schloss Alten H, Wallerstein D (2006a) Composition and antioxidant activities of the essential oil of cinnamon (Cinnamomum zeylanicum Blume) leaves from Sri Lanka. J Essent Oil-Bear Plants 9:170–182. https://doi.org/10.1080/0972060X.2006.10643490
Boniface Y, Philippe S, Lima H, Pierre N, Alain A, Fatiou T, Dominique S (2012) Chemical composition and antimicrobial activities of Cinnamomum perrottetii dry leaves essential oil against food-borne pathogens and adulterated microorganisms. Int Res J Biol Sci 1:18–25
Unlu M, Emel E, Gulhan VU, Zeytinoglu SH (2010) Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food and chemical toxicology: an international journal published for the Brit Indus Biol Res Asso 48:3274–3280. https://doi.org/10.1016/j.fct.2010.09.001
Patel K, Ali S, Sotheeswaran S, Dufour JP (2007) Composition of the leaf essential oil of Cinnamomum verum (Lauraceae) from Fiji Islands. J Essent Oil Bear Plants 10:374–377. https://doi.org/10.1080/0972060X.2007.10643569
Chakraborty A, Sankaran V, Ramar M, Chellappan DR (2015) Chemical analysis of leaf essential oil of Cinnamomum verum from Palni hills, Tamil Nadu. J Chem Pharm Sci 3:10
Monteiro IN, dos Santos MO, Costa-Junior LM, da Silva LA, de Aguiar AEH, Maia JGS, Filho VEM (2017) Chemical composition and acaricide activity of an essential oil from a rare chemotype of Cinnamomum verum Presl. on Rhipicephalus microplus (Acari: Ixodidae). Vet Parasitol 238:54–57. https://doi.org/10.1016/j.vetpar.2017.03.016
Chinh HV, Luong NX, Thin DB, Dai DN, Hoi TM, Ogunwande IA (2017) Essential Oils Leaf of Cinnamomum glaucescens and Cinnamomum verum from Vietnam. Amer J Pl Sci 08(11):2712–2721. https://doi.org/10.4236/ajps.2017.811182
Li Y, Dexin K, Lin XM, Xie ZH, Bai M, Huang S, Nian H, Hong W (2016) Quality evaluation for essential oils of Cinnamomum verum leaves at different growth stages based on GC-MS, FTIR and microscopy. Food Anal Methods 9:202–212. https://doi.org/10.1007/s12161-015-0187-6
Hema R, Kumaravel S, Martina SD (2010) Chromatograph interfaced to a mass spectrometer analysis of Cinnamomum verum. Nat Sci 8:152–155
Subki SY, Jamal JA, Husain K, Manshoor N (2013) Characterization of leaf essential oils of three Cinnamomum species from Malaysia by gas chromatography and multivariate data analysis. Pharmacogn J 5:22–29. https://doi.org/10.1016/j.phcgj.2012.12.004
Sriramavaratharajan V, Murugan R (2018) Chemical profile of leaf essential oil of Cinnamomum walaiwarense and comparison of its antioxidant and hypoglycemic activities with the major constituent benzyl benzoate. Nat Prod Commun 13:779–782. https://doi.org/10.1177/1934578X1801300633
Chen HP, Yang K, You CX, Chun Lei N, Sun R, Geng Zhu M, Ping C, Qian D, Shu D, Zhi (2014) Chemical constituents and insecticidal activities of the essential oil of Cinnamomum camphora leaves against Lasioderma serricorne. J Chem 5(1):1-5 https://doi.org/10.1155/2014/963729
Guo S, Geng Z, Zhang W, Liang J, Wang C, Deng Z, Du S (2016) The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int J Mol Sci 17(11):1836. https://doi.org/10.3390/ijms17111836
Jiang H, Wang J, Song L, Cao X, Yao X, Tang F, Yue Y (2016) GC x GC-TOFMS analysis of essential oils composition from leaves, twigs and seeds of Cinnamomum camphora (Presl.) and their insecticidal and repellent activities. Molecules 21:423. https://doi.org/10.3390/molecules21040423
Yu H, Ren X, Liu Y, Xie Y, Guo Y, Cheng Y, Qian H, Yao W (2019) Extraction of Cinnamomum camphora Chvar. Borneol essential oil using neutral cellulase assisted-steam distillation: optimization of extraction, and analysis of chemical constituents. Ind Crop Prod 141:111794
Xu Y, Qin J, Wang P, Li Q, Yu S, Zhang Y, Wang Y (2019) Chemical composition and larvicidal activities of essential oil of Cinnamomum camphora (L.) leaf against Anopheles stephensi. J Braz Soc Trop Med 53(1):1–5. https://doi.org/10.1590/0037-8682-0211-2019
Chen J, Tang C, Zhang R, Ye S, Zhao Z, Huang Y, Xu X, Lan W, Yang D (2020) Metabolomics analysis to evaluate the antibacterial activity of the essential oil from the leaves of Cinnamomum camphora (Linn.) Presl. J Ethnopharmacol 253:112–652. https://doi.org/10.1016/j.jep.2020.112652
Baruah A, SnC N (2006) Leaf essential oils of Cinnamomum glanduliferum (Wall) Meissn and Cinnamomum glaucescens (Nees) Meissn. J Essent Oil Res 18:200–202. https://doi.org/10.1080/10412905.2006.9699065
Singh C, Singh S, Pande C, Tewari G, Kharkwal GC (2014) Chemical composition of the leaves essential oil from Cinnamomum glanduliferum (Wall) Meissn from Uttarakhand, India. J Essent Oil Bear Pl 17:927–930. https://doi.org/10.1080/0972060X.2014.935027
Azab SS, Jaleel GAA, Eldahshan OA (2017) Anti-inflammatory and gastroprotective potential of leaf essential oil of Cinnamomum glanduliferum in ethanol-induced rat experimental gastritis. Pharm Biol 55(1):1654–1661. https://doi.org/10.1080/13880209.2017.1314512
Chalchat JC, Garry RP, Muhayimana A (1995) Essential oil of Tagetes from Rwanda and France: chemical composition according to harvesting, location, growth stage and part of plant extracted. J Essent Oil Res 34:375–386. https://doi.org/10.1080/10412905.1995.9698544
Kaul PN, Bhattacharya AK, Rajeswara Rao BR (1996) Seasonal variation in the composition of the essential oil of Cinnamon (Cinnamomum zeylanicum Blume) leaves. Indian Perfum 40:36–38
Inouye S, Takizawa T, Yamaguchi H (2001) Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob Chemother 47:565–573. https://doi.org/10.1093/jac/47.5.565
Rameshkumar KB, George V, Shiburaj S (2007) Chemical constituents and antibacterial activity of the leaf oil of Cinnamomum chemungianum Mohan et Henry. J Essen Oil Res 19:98–100. https://doi.org/10.1080/10412905.2007.9699238
El-Baroty GS, El-Baky HHA, Farag RS, Saleh MA (2010) Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils. Afr J Biochem Res 4:167–174
Katiyar (2010) Cinnamomum zeylanicum and Cinnamomum cassia on food spoilage bacteria and water borne bacteria. Annals Biol Res 1:200–209
Mehmet U, Ergene E, Gulhan UV, Zeytinoglu HS, Nilufer V (2010) Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem Toxicol 48:3274–3280. https://doi.org/10.1016/j.fct.2010.09.001
Goni P, Lopez P, Sanchez C, Gomez-Lus R, Becerril R, Nerin C (2009) Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem 116:982–989. https://doi.org/10.1016/j.foodchem.2009.03.058
Lambert RJW, Skandamis PN, Coote PJ, Nychas GJE (2001) A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91:453–462. https://doi.org/10.1046/j.1365-2672.2001.01428.x
Friedman M, Henika PR, Levin CE, Mandrell RE (2004) Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J Agric Food Chem 52:6042–6048. https://doi.org/10.1021/jf0495340
Prabuseenivasan S, Jayakumar M, Ignacimuthu S (2006) In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med 6:1–8. https://doi.org/10.1186/1472-6882-6-39
Shafreen B, Mohmed R, Selvaraj C, Singh SK, Pandian SK (2014) In silico and in vitro studies of cinnamaldehyde and their derivatives against LuxS in Streptococcus pyogenes: Effects on biofilm and virulence genes. J Mol Recog 27:106–116. https://doi.org/10.1002/jmr.2339
Gill AO, Holley RA (2004) Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl Environ Microbiol 70:5750–5755. https://doi.org/10.1128/AEM.70.10.5750-5755.2004
Oussalah M, Caillet S, Lacroix M (2006) Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J Food Prot 69:1046–1055. https://doi.org/10.4315/0362-028X-69.5.1046
Carson CF, Mee BJ, Riley TV (2002) Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 46:1914–1920. https://doi.org/10.1128/AAC.46.6.1914-1920.2002
Bouhdid S, Abrini J, Amensour M, Zhiri A, Espuny MJ, Manresa A (2010) Functional and ultrastructural changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Cinnamomum verum essential oil. J Appl Microbiol 109:1139–1149. https://doi.org/10.1111/j.1365-2672.2010.04740.x
Yang SK, Yusoff K, Ajat M, Thomas W, Abushelaibi A, Akseer R, Lim E, Lai KS (2019) Disruption of KPC-producing Klebsiella pneumoniae membrane via induction of oxidative stress by cinnamon bark (Cinnamomum verum J. Presl) essential oil. PLoS ONE 14:e0214326. https://doi.org/10.1371/journal.pone.0214326
Narayanankutty A, Kunnath K, Alfarhan A, Rajagopal R, Ramesh V (2021) Chemical composition of Cinnamomum verum leaf and flower essential oils and analysis of their antibacterial, insecticidal, and larvicidal properties. Mol 26(20):6303. https://doi.org/10.3390/molecules26206303
Marasini BP, Baral P, Aryal P, Ghimire KR, Neupane S, Dahal N, Singh A, Ghimire L, Shrestha K (2015) Evaluation of antibacterial activity of some traditionally used medicinal plants against human pathogenic bacteria. Bio Med Res D 265425:6. https://doi.org/10.1155/2015/265425
Brodowska K, Sykula A, Garribba E, Chruscinska LE, Sojka M (2016) Naringenin Schiff base: antioxidant activity, acid-base profile, and interactions with DNA. Transition Met Chem 41:179–189. https://doi.org/10.1007/s11243-015-0010-7
Miguel MG (2010) Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr J 25:291–312. https://doi.org/10.1002/ffj.1961
Palozza P, Krinsky NI (1992) Antioxidant activity of carotenoids in vivo and in vitro: An overview. Meth Enzymol 213:403–420. https://doi.org/10.1016/0076-6879(92)13142-K
Farag RS, Daw ZY, Hewedi FM, El- Baroty GSA (1989) Antimicrobial activity of some Egyptian spice essential oils. J Food Prot 52:665–667. https://doi.org/10.4315/0362-028X-52.9.665
Evans RCA, Miller NJ, Paganga G (1996) Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol Med 20:933–956. https://doi.org/10.1016/0891-5849(95)02227-9
Wang W, Wu N, Zu YG, Fu YJ (2008) Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem 108:1019–1022. https://doi.org/10.1016/j.foodchem.2007.11.046
Prakash D, Upadhyay G, Pushpangadan P, Gupta C (2011) Antioxidant and free radical scavenging activities of some fruits. J Complement Int Med 8:1–16. https://doi.org/10.2202/1553-3840.1513
Tamil Selvi M, Thirugnanasampandan R, Sundarambal S (2015) Antioxidant and cytotoxic activities of essential oil of Ocimum canum Sims. from India. J Saudi Chem Soc 19:97–100. https://doi.org/10.1016/j.jscs.2011.12.026
Schmidt Co E, Kitzing K, Schloss Alten H, Wallerstein D (2006b) Composition and antioxidant activities of the essential oil of cinnamon (Cinnamomum zeylanicum Blume) leaves from Sri Lanka. J Essent Oil-Bear Plants 9:170–182. https://doi.org/10.1080/0972060X.2006.10643490
Jayaprakasha GK, Rao LJM (2011) Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit Rev Food Sci Nutr 51:547–562. https://doi.org/10.1080/10408391003699550
Amma KPP, Rani MP, Sasidharan I, Sreekumar MM (2012) Comparative chemical composition and in vitro antioxidant activities of essential oil isolated from the leaves of Cinnamomum tamala and Pimenta dioica. Nat Prod Res 27:290–294
Abdelwahab SI, Mariod AA, Tahaa MME, Zamanc FQ, Abdelmageed AHA, Khamis S, Sivasothyen Y, Awing K (2017) Chemical composition and antioxidant properties of the essential oil of Cinnamomum altissimum Kosterm. (Lauraceae). Arab J Chem 10:131–135
Gulcin I (2011) Antioxidant activity of food constituents: an overview. Arch Toxicol 86:345–391. https://doi.org/10.1007/s00204-011-0774-2
Hidalgo ME, De la Rosa C, Carrasco H, Cardona W, Gallardo C, Espinoza L (2009) Antioxidant capacity of eugenol derivatives. Quimica Nova 32:1467–1470. https://doi.org/10.1590/S0100-40422009000600020
Govindarajan M (2010) Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (willd.) Hook. F. Benth (Rutaceae) against three mosquito species. Asian Pac J Trop Med 3:874–877
Govindarajan M (2011) Larvicidal and repellent properties of some essential oils against Culex tritaeniorhynchus Giles and Anopheles subpictus Grassi (Diptera : Culicidae). Asian Pac J Trop Med 4:106–111. https://doi.org/10.1016/S1995-7645(11)60047-3
Liu C, Liu QY, Zhou L, Liu ZL (2015) Larvicidal activity of essential oil derived from Illicium henryi Diels (Illiciaceae) leaf. Trop J Pharm Res 14(1):111–116. https://doi.org/10.4314/tjpr.v14i1.16
Samarasekera R, Kosmulalage SK, Indira S, Weerasinghe (2005) Mosquitocidal activity of leaf and bark essential oils of Ceylon Cinnamomum zeylanicum. J Essen Oil Res 17:301–303. https://doi.org/10.1080/10412905.2005.9698909
Pandiyan G, Mathew N, Munusamy S (2019) Larvicidal activity of selected essential oil in synergized combinations against Aedes aegypti. Ecotoxicol Environ Saf 174:549–556. https://doi.org/10.1016/j.ecoenv.2019.03.019
Barbosa JDF, Silva VB, Alves PB, Gumina G, Santos R, Sousa DP, Socrates CH (2012) Structure–activity relationships of eugenol derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag Sci:124–142. https://doi.org/10.1002/ps.3331
Thomas A, Mazigo HD, Manjurano A, Morona D, Kweka EJ (2017) Evaluation of active ingredients and larvicidal activity of clove and cinnamon essential oils against Anopheles gambiae. Parasites Vectors 10:411. https://doi.org/10.1186/s13071-017-2355-6
Dias T, Brito I, Moujir L, Paiz N, Darias J, Cueto M (2005) Cytotoxic Sesquiterpenes from Aplysia dactylomela. J Nat Prod 68:1677–1679. https://doi.org/10.1021/np050240y
Barnard DR (1999) Repellency of essential oils to mosquitoes (Diptera: Culicidae). J Med entomol 36:625–629
Cheng SS, Liu JY, Tsai KH, Chen WJ, Chang ST (2004) Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J Agric Food Chem 52:4395–4400. https://doi.org/10.1021/jf0497152
Traboulsi AF, El-Haj S, Tueni M, Taoubi K, Nader NB, Mrad A (2005) Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiensmolestus (Diptera: Culicidae). Pest Manag Sci 61:597–604. https://doi.org/10.1002/ps.1017
Tawatsin A, Wratten SD, Scott RR, Thavara U, Techandamrongsin Y (2001) Repellency of volatile oils from plants against three mosquito vectors. J Vector Ecol 26:76–82
Tawatsin A, Asavadachanukorn P, Thavara U, Wongsinkongman P, Bansidhi J, Boonruad T (2006) Repellency of essential oils extracted from plants in Thailand against four mosquito vectors (Diptera: Culicidae) and oviposition deterrent effects against Aedes aegypti (Diptera: Culicidae). Southeast Asian J Trop Med Public Health 37:915–931
Amer A, Mehlhorn H (2006) Repellency effect of forty-one essential oils against Aedes, Anopheles and Culex mosquitoes. Parasitol Res 99:478-90. https://doi.org/10.1007/s00436-006-0184-1
Pushpanathan T, Jebanesan A, Govindarajan M (2008) The essential oil of Zingiber officinalis Linn (Zingiberaceae) as a mosquito larvicidal and repellent agent against the filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 102:1289–1291. https://doi.org/10.1007/s00436-008-0907-6
Intirach J, Junkum A, Tuetun B, Choochote W, Chaithong U, Jitpakdi A, Pitasawat B (2012) Chemical constituents and combined larvicidal effects of selected essential oils against Anopheles cracens (Diptera : Culicidae). J Chem 2012:591616. https://doi.org/10.1155/2012/591616
Sarma R, Adhikari K, Mahanta S, Khanikor B (2019) Combinations of plant essential oil based Terpene compounds as larvicidal and adulticidal agent against Aedes aegypti (Diptera : Culicidae). Sci Rep 9:9471. https://doi.org/10.1038/s41598-019-45908-
Santos SRL, Melo MA, Valença A, Santos RLC, SCH SDPDC (2011) Structure – activity relationships of larvicidal monoterpenes and derivatives against Aedes aegypti Linn. Chemosphere 84(150):153. https://doi.org/10.1016/j.chemosphere.2011.02.018
Rey D, Cuany A, Pautou MP, Meyran JC (1999) Differential sensitivity of mosquito texa to vegetable tannins. J Chem Ecol 25:537–548. https://doi.org/10.1023/A:1020953804114
David JP, Rey D, Pauntou MP, Meyran JC (2000) Differential toxicity of leaf litter to dipteran larvae of mosquito developmental sites. J Invertebr Pathol 75:9–18. https://doi.org/10.1006/jipa.1999.4886
Jantan I, Yalvema MF, Ahmad NW, Jamal JA (2005) Insecticidal activities of the leaf oils of eight Cinnamomum species against Aedes aegypti and Aedes albopictus. Pharm Bio 43:526–532. https://doi.org/10.1080/13880200500220771
Funding
We thank the National Medicinal Plant Board, New Delhi for providing financial support through a major research project (F.No. Z. 18017/187/CSS/ R&D/TN-01/2023-24- NMPB-IV A).
Author information
Authors and Affiliations
Contributions
Saranya Surendran; execution of laboratory studies, preparation of the manuscript; Smija, K.P: execution and assistance of laboratory studies; Raju Ramasubbu: planning of research problem, work designed, lead, review and editing of the manuscript, Azhagu Madhavan Sivalingam and Arjun Pandian reviewed, edited revised article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 728 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Surendran, S., KP, S., Pandian, A. et al. Analysis of chemical composition and biological efficiency of leaf essential oils isolated from seven species of Cinnamomum of the Western Ghats, India. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05653-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05653-8