Abstract
The study explores the effectiveness of alkaline pretreatment using various concentrations of NaOH (2%, 4%, 6%, 8%, and 10%) on lignocellulosic biomass, specifically paddy straw and sugarcane bagasse, and its impact on the biomass structure and subsequent bioethanol production. Among the 2–10% NaOH pretreatment, samples at 2% NaOH exhibited a significant removal of lignin and increased reducing sugar yields of 8.369 g/L from paddy straw and 7.635 g/L from sugarcane bagasse compared to untreated samples. The growth patterns of two yeast strains, Saccharomyces cerevisiae and Yarrowia lipolytica, were analyzed under different treatment conditions using paddy straw and sugarcane bagasse as carbon sources. The results indicated that S. cerevisiae showed enhanced growth when dextrose was present with paddy straw but exhibited lower growth with sugarcane bagasse. In contrast, Y. lipolytica exhibited higher growth with the addition of dextrose regardless of the carbon source. Scanning electron micrographs (SEM) and Fourier transform infrared (FTIR) analysis further confirmed the structural changes and lignocellulose degradation after 2% NaOH pretreatment. Among the treatment conditions, the highest ethanol yield of 65.6 ml/L was obtained when S. cerevisiae and Y. lipolytica were co-fermented with sugarcane bagasse as the carbon source without the addition of dextrose. Overall, this study demonstrates the effectiveness of alkaline pretreatment using 2%NaOH in removing lignin from paddy straw and sugarcane bagasse, resulting in improved bioethanol production. The findings highlight the potential of utilizing lignocellulosic biomass as a sustainable feedstock for biofuel production through efficient treatment methods.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Climate change and energy security are the prime concern in the twenty-first century. Researchers actively seek alternative energy sources to mitigate their drawbacks in response to fossil fuels’ depletion and detrimental effects. Bioethanol has emerged as a promising substitute for fossil fuels because it controls oil price fluctuations and reduces greenhouse gas emissions. Additionally, it is non-toxic, biodegradable in nature, and carbon–neutral [1]. While bioethanol has traditionally been produced from food crops such as cassava, wheat, and sugarcane molasses, this practice has raised concerns due to its negative impact on the food chain [2]. Consequently, researchers have turned their attention towards utilizing agricultural waste for bioethanol production, which mitigates environmental pollution caused by waste disposal and addresses concerns related to food availability.
Agricultural crop residues such as paddy straw and sugarcane bagasse serve as a potential feedstock for bioethanol production. Paddy straw, characterized by its low-cost nature as a byproduct of rice cultivation and boasting a substantial annual output volume of 731 million tons, comprises approximately 32–47% cellulose, 19–27% hemicellulose, 5–24% lignin, and 18% ashes, whereas sugarcane bagasse has 40–50% cellulose, 25–35% hemicellulose, and 17–20% lignin [3, 4]. Efficient bioethanol production from these lignocellulosic biomasses necessitates their decomposition into cellulose, lignin, and hemicellulose. This decomposition is achieved through pretreatment, which disrupts the recalcitrant structure of lignocellulose, enabling enzymatic degradation of cellulose and hemicellulose [5]. Various pretreatment methods, including autohydrolysis and physical, chemical, and physicochemical treatment, can deconstruct lignocellulosic biomass. Chemical pretreatment is the most commonly used pretreatment lignocellulosic biomass among these methods. Certain chemicals such as oxidizing agents, alkali, acids, and salts are used for the chemical pretreatment that aids in the degradation of lignin, hemicelluloses, and celluloses by breaking down ester bonds from lignocellulosic waste [6]. Alkali pretreatment also referred to as peroxide, ozonolysis, or wet oxidation pretreatment involves the use of highly concentrated alkaline solutions, such as potassium hydroxide or sodium hydroxide. Hydrolysis of lignocellulose by alkaline pretreatment is one of the most efficient treatments in reducing cellulose crystallinity compared to other pretreatment methods [7]. Hemicellulose hydrolysis can be aided by a dilute acid pretreatment; however, delignification occurs with low hydrolysis of polysaccharide components after alkaline pretreatment, resulting in increased biomass digestibility. So, both of these processes are used as a combined process for the effective pretreatment of lignocellulosic biomass with less chemical consumption resulting in less chemical cost and less generation of chemical waste [8]. By utilizing enzyme lysates from a mixture of seven bacterial strains, the study demonstrates significant delignification of rice straw, resulting in enhanced fermentation rates and a substantial increase in cumulative biogas production compared to untreated straw, highlighting the efficacy of synergistic enzymatic treatment in waste biomass hydrolysis for bioprocess technology [9]. Protease and amylase, being highly valuable microbial enzymes, find widespread applications in diverse industries, including organic waste degradation, biofuels, agriculture, pharmaceuticals, chemicals, and biotechnology, owing to their versatile capabilities [10]. The conversion of sugar into bioethanol requires innovative fermentation techniques to enhance the cost-effectiveness of the process [11].
Paddy straw contains soluble polysaccharides conjugated with high levels of phenolic compounds. These soluble polysaccharides are released during the pretreatment of paddy straw. The study by Zhu Wang et al. [12] showed that the oligosaccharides and phenolic compound conjugates promote better growth of yeast and ethanol fermentation. Saccharomyces cerevisiae, a widely used yeast, efficiently converts glucose and other sugars from cellulosic biomass into bioethanol through fermentation [13]. Yarrowia lipolytica also provides better ethanol yield through fermentation [14]. Because of its metabolic properties and potential applications, Y. lipolytica is one of the most studied “non-conventional” yeast strains. The organism is considered the most beneficial in biotechnology because it produces many valuable things such as sweeteners, organic acids, fatty acids, enzymes, and biomass [15]. The high lipid content of the organism makes it an excellent candidate for co-cultivation with microalgae, leading to an increased yield of lipids for biofuel production [16]. The utilization of Napier grass as a feedstock resulted in the highest bioethanol yield, when the mixed crude co-culture of Trichoderma reesei and S. cerevisiae was employed [17]. Another research showed the highest bioethanol production, achieved by combining two different feedstocks, cassava and sugarcane bagasse, using S. cerevisiae as a fermentation enzyme [18].
The present research aims to determine the optimal alkaline pretreatment conditions for agricultural biomass waste, namely sugarcane bagasse and paddy straw. The study uses FTIR and SEM analyses to assess the impact of alkaline pretreatment on the structural changes of lignocellulose and delignification efficiency. Furthermore, different treatment methods for bioethanol production by conventional yeast strain Saccharomyces cerevisiae (NCIM 3052) and non-conventional yeast strain Yarrowia lipolytica (NCIM 3229) are evaluated. The outcomes of this research will make a valuable contribution towards advancing sustainable and efficient bioethanol production methods, which harness agricultural waste resources while minimizing their environmental footprint.
2 Materials and methods
2.1 Raw materials
Agricultural waste samples, specifically paddy straw and sugarcane bagasse, were obtained from farmers’ fields and local stalls in Thiruvarur District, Tamil Nadu, India (latitude, 10.819990; longitude, 79.597820). The samples were subjected to air drying to reduce their moisture content. Subsequently, the samples were chopped, grounded, and sieved through a mesh size of 10, resulting in particle sizes of approximately 2 mm as shown in supplementary material 1.
2.2 Microorganisms
The yeast strains Saccharomyces cerevisiae (NCIM 3052) and Yarrowia lipolytica (NCIM 3229) were procured from the CSIR-NCL-NCIM, Pune, Maharashtra, India. Both the strains were cultured and maintained on a YPD medium consisting of peptone 20 g/L, yeast extract 10 g/L, and dextrose 20 g/L at 30 °C for 48 h, with a pH of 5.0. Regular subculturing was performed to ensure strain viability, and the cultures were subsequently stored in glycerol stock at 4 °C for future use.
2.3 Alkaline pretreatment
Dried and powdered samples were sieved through a 2-mm sieve and subsequently immersed in sodium hydroxide solutions of varying concentrations (2%, 4%, 6%, 8%, and 10%) at a solid-to-liquid ratio of 1:10. The mixtures were autoclaved at 15 psi for 30 min. After boiling, the agricultural waste samples were subjected to centrifugation for a duration of 10 min. Subsequently, the samples were filtered using a muslin cloth, and the resulting filtrate was collected for subsequent analysis. Then the pretreated samples were washed twice with distilled water to remove residual alkali and for pH neutralization. The dried biomass was then hydrolyzed using 4% sulfuric acid and placed in the water bath at a reaction temperature of 100 °C for 1 h [14]. The total reducing sugar was estimated in the supernatant by the 3,5-di-nitro salicylic acid (DNS) method [19].
2.4 Growth of yeast cultures on agricultural wastes
YPD broth and YP (yeast extract, peptone) broth were prepared facilitating the growth of Saccharomyces cerevisiae and Yarrowia lipolytica. The 50 ml of growth media was used to analyze the growth patterns of yeast cultures on paddy straw and sugarcane bagasse. Subsequently, 1 g of pretreated agricultural waste, paddy straw, or sugarcane bagasse was added. The media in the conical flasks were autoclaved and then inoculated with yeast culture at the exponential phase, either S. cerevisiae or Y. lipolytica. The experimental setups were conducted following the treatment details outlined in Table 1. To analyze the growth pattern, optical density readings of the samples were obtained at a wavelength of 600 nm using a UV–visible spectrophotometer. The readings were recorded daily over a period of 9 days.
2.5 SEM-EDXA
The morphology of raw and pretreated paddy straw and sugarcane bagasse was observed by using a scanning electron microscope (SEM)-energy-dispersive X-ray (EDX) analysis (Tescan Vega 3). To prepare the samples for imaging, a uniform Au layer was applied to the surfaces through ion sputtering to ensure enhanced conductivity and minimized charging effects during imaging. In addition to SEM imaging, the energy-dispersive X-ray (EDX) analyzer was employed to detect and quantify the inorganic constituents in the samples. The EDX analysis provided X-ray counts, offering valuable information about the elemental composition of the examined materials.
2.6 FTIR analysis
The qualitative analysis in the characteristic functional group of untreated paddy straw and sugarcane bagasse with the alkaline pretreated paddy straw and sugarcane bagasse was characterized using FTIR analysis (BRUKER Optik GmbH). The sample mixture was scanned in the middle infrared light (MIR) range specifically from 4000 to 400 cm−1. The scanning process involved an average of 64 scans and was performed at a spectral resolution of 4 cm−1. The analysis was conducted in transmittance mode.
2.7 X-ray diffraction (XRD) analysis
The crystalline characteristics of untreated paddy straw and sugarcane bagasse, as well as alkali-pretreated paddy straw and sugarcane bagasse, were analyzed using X-ray diffractometry (XRD) with a Malvern Panalytical Empyrean instrument employing CuKα radiation at a wavelength (λ) of 1.5406 Å, operated at 40 kV and 30 mA. The recorded intensity profiles spanned a 2θ range from 5° to 60° with a step size of 0.008° and a scanning rate of 2°/min. The determination of the crystallinity index (CrI) followed the empirical method proposed by Segal et al. [20]. The CrI was calculated using the formula:
where I002 represents the maximum diffraction intensity at the peak position of 2θ = 22.03°, and Iam is the intensity at 2θ = 18°.
2.8 Fermentation
For fermentation, 100 ml of YP medium containing pretreated paddy straw and sugarcane bagasse samples in conical flasks were autoclaved. The sterilized medium was then inoculated with S. cerevisiae and Y. lipolytica obtained from the 48-h-old culture, which had a cell density of 9.72 × 107 cells/ml of S. cerevisiae and 7 × 107 cells/ml of Y. lipolytica. The fermentation experiments were carried out by yeast strains under various treatment conditions involving paddy straw and sugarcane bagasse. After inoculation, the conical flasks were tightly sealed with rubber septa, and the cultures were incubated in an incubator shaker at 30 °C at 110 rpm for a duration of 96 h [21]. At the end of the fermentation period, broth samples containing yeast cells were collected by extracting 600 µl using a thin needle through the rubber septum. These samples were then transferred to clean 2-ml Eppendorf tubes. The tubes were centrifuged at 8000 g for 2 min to separate the yeast cells. Following centrifugation, 500 µl of the supernatant was transferred to another 2 ml Eppendorf tube without disturbing the pellet. Then, 5 µl of butanol was added to the tube, followed by vortexing at maximum speed for 30 s. Next, 1 ml of ethyl acetate was added to the mixture, which was vortexed again at maximum speed for 5 min. Subsequently, the tubes were centrifuged at 5000 g for 2 min, facilitating the separation of phases. The organic upper phase was collected and used for gas chromatography (GC-FID) analysis for ethanol estimation.
2.9 Bioethanol estimation by gas chromatography
The ethanol in the culture samples was quantified using the gas chromatographic technique. ThermoFisher Scientific gas chromatograph Trace 1110 equipped with a flame ionization detector (FID) was used to estimate the bioethanol present in the samples. GC column TG-5MS 30 m × 0.25 mm × 0.25 µm was fitted to provide on-column injection with FID conditions: 250 °C; H2 (flow rate, 25 ml/min); air (flow rate, 100 ml/min); N2 carrier gas (flow rate, 27 ml/min). The initial oven temperature of GC was set to 50 °C, which was subsequently increased at a rate of 7 °C per minute until it reached 100 °C. Ethyl acetate was used as blank. Two microliters of the sample was injected into the injector using a syringe. In between injections syringe was washed thoroughly with ethyl acetate to avoid cross-contamination. Generally, the retention time of ethanol was equivalent to 65 °C.
2.10 Statistical analysis
The statistical significance of the results was determined by two-way analysis of variance (ANOVA) with a 95% confidence interval and a significance level set at p = 0.05. GraphPad Prism version 8.0 and OriginPro 8.5 software were employed for all the data analysis in this research article.
3 Results and discussion
3.1 Alkaline pretreatment of paddy straw and sugarcane bagasse
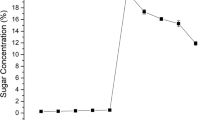
Alkaline pretreatment results in the effective removal of lignin from lignocellulosic biomass. It disrupts the biomass structure, breaking the cellulose and hemicellulose bonding and dignifying the biomass’s lignin content [22]. Compared to acidic reagents such as sulfuric acid and sulfite, alkaline pretreatment reagents, such as sodium hydroxide (NaOH), are less corrosive. Alkaline pretreatment using NaOH is considered the most effective due to certain advantages over other methods. NaOH is highly effective in removing lignin content from lignocellulosic biomass by disrupting ester bonds, a non-corrosive and cost-effective pretreatment method [23]. In this study, paddy straw and the sugarcane bagasse were pretreated with different percentages (2%, 4%, 6%, 8%, and 10%) of sodium hydroxide solution. It was observed that at 2% NaOH pretreatment, the paddy straw produced 8.369 g/L and sugarcane bagasse produced 7.635 g/L of reducing sugar compared to significantly lower yields from untreated paddy straw and sugarcane bagasse which produced only 4.874 g/L and 4.055 g/L respectively. The corresponding reducing sugar yields of untreated and pretreated samples are presented in Fig. 1. Previous research studies have demonstrated the potential of different pretreatment methods for lignocellulosic biomass. For instance, NaOH steam explosion pretreatment of sugarcane bagasse resulted in the highest production of reducing sugars, reaching 9.07 g/L [24]. Another study combining sodium carbonate and sodium sulfide in a 1:2 ratio for pretreating sugarcane bagasse achieved the highest reducing sugar yield of 592 mg/g [25]. Similarly, wheat straw exhibited the highest reducing sugar yield of 7.06 g/L when treated with 10% NaOH [5]. Additionally, rice straw pretreated with 2% NaOH yielded 8.17 g/L of reducing sugars [26]. The obtained reducing sugars from pretreated biomass can be further converted into fuel alcohol through the fermentation process facilitated by yeast.
3.2 Growth pattern of yeast cultures with paddy straw and sugarcane bagasse
The growth patterns of S. cerevisiae and Y. lipolytica were observed under different treatment conditions (shown in Table 1) using paddy straw and sugarcane bagasse as carbon sources. The results shown in Fig. 2 indicate that S. cerevisiae exhibited higher growth when dextrose was present in addition to paddy straw (T4), compared to the treatment without dextrose (T3). This suggests that S. cerevisiae, the conventional yeast, shows enhanced growth when the media contains dextrose and paddy straw. However, when sugarcane bagasse was used as the carbon source, S. cerevisiae exhibited lower growth when dextrose was added (T9) but showed higher growth without dextrose (T8). S. cerevisiae showed the highest growth on day 6. In contrast, Y. lipolytica showed the highest growth when dextrose was present, irrespective of whether paddy straw (T6) or sugarcane bagasse (T10) was used as the carbon source. T1 and T2, which served as control treatments, did not include paddy straw or sugarcane bagasse but contained dextrose. Y. lipolytica showed the highest growth on day 7. The growth readings initially increased until day 2, decreased subsequently, and then increased again after day 5. This phenomenon can be attributed to diauxic growth, where the yeast initially utilizes dextrose as the preferred carbon source. Upon its depletion, the yeast switch to utilizing sugarcane bagasse and paddy straw for their growth [3].
3.3 SEM-EDXA
Scanning electron micrographs of both untreated and 2%NaOH pretreated paddy straw and sugarcane bagasse samples are presented in Fig. 3. The pretreated samples show significant changes in their surface morphology compared to the untreated samples. These changes indicate the removal of lignin and the release of cellulose by the reaction of 2% NaOH with the ester bonds present in the biomass. The FTIR analysis further supports this observation. Prior to pretreatment, the untreated paddy straw and sugarcane bagasse samples exhibited a rigid and compact surface structure. A previous study investigating wheat straw pretreatment with increasing concentrations of NaOH demonstrated that higher NaOH concentrations led to increased abrasion, fiber splitting, scaling, and layering on the sample’s surface. These morphological changes suggested greater cellulose release from the biomass as the NaOH concentration during pretreatment increased [5]. Additionally, Fig. 4 presents the EDX element maps of the untreated and pretreated paddy straw and sugarcane bagasse samples, providing visual representations of the elemental distribution, while a table within these figures summarizes the elements detected.
3.4 FTIR analysis of paddy straw and sugarcane bagasse
FTIR analysis was conducted to examine the chemical and structural changes in the paddy straw and sugarcane bagasse before and after pretreatment with 2% NaOH. It showed increased complexity of spectra in pretreated samples compared to the untreated samples indicating the degradation of the lignocellulose components, shown in Fig. 5. The broadband transmittance at 3336.16 cm−1, 2899.75 cm−1, 1425.50 cm−1, and 1034.64 cm−1 in untreated paddy straw and broadband signals at 3337.01 cm−1, 2890.49 cm−1, 1426.14 cm−1 and 1028.87 cm−1 in untreated sugarcane bagasse are the characteristic peaks of cellulose [27]. The peak at 3336.16 cm−1 of untreated and 3775.13 cm−1, 3715.34 cm−1, 3660.32 cm−1 of pretreated paddy straw and peak at 3337.01 cm−1 of untreated and 3857.05 cm−1, 3758.12 cm−1, 3663.79 cm−1 of pretreated sugarcane bagasse indicates the O–H stretching of hydrogen bond [28]. In paddy straw, the peaks at 2899.75 cm−1 of untreated and 2893.59 cm−1 of pretreated correspond to weak C-H stretching in cellulose. Similarly, in sugarcane bagasse, the peak at 2890.49 cm−1 of untreated and 2876.82 cm−1 of pretreated shows the weak C-H stretching [29]. The peak at 1977.79 cm−1 and 2041.11 cm−1 indicates the aromatic C = C stretching in the lignin of untreated paddy straw and sugarcane bagasse respectively. The pretreated paddy straw and the sugarcane bagasse showed the C = C stretch vibration at 1980.80 cm−1 and 2040.35 cm−1 respectively [30]. Additional peaks were observed after pretreatment of sugarcane bagasse at around 1990–2500 cm−1 that indicates the degradation of lignocellulose structure. The signal peak at 897.97 cm−1 and 896.37 cm−1 in untreated paddy straw and sugar bagasse respectively indicates the C-O group of the β-1,4-glycosidic linkage present in cellulose moiety. There was intensity difference in the β-glycosidic linkage of the pretreated paddy straw with additional peak formation at 663.76 cm−1, 607.72 cm−1, and pretreated sugarcane bagasse at 602.13 cm−1 denoting the cleavage of β-1,4-glycosidic bond [31] Multiple peaks of CO stretching were observed in both the untreated and pretreated samples of paddy straw and sugarcane bagasse around 1400–1000 cm−1 region. The signal at 1726.62 cm−1 of untreated and 1725.53 cm−1 of pretreated paddy straw corresponds to C = O functional group which is a characteristic peak of hemicellulose [32]. Overall analysis of both paddy straw and sugarcane bagasse samples indicates the similarity in the raw material and decrease in cellulose and lignin content after pretreatment using 2% NaOH.
3.5 X-ray diffraction (XRD) analysis
The XRD analysis provides valuable insights into the structural changes induced by alkali pretreatment in both paddy straw and sugarcane bagasse. The CrI serves as a quantitative measure of crystallinity, offering a systematic approach to evaluating the impact of the pretreatment process on the biomasses under investigation. The crystallinity indices (CrI) of untreated paddy straw and sugarcane bagasse were determined to be 45.5% and 50.4%, respectively. Following treatment with 2% NaOH alkali, the CrI increased to 67.8% for paddy straw and 75.3% for sugarcane bagasse. This observed enhancement in CrI is attributed to the transformation of crystalline cellulose into its amorphous constituents, such as hemicellulose, lignin, and silica, during the alkali pretreatment process. The alkaline pretreatment not only induces a shift in the CrI but also facilitates the biodegradability of cellulose, concurrently augmenting the inner surface area to pore ratio [33]. These results align with existing studies. Chandel et al. [34] demonstrated a notable increase in CrI from 48.8 to 71.87% for native sugarcane bagasse upon alkali pretreatment. Similarly, Gabhane et al. [35] reported an elevation in CrI from 42.1 to 48% for raw paddy straw subjected to alkali pretreatment. Additionally, Cai et al. [36] observed an increase in CrI from 46.63 to 61.11% for unpretreated paddy straw following alkali pretreatment. These findings collectively underscore the efficacy of alkali pretreatment in modulating the crystalline structure of lignocellulosic biomass.
3.6 Estimation of bioethanol using gas chromatography
The results of the fermentation experiments unequivocally confirmed the presence of ethanol in all treatment conditions shown in Fig. 6. Among the treatment conditions, both S. cerevisiae and Y. lipolytica were employed together to ferment paddy straw and sugarcane bagasse separately, and the bioethanol production was estimated. Remarkably, the highest ethanol yield of 65.6 ml/L was achieved when S. cerevisiae and Y. lipolytica were grown with sugarcane bagasse as the carbon source without the addition of dextrose (T12). However, there were no significant differences observed in the ethanol yield when comparing all other treatment conditions (Fig. 7; supplementary material 2). Table 2 illustrates the bioethanol production from agricultural waste through fermentation utilizing yeast culture. Previous studies have highlighted the potential of S. cerevisiae for ethanol fermentation. Ahmad et al. [37] recommended S. cerevisiae due to its ability to consume more sugar, leading to higher ethanol production with a 25% inoculum. Tsigie et al. [14] obtained an ethanol yield of 0.084 g ethanol/g dry weight when using S. cerevisiae for fermentation with Y. lipolytica biomass hydrolysate as the medium. Onoghwarite [38] achieved a maximum ethanol yield of 143.15 mg/L under specific conditions while producing bioethanol from corn stover using S. cerevisiae. Muthusamy et al. [39] achieved a higher ethanol yield of 3.48 g/L at 72 h of incubation by fermenting sugarcane juice with Streptomyces olivaceus among five agro-residues tested. Wine et al. [40] estimated a bioethanol production of 9.10 g/l from corncob hydrolysate using S. cerevisiae after 48 h of incubation [40]. In a research article by Wang et al. [41], they reported the successful production of bioethanol from hot water sugar maple wood extract hydrolysate. They achieved a maximum bioethanol yield of 24.05 g/L using a recombinant strain of Escherichia coli FBWHR. The production process involved a 30-h incubation period. Ezebuiro et al. [42] demonstrated efficient bioethanol production using sugarcane bagasse and cassava peels as substrates with Bacillus cereus GBPS9, resulting in ethanol contents of 18.40 g/L and 17.80 g/L, respectively. Nikhil et al. [43] analyzed bioethanol production from saccharified sugars of agro-wastes (pearl millet bran) using Aspergillus flavus FPDN1 and found an ethanol concentration of 88% with a retention time of 3.31 min. Mushimiyamana et al. [44] also investigated bioethanol production using S. cerevisiae inoculated in a medium containing agro-wastes such as sugar beet peel, potato peel, onion peel, and carrot peel. Among these, the maximum ethanol yield of 17.3% was obtained from sugar beet peel after 28 days.
4 Conclusions
In conclusion, alkaline pretreatment using 2% sodium hydroxide (NaOH) has proved to be an effective method for lignin and cellulose release from lignocellulosic biomass of paddy straw and sugarcane bagasse. The pretreatment with 2% NaOH resulted in higher yields of reducing sugars compared to untreated biomass samples. The growth patterns of S. cerevisiae and Y. lipolytica varied under different treatment conditions, with S. cerevisiae showing enhanced growth in the presence of dextrose along with paddy straw, but lower growth when dextrose was added to sugarcane bagasse. In contrast, Y. lipolytica exhibited the highest growth when dextrose was present regardless of the carbon source used. SEM and FTIR analysis confirmed the morphological and chemical changes in the biomass after 2% NaOH pretreatment, indicating the removal of lignin and degradation of lignocellulosic components. Furthermore, the fermentation experiments demonstrated bioethanol production under all treatment conditions. Interestingly, the highest ethanol yield was obtained when S. cerevisiae and Y. lipolytica were grown with sugarcane bagasse as the carbon source without adding dextrose. However, there were no significant differences in ethanol yield among the other treatment conditions. This research contributes to the development of efficient and sustainable strategies for biomass utilization and biofuel production. Further optimization and scale-up studies are necessary to fully explore the potential of these pretreatment methods and microbial fermentation for bioethanol production.
Data availability
All the datasets are presented in the main manuscript.
References
Toor M, Kumar SS, Malyan SK, Bishnoi NR, Mathimani T, Rajendran K et al (2020) An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 242:125080
Awoyale AA, Lokhat D (2021) Experimental determination of the effects of pretreatment on selected Nigerian lignocellulosic biomass in bioethanol production. Sci Rep 11:557
Sonwani R, Gupta SB, Soni R (2020) Production of bioethanol from biodegraded alkali pretreated rice straw. Vegetos 33:128–134
Aziz T, Shah Z, Sarwar A, Ullah N, Khan AA, Sameeh MY et al (2023) Production of bioethanol from pretreated rice straw, an integrated and mediated upstream fermentation process. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-023-04283-w
Tsegaye B, Balomajumder C, Roy P (2019) Alkali pretreatment of wheat straw followed by microbial hydrolysis for bioethanol production. Environ Technol 40:1203–1211
S Rakesh K Subburamu N Arunkumar 2021 Pretreatment of paddy straw for sustainable bioethanol production Sustainable Bioprocessing for a Clean and Green Environment CRC Press 93 102
Mirahmadi K, Kabir MM, Jeihanipour A, Karimi K, Taherzadeh MJ (2010) Alkaline pretreatment of spruce and birch to improve bioethanol and biogas production. Bioresour 5(2):928–38
Ashoor S, Mallapureddy KK, Sukumaran RK (2022) Sequential mild acid and alkali pretreatment of rice straw to improve enzymatic saccharification for bioethanol production. Prep Biochem Biotechnol 53(3):231–8
Shah TA, Majeed T, Rahman SU, Ihsan T, Aziz T, Alharbi M et al. (2023) Synergistic treatment of crude enzymes from Bacillus sp. strains to boost anaerobic fermentation of rice straw. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-023-05090-z
Ullah N, Rehman MU, Sarwar A, Nadeem M, Nelofer R, Shakir HA et al (2022) Purification, characterization, and application of alkaline protease enzyme from a locally isolated Bacillus cereus strain. Fermentation 8:628
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–27
Zhu Wang Y, Zheng J, Nawaz M, Yang F, Hu J, Gao MT.(2022) Oligosaccharide-phenolic compound conjugates in soluble polysaccharides from rice straw alleviate ethanol fermentation stresses in Saccharomyces cerevisiae. Ind Crops Prod 181:114782
Zou J, Chang X (2022) Past, present, and future perspectives on whey as a promising feedstock for bioethanol production by yeast. J Fungus 8:395
Tsigie YA, Wu C-H, Huynh LH, Ismadji S, Ju Y-H (2013) Bioethanol production from Yarrowia lipolytica Po1g biomass. Bioresour Technol 145:210–216
Drzymała K, Mirończuk AM, Pietrzak W, Dobrowolski A (2020) Rye and oat agricultural wastes as substrate candidates for biomass production of the non-conventional yeast Yarrowia lipolytica. Sustainability 12:7704
T Jayakumar S Rakesh 2022 A study exploring the effects of cell disruption techniques on lipid recovery in co-cultivated microalgae and oleaginous yeast Bioenergy Res 16 3 1537 47
Mueansichai T, Rangseesuriyachai T, Thongchul N, Assabumrungrat S (2022) Lignocellulosic bioethanol production of napier grass using Trichoderma reesei and Saccharomyces cerevisiae co-culture fermentation. Int J Renew Energy Dev 11:423–433
Otoikhian SK, Amune OU (2022) Comparative assessment of the co-production of bioethanol and single cell proteins from sugarcane bagasse, rice husks, and cassava peels using Saccharomyces cerevisiae. GSC Adv Eng Technol 3:001–009
Miller GL (1972) Analytical chemistry. McGraw Hill Book, New York
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Sharma S, Nandal P, Arora A (2019) Ethanol production from NaOH pretreated rice straw: a cost effective option to manage rice crop residue. Waste Biomass Valorization 10:3427–3434
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48
Goshadrou A (2019) Bioethanol production from Cogongrass by sequential recycling of black liquor and wastewater in a mild-alkali pretreatment. Fuel 258:116141
Silva TAL, Zamora HDZ, Varão LHR, Prado NS, Baffi MA, Pasquini D (2018) Effect of steam explosion pretreatment catalysed by organic acid and alkali on chemical and structural properties and enzymatic hydrolysis of sugarcane bagasse. Waste Biomass Valorization 9:2191–2201
Saratale GD, Saratale RG, Kim SH, Kumar G (2018) Screening and optimization of pretreatments in the preparation of sugarcane bagasse feedstock for biohydrogen production and process optimization. Int J Hydrogen Energy 43:11470–11483
Kumar N, Yadav A, Singh G, Singh A, Kumar P, Aggarwal NK (2023) Comparative study of ethanol production from sodium hydroxide pretreated rice straw residue using Saccharomyces cerevisiae and Zymomonas mobilis. Arch Microbiol 205:146
El-Sakhawy M, Kamel S, Salama A, Tohamy H-AS (2018) Preparation and infrared study of cellulose based amphiphilic materials. Cellul Chem Technol 52:193–200
Cao Y, Tan H (2002) Effects of cellulase on the modification of cellulose. Carbohydr Res 337:1291–1296
Binod P, Satyanagalakshmi K, Sindhu R, Janu KU, Sukumaran RK, Pandey A (2012) Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew Energy 37:109–116
Md Salim R, Asik J, Sarjadi MS (2021) Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci Technol 55:295–313. https://doi.org/10.1007/s00226-020-01258-2
Reddy KO, Ashok B, Reddy KRN, Feng YE, Zhang J, Rajulu AV (2014) Extraction and characterization of novel lignocellulosic fibers from Thespesia lampas plant. Int J Polym Anal Charact 19:48–61
Nath Barman D, Haque MA, Kang TH, Kim GH, Kim TY, Kim MK et al (2014) Effect of mild alkali pretreatment on structural changes of reed (Phragmites communis Trinius) straw. Environ Technol 35:232–241
Kumari D, Singh R (2022) Rice straw structure changes following green pretreatment with petha wastewater for economically viable bioethanol production. Sci Rep 12:10443
Chandel AK, Antunes FA, Anjos V, Bell MJ, Rodrigues LN, Polikarpov I et al (2014) Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid–base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels 7:63. https://doi.org/10.1186/1754-6834-7-63
Gabhane J, Kumar S, Sarma AK (2020) Effect of glycerol thermal and hydrothermal pretreatments on lignin degradation and enzymatic hydrolysis in paddy straw. Renew Energy 154:1304–1313
Cai J, Wang Y, Liu J, Zhang X, Li F (2022) Pretreatment enhanced structural disruption, enzymatic hydrolysis, fermentative hydrogen production from rice straw. Int J Hydrog Energy 47:11778–11786
Ahmad A, Naqvi SA, Jaskani MJ, Waseem M, Ali E, Khan IA et al (2021) Efficient utilization of date palm waste for the bioethanol production through Saccharomyces cerevisiae strain. Food Sci Nutr 9:2066–2074
Onoghwarite OE, Obiora NVI, Ben EA, Moses N-OE (2016) Bioethanol production from corn stover using Saccharomyces cerevisiae. Int J Sci Eng Res 7:290–293
Muthusamy S, Selvan ST, Arunachalam P, Grasian I (2019) Bioconversion and bioethanol production from agro-residues through fermentation process using mangrove-associated actinobacterium Streptomyces olivaceus (MSU3). Biofuels 10:167–179
Wine P, Jerry O, Aleke K, Chika E, Anthonia N, Ikechukwu M (2016) Bioethanol production from corncob hydrolysed by cellulase of Aspergillus niger using zymomonas mobilis and Saccharomyces cerevisiae isolated from International Journal of Current Research in Biosciences and Plant Biology bioethanol production from Cornco. Int J Curr Res Biosci Plant Biol 3:39–45
Wang Y, Liu S (2014) Kinetic modeling of ethanol batch fermentation by Escherichia coli FBWHR using hot-water sugar maple wood extract hydrolyzate as substrate. Energies 7:8411–8426
Ezebuiro V, Ogugbue CJ, Oruwari B, Ire FS (2015) Bioethanol production by an ethanol-tolerant Bacillus cereus strain GBPS9 using sugarcane bagasse and cassava peels as feedstocks. J Biotechnol Biomater 5:1
Nikhil B, Adhyaru D, Thakor P (2012) Production of xylanase by Aspergillus flavus FPDN1 on pearl millet bran: optimization of culture conditions and application in bioethanol production. Int J Res Chem Environ 2:204–210
Mushimiyimana, I. and Tallapragada, P. (2017) Bioethanol production from agro wastes by acid hydrolysis and fermentation process. J Sci Ind Res
Wu X, Zhang J, Xu E, Liu Y, Cheng Y, Addy M et al (2016) Microbial hydrolysis and fermentation of rice straw for ethanol production. Fuel 180:679–686
Kim I, Lee B, Park J-Y, Choi S-A, Han J-I (2014) Effect of nitric acid on pretreatment and fermentation for enhancing ethanol production of rice straw. Carbohydr Polym 99:563–567
Arora A, Priya S, Sharma P, Sharma S, Nain L (2016) Evaluating biological pretreatment as a feasible methodology for ethanol production from paddy straw. Biocatal Agric Biotechnol 8:66–72
Canilha L, Carvalho W, De Almeida Felipe MDG, De Almeida E, Silva JB, Giulietti M (2010) Ethanol production from sugarcane bagasse hydrolysate using Pichia stipitis. Appl Biochem Biotechnol 161:84–92. https://doi.org/10.1007/s12010-009-8792-8
da Silveira dos Santos D, Camelo AC, Rodrigues KC, Carlos LC, Pereira N (2010) Ethanol production from sugarcane bagasse by Zymomonas mobilis using simultaneous saccharification and fermentation (SSF) process. Appl Biochem Biotechnol 161:93–105. https://doi.org/10.1007/s12010-009-8810-x
de Carvalho DM, de Queiroz JH, Colodette JL (2016) Assessment of alkaline pretreatment for the production of bioethanol from eucalyptus, sugarcane bagasse and sugarcane straw. Ind Crops Prod 94:932–941
Acknowledgements
The authors thank the Biofuel Research Laboratory of the Department of Microbiology, Central University of Tamil Nadu, India.
Funding
This project is supported by the Central University of Tamil Nadu, Thiruvarur. This work is financially supported by the SERB DST Project (EEQ/2023/000530).
Author information
Authors and Affiliations
Contributions
TJ: writing the original draft and carrying out the work; AVK: alkali pretreatment and ethanol estimation; AKB: reviewing and suggestions for improvement; SR: conceptualization, reviewing, and final editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tharunkumar, J., Arosha, V.K., Bajhaiya, A.K. et al. Optimizing alkaline pretreatment for delignification of paddy straw and sugarcane bagasse to enhance bioethanol production. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05458-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05458-9