Abstract
The need to reduce the cost of production of highly demanding nanoparticles is receiving some challenges, especially the availability of the needed raw materials. Calcium oxide nanoparticles are one of the most useful nanoparticles because of their unique properties that favour environmental applications. In this study, costless raw materials (periwinkle shells) of raw materials for the production of calcium oxide nanoparticles using a sol–gel method of synthesis. The nanoparticles were characterized using XRD, FT-IR, UV visible spectrophotometer, SEM, BET, and ICP instrumentation. Information obtained from the characterization showed that the material has a band gap of 4.12 eV, average crystallite size of 18 nm, BET surface area 220.11 m2/g, pore volume of 8.43 cc/g and mesoporous property defined by average porosity of 59%, and pore diameter of 3.02 nm. The XRD peaks. The XRD spectrum of the synthesized product showed a principal diffraction peak at 34.2163° with other peaks that confirmed CaO nanoparticle phases. The specific surface area deduced from the XRD spectrum (average = 98 m2/g) was lower than that value obtained from the nitrogen adsorption study. Based on the characters observed for the nanoparticles, they were applied for adsorption removal of lead under different conditions (including pH, [Pn2], time, and adsorbent dosage), and the evaluated adsorption efficiency was 99 g/mg while the optimum efficiency of 98% was observed at the normal experimental condition and 100% under photoactivated adsorption. The nanoparticles showed high recyclability (< 90%) and their tendency to adsorb increases with a decrease in temperature. Tests conducted on different kinetic and adsorption models support the fitness of pseudo-second-order kinetic, liquid film diffusion, Freundlich, Halsey, and Elovich adsorption models. The impact of the interaction of the tested factors on adsorption was investigated using response surface analysis, and it was observed that the optimum adsorption efficiency can be reduced when all the factors are combined. Therefore, an optimum efficiency of 80% at an adsorbent dosage of 0.5, time = 75 min, temperature = 318 K, and concentration of 250% was statistically significant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In current times, the literature is highly dominated by reports on the toxicity of heavy metal ions in water due to the established potential toxic impact of these metal ions on public health [1]. Heavy metals such as lead are persistent and cannot easily be destroyed by biological organisms [2]. Unfortunately, one of the easiest routes for heavy metals to enter the environment is the aqueous media [3]. The after-effect of heavy metal ions’ intrusion into the aquatic environment is interference with the global demand for potable water and the increasing risk of health challenges. Consequently, the associated problems are expanding daily, especially when considering the usefulness of water for the survival of living organisms, irrigation, agricultural activities, and various domestic and industrial applications [4]. The beneficial roles of water can deeply be limited by the presence of heavy metal contaminants and can progress further to initiate toxic impacts ranging from mild effects to death. Much has been reported on the short, medium, and long-term toxicity impacts of lead ions, but the terminal effect is dead [5,6,7].
Given their confirmed poisonous nature, some technologies have been developed to enhance the withdrawal of lead ions from water, such as adsorption, dialysis, and bioremediation [8, 9]. However, some of the major challenges associated with the achievement of optimum efficiency from most of these technologies are high materials and implementation costs, scarcity of raw materials, materials limitation, lack of or insufficient power supply, secondary waste management, etc. [10, 11].
Adsorption is one of the widely accepted methods that can easily be modelled towards the achievement of excellent efficiency through materials selection, eco-friendly practices, and limited input of resources [12]. There are different materials suitable as adsorbents for the removal of lead (II) from an aqueous solution, such as plant and animal materials [13], inorganic materials [14], organic and inorganic polymers [15], and synthetic materials [16]. However, nanoparticles have remarkable advantages over several classical counterparts because they possess high thermally and mechanical stability, porosity [17], reusability [18], and high adsorption surface area due to their nano sizes [19]. Also, efforts are currently on the way to harness the outstanding advantages offered by nanoparticles towards the enhancement of adsorption for the improvement of the overall quality of wastewater instead of the removal of heavy metal alone [20]. Probably some precursors for the synthesis of nanoparticles are relatively toxic and may constitute secondary toxicity [21]. The cost of synthesis may be extremely high when subjected to cost–benefit analysis, and some of the raw materials or the required instrumentation are not easily accessible, thereby defiling the required environmental and economic protocols. Cost–benefit analysis can easily be achieved through the utilization of waste materials that do not have immediate benefit or usefulness to man and need to be disposed of. Some solid waste such as shells of crustaceans and bones of animals have chemical contents that can be converted to precursors for nanomaterials synthesis. For example, Essien et al. [22] successfully synthesized calcium oxide nanoparticles (CaO–NP) from snail shells for the adsorption removal of amoxicillin water and the results obtained indicated almost complete removal efficiency. In our research group, we have obtained good surface properties for calcium oxide nanoparticles synthesized from oyster shells, and further applications in the removal of contaminants indicated excellent removal efficiencies for methyl orange [23], procaine penicillin [24], bromocresol green [25], and methylene blue [26]. Crab [27] and mussel [28] shells have also been found to be excellent precursors for the synthesis of CaO–NP, and their adsorption properties were properly affirmed based on the evaluated properties. A critical examination of the highlighted and other crustacean shells indicates that they are rich in CaCO3, which can be easily converted to CaO–NP.
Given the yet-to-be-developed procedures in the management of periwinkle shell wastes, the high CaCO3 content and the increasing demand for nontoxic raw materials (such as calcium oxide nanoparticles) for the treatment of industrial and other forms of effluence against lead toxicity, the present study is aimed at converting CaCO3 (in periwinkle shell) to CaO nanoparticles (CaO–NPs) for immediate application in the adsorption removal of Pb(II) from aqueous solution. To fully achieve this aim, the study shall also assess the possibility of using and re-using the CaO–NPs and the influence of the adsorption process on the physicochemical quality of the water.
2 Materials and msethods
2.1 Synthesis of CaO–NPs
Periwinkle shells were used as precursors for the synthesis of CaO–NPs. In the periwinkle processing zone (where the samples were collected), the routine practice is to remove the flesh and dispose of the shell as solid waste. The collected samples were washed, dried, and crushed to powder form using an electric motor connected to a crusher. The grounded samples (with reduced particle size) were reacted with 2 M HCl to convert the CaCO3 to CaCl2. The formed sol was reacted with a 50% solution of NaOH to yield Ca(OH)2 gel. The system was allowed to stay for 12 h after which it was thoroughly washed with distilled water, dried to constant weight, and calcined at 800 °C for 2 h.
2.2 Characterization
The prepared CaO–NPs were characterized using an X-ray diffractometer, Fourier transformed infrared (FT-IR), Braeuner-Emmett-Teller analyzer (BET), UV–visible spectrophotometer, and scanning electron microscope. All measurements for the concentration of Pb(II) were done using inductive coupling plasma (ICP).
2.3 Adsorption experiment
Batch adsorption experiments were implemented using the method widely reported in the literature [29]. The experimental steps started with the preparation of various concentrations of lead (II) (10, 20, 30, and 40 ppm) using Pb(NO2)2. The influence of concentrations was investigated using the listed concentrations and the time involved was 60 min. The contribution of the period of contact, pH, the dosage of CaO–NPs, and temperatures to the removal of Pb(II) were also investigated by varying these factors over reasonable ranges. In each case, the equilibrium amount of the metal on removed by the adsorbent (\({Q}_{e})\) and the percentage removal was computed using Eq. 1 and 2 [30].
The initial or intake and outlet concentrations are represented as \({C}_{0}\) and \({C}_{e}\) respectively in the above equations.
2.4 Process optimization and interaction
Design expert 13 was used to design a 2-by-4, fifteen-sample runs consisting of various combinations of temperature, concentration, adsorbent dosage, and time. Analyses were performed to establish interaction parameters for various combinations of the listed parameters and to establish the optimum conditions associated with optimum adsorption efficiency.
3 Results and discussion
3.1 Characterization of adsorbent
The particle size, surface area, adsorption energy, porosity, and other pore parameters of the nanocomposite (CaO–NPs) were evaluated using Bruaner-Emmett-Teller (BET) and various isotherms developed from data associated with the nitrogen adsorption (such as multiple BET) were employed to evaluate the pore parameters for the adsorbent.
The multi BET (M-BET) model can be represented as a linear shown in Eq. 3 [31],
In the above Eq. (3), N represents the amount of N2 adsorbed at a pressure, P, giving an initial pressure, P0, \({N}_{m}\) stands for the adsorption capacity of the monolayer, and C is a constant, whose value is related to the heat capacity, \(C=\left[{q}_{ads}-{q}_{cond}\right]/RT.\) Also, qads is the adsorption heat while qcond is the heat of condensation of the adsorbate. The M-BET surface area was evaluated from the slope and intercept of the M-BET plot (Fig. 1) [32], and the values obtained are recorded in Table 1. Other pore parameters (also recorded in Table 1) were also obtained from other isotherms, including Dubinin-Ashtakov (D-A), Barreett-Joyner-Halenda (B-JH), Dubinin-Radushkevich (D-R), Density functional theory (DFT) and Horvath-Kawazoe (HK) methods. The measured surface area showed the lowest from the DFT model (54.32 m2/g) while the D-H model revealed the highest (265.14 m2/g). Also, the pore volume parameters obtained from the various models (excluding M-BET) showed a range of 3.58 to 13.04 cc/g) while the ratio of the surface area (in m2/g) to the pore volume (in m3/g) ranged from 8.46 × 106 to 1.90 × 108 m−1. On the other hand, the measured particle size from the different models showed a range of 1.923 (HK to 5.923 nm (S-F). Based on the range observed for the pore parameters of the nanoparticles from the different models, it is most likely that the synthesized material is a mesoporous nanoparticle. Porosity classification for mesoporous materials is in the range of 2 to 50 nm for mesoporous, 0.2 nm for microporous, and > 50 to 100 nm for microporous. The significance of porosity in the determination of the effectiveness of nanomaterials in surface applications agrees with the trend that the lower the pore diameter and the higher the surface area, the better the expected performance. Based on the R2 values obtained for the different models, the M-BET and B-JH model. The evaluated M-BET surface area is lower than the value of 785 and 747 m3/g reported by Odiongenyi et al. [33] and Mostapha et al. [34] for CaO–NP obtained from bonnet shells and mud waste; they also reported pore diameter in the mesoporous dimension. However, the measured particle size showed a significant improvement over average values of 6.17 and 77.4 m2/g, as reported by Khine et al. [35] and Jalu et al. [36], both for mesoporous CaO–NP. Another significant performance index for nanoparticles is the surface area to volume ratio which are related higher than those evaluated for the listed examples (Table 1).
The variation in the observed pore parameters of the synthesized CaO–NPs (compared to some reported works) can be related to alteration in some factors, such as the method of synthesis, source of raw materials, calcination temperature, and duration of calcination [37, 38]. The calculated dimension of the nanoparticles indicates that the CaO–NPs is under the mesoporous class of nanoparticles [39]. A similar dimension has been reported for CaO–NPs, which we synthesized from different oyster shells [40].
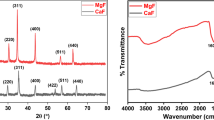
The scanning electron micrograph and the Fourier-transformed infrared (FT-IR) of the CaO–NPs are shown in Fig. 2. The micrograph reveals the dispersing of porous appearance in the internal structure of the CaO–NPs. On the other hand, the infrared absorption bands manifested at the following frequencies: 3642, 1407, and 872 cm−1. The narrow band at 3642 cm−1 is due to OH stretching from the traces of H2O [41], while the peak at 1407 cm−1 is typical for Ca–O vibration in the CaO–NPs [42].
The diffractogram obtained from the XRD analyses of the CaO–NPs is shown in Fig. 3a. The most intense peak was observed at [2Theta] = 34.2163° with an intensity of 1706.24 cps while others were found at 18.1561, 29.4876, 47.1828, and 50.917°. The corresponding intensities were 1011.77, 1466.09, 694.78, and 50.92° respectively, and the full width at half maxima (\(\beta\)) are shown in Table 2. Such reference peaks (especially at 34.2163°) have been observed for CaO–NPs by other researchers, thereby confirming that the synthesized materials are CO–NP [43,44,45]. The application of Scherrer’s equation (Eq. 4) was useful in the determination of the size (dx) of the CaO–NPs crystallite [46, 47].
In the above equation, k and λ represent Scherrer’s constant and the X-ray wavelength, respectively. The evaluated particle sizes for the various peaks are also shown in Table 2. Consequently, the average size of the crystallite is evaluated as 18.57 nm, which is comparable to results from works done on similar products using different precursors [48, 49].
The dislocation density for the nanoparticles was observed to range from 0.0524 to 0.0563 with an average value of 0.0539 nm−1 (Table 1). The evaluated δ values showed a decreasing trend with an increase in the diffraction angle (from 0.0563 to 0.0514 nm−1). However, \({d}_{x}\) values tend to increase with an increase in the angle of diffraction from 17.77 to 19.44 nm (Table 1). Generally, the observed δ values are relatively low, which suggests that the CaO–NP is relatively stable against deformation. The reported mean crystallite size also confirms that the synthesized product has a nano dimension. The crystallite size can also be evaluated using the Wiliamson-Hall equation. This equation is based on the assumption that the total broadening is due to microstrain (i.e. \({\beta }_{Micro} = 4\epsilon tan\theta )\) and crystallite size (defined as \({\beta }_{Cryst} = \frac{k\lambda }{{d}_{x}cos\theta }\)). Therefore, the total broadening can be represented as,
The rearrangement and simplification of Eq. 5 generated Eq. 6 as follows:
The linearity of Eq. 6 for the tested CaO–NP was confirmed by an R2 value equal to 0.9776 while the crystalline, deduced from the intercept (i.e., 29.8 nm), showed some degree of agreement with the average value (18.574 nm) obtained from the Scherer equation. However, the slightly higher values obtained from the Williamson-Hall model are in agreement with most experimental findings that the Williamson-Hall model tends to overestimate crystallite size by over 35% [50].
In the crystal system, the interplanar distance is related to the Miller indices and the lattice constant of the CaO–NP according to Eq. 7.
Since CaO–NP is a cubic crystal, a = b = c. Therefore, Eq. 7 can be simplified to the following equations (Eqs. 8 and 9).
However, the Bragg equation relates the \({d}_{hkl}\) with the diffraction angle according to Eq. 10, which is simplified to Eq. 11
For a first-order diffraction order, n = 1, and Eqs. 9 and 11 were equated to obtain the lattice parameter for the synthesized CaO–NP as follows:
The miller indices were obtained from the JCPD database corresponding to the different angles of diffraction and \(\lambda =1.5406\) Å. Based on the above equations, the calculated values of the lattice parameters (average = 4.2741 Å) and \({d}_{hkl}\) (average = 2.8485 Å) are recorded in Table 2. The specific surface area of the CaO–NP was also calculated using the Sauter equation given as Eq. 13:
The symbol \(\rho\) in Eq. 13 defines the density of the CaO–NP, and its crystallite size is denoted by \({d}_{x}.\) Based on the reported crystallite sizes, the evaluated SSA ranged from 89.72 to 98.15 m3/g, which is relatively higher than some literature values for CaO–NP, such as 77.4 m2/g reported by Jalu et al. [35] for CaO–NP obtained from egg shells. This suggests that with an enhanced specific surface area, the tendency towards photodegradation and adsorption is expected to be higher [51]. The evaluated surface area is lower than the value obtained from the BET method isotherm, which can be accounted for by the difference in the method of approach, explained by the consideration of pore diameter and crystallite size by the former and later methods, respectively.
However, the porosity of the nanoparticles based on the XRD density and the bulk density of the CaO–NP by applying Eq. 14.
The evaluated porosity is 59.74% and compares with the value of 47.84% deduced from the work of Jalu et al. [35] and therefore confirms that the synthesized CaO–NP is a mesoporous nanomaterial.
The spectrum arising from the absorption of UV by the CaO–NPs is shown in Fig. 3b. The spectrum reveals that the maximum wavelength (\({\lambda }_{{\text{max}}(CaO-NPS)}\)) of UV absorption by the nanoparticles is 239 nm, and therefore, we infer that the nanoparticles absorb in the UV range and have a characteristic band gap of 4.12 eV. This band gap (\({E}_{BG (CaO-NPS)}\)) was evaluated from the Planck equation (Eq. 5) [52],
Other reported works have confirmed closely related \({\lambda }_{{\text{max}}(CaO-NPS)}\) and \({E}_{BG (CaO-NPS)}\) for CaO–NPs [52].
3.2 Variables influencing the adsorption of Pb 2+ by CaO–NPs
The pH for optimum absorption of the Pb2+ was evaluated at around 6.4, as shown in Fig. 3c. The rise in adsorption became sharper above the pH of 3 and displayed an almost steady trend at pH close to 6. The adsorption removal of the heavy metal ion progresses with increasing pH up to this optimum value. We could not continue the experiment beyond the pH of 6.4 to eliminate errors that could be introduced by precipitation. Such observations have been reported elsewhere with close pH values for the adsorption removal of Pb2+ from aqueous solution [53,54,55]. The observed trend reveals that the adsorption of the positively charged Pb2+ is favoured by alkaline pH, which is dominantly negative. At alkaline pH, the attraction of the metal ion is most likely because of the prevailing negatively charged surface. Still, at acidic pH, the surface can repel the lead ion such that as the population of positive charges increases, the uptake of Pb2+ also increases. The observed pH for maximum adsorption is also consistent with reported values of the pH at the point of zero charge for CaO–NP but with irregularity after that optimum pH due to the changes in the mechanism from adsorption to precipitation [55, 56].
Figure 4 contains plots demonstrating the changes in the percentage lead (II) adsorbed under different experimental controlled conditions. Figure 4a shows that the magnitude of adsorption increases with an increase in the concentration of the heavy metal. The initial rise in the percentage of dye adsorbed with [Pb2+] was very sharp between [Pb2+] of 100 and 200 ppm corresponding to adsorption efficiency of 61 and 94%, respectively. However, above the [Pb2+], the efficiency progressively rises to about 98%. Lead ion adsorption can be significantly dependent on the number of available surface area. The large surface area and surface area to volume ration that characterized nanoparticles such as CaO–NP suggest that the adsorption will progress until all the available site is occupied.
A consideration of interplay between the mass of the adsorbent and the concentration of lead ion on adsorption indicated that although adsorption was higher at higher concentrations, similarities in the pattern became obvious irrespective of the mass of the adsorbent dosages (Fig. 4a), indicating that the mechanism of adsorption is independent on the concentration of the lead ions or the mass of the adsorbent. The observed increase in percentage adsorption with increasing initial Pb2+ concentration also confirmed that the number of lead ions that are transported to the surface of the CaO–NPs increases as the concentration increases.
Adsorbent dosage can display a significant influence on the adsorption of lead ions through its influence on the number of available adsorption sites and available surface area. Based on the pattern of plots displayed in Fig. 4b, maximum adsorption approaching 100% was observed at a lead concentration of 400 ppm and adsorbent dosage of 0.8 g. This optimum value is almost equal to the optimum observed when lead ion was a major factor under consideration. A short fall of only 2 from 100% efficiency (at a lower concentration of Pb2+) is excellent and therefore positioned the used CaO–NP as an excellent adsorbent for the removal of Pb2+. A combination of the influence of the two factors showed an increase in the percentage removal of Pb2+, tending to an optimum value of almost 100% within 70 min before a slight drop and steady trend. The observed trend for the variation of the amount of lead ions adsorbed with adsorbent dosage is consistent with some documented results concerning the adsorption of Pb2+ by watermelon waste [57], wall nutshell [58], where an increase in adsorption was also observed as the adsorbent dosage increase.
The use of CaO–NPs for separating Pb2+ from the aqueous solution also displayed better feasibility with time, as shown in Fig. 4c for various concentrations of Pb2+. The effectiveness of time factors also became more favourable for higher concentrations of heavy metal ions. The drifting of the Pb2+ towards the CaO–NPs is a function of the time required for the Pb2+ to approach the adsorption sites and become equilibrated before the actual adsorption. Under the present study, The progress of adsorption, therefore, becomes more effective as the extent of interaction between the adsorbate and the adsorbent becomes enhanced with time and concentration. Higher concentrations of Pb2+ are also observed to show slight enhancement in adsorption, although the observed pattern of the plots for all concentrations follows a similar trend (Fig. 4c). A linear pattern between adsorption and period of contact was observed, and this corresponded to efficiency ranging from 83 to 99% within the first 70 min. After this, a further increase in time did not reflect a significant rise in adsorption. At a concentration of 100 to 400 ppm, the maximum adsorption ranged from about 93 to 99%, respectively. This also agrees with the role of adsorbate concentration in adsorption efficiency. When adsorption starts, it requires time to progress and two options are possible, namely, a decrease in adsorption with time that maybe attributed to an increased rate of desorption or a limited number of active and vacant adsorption sites. On the contrary, the observed increase in adsorption with time shows that the tendency towards adsorption is higher than the tendency towards desorption and that the surface of the adsorbent was not significantly deactivated as the adsorption progressed. Published works showing similarity in trend for the variation of Pb2+adsorption with time have been documented for some adsorbents [59, 60].
Any factor that can slow down the rate of migration of the lead ion from the bulk solution to the surface of the adsorbent can affect the extent of adsorption. Such factors may result in physical changes. However, the effect of temperature can become more severe on the adsorption of ions to the surface of the nanoparticles. We observed from Fig. 4d that between a temperature of 303 and 333 K, adsorption efficiency achieved a maximum decrease to 92, 91.6, 91.2, and 9.05% for [Pn2+] corresponding to 100, 200, 300, and 400 ppm, respectively. The general trend shows a decrease in adsorption with an increase in temperature and confirms that the mechanism of adsorption is physical adsorption. This trend can be due to the deactivation of the adsorption surface, which may lead to an increase in desorption. We confirmed by some reported works that our observation has some support from some published works [61].
3.3 Kinetic of the adsorption
Although several kinetic models were tested for their fitness through linear plots and evaluated error values, the models that generated good fits for the adsorption of Pb2+ were pseudo 1st order (PDFO) [62], pseudo 2nr order (PDSO) [63], Elovich (EL) [64], Weber Morris (W-M) [65], and Liquid film (L-F) [66] kinetics. Therefore, these models are hereby assessed in this sub-section. The linear forms of the listed models are represented by Eq. 6, 7, 8, 9 and 10), while the respective plots are shown in Fig. 5a–d respectively [67,68,69,70].
Also, from the slopes and intercepts of the plots, data calculated for the various kinetic parameters are provided in Table 3. The mean square errors are also provided for the respective model [71] The PDSO presents higher values of R2 and lowers the error values (Fig. 5a and Table 3) than the PDFO model (Table 3). Therefore, the assumptions behind the PDSO can explain the adsorption of Pb2+ better than that of the PDFO. The PSFO model assumes that the diffusion through the interface is a significant mechanism in the adsorption of the metal ion. Theoretical equilibrium adsorption capacity evaluated from the PSFO plots ranges from 244.29 to 613.99 mg/g and tends to increase with an increase in the concentration of Pb2+. From 100 to 400 ppm. On the other hand, Qe values obtained from the PDSO plots ranged from 104.09 to 104.49 mg/g and showed significant closeness with experimental values. The initial adsorption rate obtained from the Qe values \(\left(i.e h= {k}_{2}{Q}_{e}^{2}\right) \left[76\right]\), ranged from 18.66 to 28.62 and tended to increase with an increase in the concentration of the lead ion. The PDFO rate constants for the different concentrations of the Pb2+ ranged from 0.0015 to 0.0084 /min, while those of PDSO showed a range of 0.001905 to 0.002622 /min. Both kinetic rate data showed an increasing trend as the concentration of the metal ion increased. This indicates that the adsorption is faster as the adsorbent dosages increase. Reported studies showing similarity in the fitness of PDFO kinetics concerning Pb2+ adsorption have been documented in the literature [72,73,74]. However, overwhelming literature supports the PDSO model. Based on the above analysis and the information presented in Table 3, the PDSO model offers a better description of the kinetics of the adsorption removal of the Pb2+ than the PDFO model because the PDSO presents higher values of R2 and lower values of the sum of squares as well as the mean square errors (Table 3). The applicability of the PDSO model suggests that the chemisorption mechanism tended to occur after the initial physical adsorption.
The EL plot is shown in Fig. 5b, and from a theoretical background, the EL is typical for the chemisorption mechanism [44]. However, sensing from the trends observed for the variation of the amount of Pb2+ adsorbed with temperature, the initial adsorption mechanism for Pb2+ is physical adsorption; therefore, chemisorption might have been a succeeding mechanism to the initial adsorption. However, the calculated sum of square (SSE) and mean square (MSE) errors are much larger than those observed for the PDSO. Relatively, lower R2 values were also observed; suggesting that the Elovich model ix not the most suitable model for the explanation of the kinetic of the adsorption of Pb2+ by the synthesized CaO–NPs.
Diffusion of the metal ions from the bulk solution to the surface of the nanoparticles is a significant factor in the adsorption kinetics. Consequently, the major diffusion models were tested, which are the L-F (Eq. 9) and M-W (Eq. 10) models. Based on the MSE, SSE, and is2 values, lower errors, zero slopes, and better R2 are predicted by the L-F model than the W-M model, which suggests that thin film diffusion dominates the adsorption kinetics. Similar diffusion models have been observed for the adsorption of lead (II) by some adsorbents such as biosorbents [75], metal–organic framework [76], and biowaste [77]. The rate constants evaluated from the L-F models are in good agreement with those associated with the PDSO. The model shows some dependency on the concentration of Pb2+, as shown by the observed variation in the diffusion constant and the liquid film boundary parameters (Table 3). This conclusion does not however rule out the influence of the intraparticle diffusion model completely, especially when considering the influence of concentration on the diffusion of the lead ion. The observation of the W-M boundary parameter showed an increased improvement with an increase in concentration but higher rate values compared to those reported for the L-F model (Table 3).
From the presented data (Table 3), the kinetic of the adsorption removal of Pb2+ from aqueous solution is best described by the PDSO and L-F models because the two models present the least error values and higher R2 values compared to other models.
3.4 Adsorption isotherms for Pb 2+
Different adsorption models were tested for their applicability in the explanation of the adsorption characters of Pb2+ unto the surface of CaO–NPs. Tested models were evaluated based on the R2, SSE, and MSE values, and those with R2 > 0.9 were selected for further analysis. The models that met the listed conditions were the Freundlich (F-R), Temkin (T-N), Langmuir (L-N), Elovich (E-L), Dubinin-Raduskevich (D-R), and Halsey (H–S) isotherms. Expressions for the listed isotherms are shown in Eqs. 11 to 1, respectively. Figure 6 shows representative plots for the fitted models and parameters deduced from all the plots are recorded in Table 4 [78, 79].
The Freundlich model (Eq. 11) explains the heterogenous adsorption project with the Freundlich constants (kF-R and 1/\({n}_{F-R}\)) representing the adsorption capacity and intensity of the adsorption, respectively [80]. Judging from the value of \({n}_{F-R}\) adsorption can be predicted to be linear, cooperative, favourable, or unfavourable [81]. Also, the Langmuir isotherm (Eq. 13) has two major constants, namely, the Langmuir maximum adsorption capacity (Qmax) and the adsorption–desorption equilibrium constant (kL-N). Qmax values are informative in the provision of the reference point for the prediction of favourable adsorption based on the magnitude of the separation factor (calculated values not shown) and the intensity factor [82]
Since the F-R model gave better R2 values and lower errors than the Langmuir model. The tendency for the adsorption of Pb2+ largely included the multilayer process, which has also been observed for the adsorption of Pb2+ by other adsorbents [81]. The evaluated values of \({n}_{F-R}\) are less than unity and show no significant difference for the various masses of the adsorbent. Literature concerning the \({n}_{F-R}\) is in support of normal adsorption for \(\frac{1}{{n}_{F-R}}<1\) while cooperative adsorption is feasible for \(\frac{1}{{n}_{F-R}}>1\). Also, the values of \({n}_{F-R}\) within the range of 1 and 10 are consistent with favourable adsorption [83]. Based on the documented values of \({n}_{F-R}\) calculated for the adsorption of Pb2+ by the nanoparticles (Table 4), it can be concluded that cooperative and favourable adsorption defined the adsorption characteristics of the adsorbate on the CaO–NPs.
The error values for the T-N model (Eq. 12) were relatively high compared to other models (Table 4), and the R2 values were comparable. Generally, the T-N isotherm is founded on the fundamental that the adsorption heat decreases as the surface coverage of the adsorbent increases [84]. Given the present results (Table 4), the values of the heat of adsorption for the various masses of the adsorbents are significantly low and technically support the involvement of physical adsorption, as observed in this study [85].
Although evaluated error values for the fitness of the D-R model were relatively high, the model displayed good R2 values and from the slope and intercept values of the plots, the D-R adsorption variables for the different masses of the CaO–NPs were obtained and recorded in Table 3. The essential significance of the model is that the adsorption energy was evaluated as the inverse of \(\sqrt{{2k}_{D-R}}\). Consequently, the calculated adsorption energies, which ranged from 24.1 to 34.4 J/mol, are below the 16 kJ/mol threshold acceptable for the mechanism of chemical adsorption. Therefore, the operation of physic adsorption is confirmed. Such a report has been confirmed for the adsorption of Pb2+ onto rice bran surfaces [86].
The Elovich model also displayed excellent adsorption parameters based on the evaluated coefficient of determination and error functions, as shown in Table 4. The model is aligned with multilayer adsorption and is founded on the basis of exponential increment of adsorption site with adsorption [87]. The model also displayed a better prediction of maximum adsorption capacity compared to the prediction from the L-N model. The adsorption removal of Pb2+ has been reported elsewhere to be consistent with the Elovich isotherm, as observed in this work [88, 89].
The Halsey isotherm also shows some degree of fitness based on the calculated R2 and error values. The essential significance of this isotherm is that it is also applicable to multilayer adsorption processes involving porous material [90]. The fitness of the Halsey isotherm for the adsorption of Pb2+ on silica-based adsorbent was confirmed by Chu et al. [91]. and the reported Halsey adsorption parameters are relatively comparable to those obtained in this work. However, the adsorption parameters we have obtained from the slope and intercept of the plots do not show significant differences at all levels of dosages of the nanoparticles (Table 4), suggesting that the isotherm may not be dosage-dependent concerning the nanoparticles. The error values were relatively minimal (i.e., within two decimal places), and the R2 values for the plots extended from a minimum value of 0.9051 to 0.9599. The intensity parameters obtained from F-R (\({{\varvec{n}}}_{F-R})\) and H–S (\({{\varvec{n}}}_{F-R}\)) isotherms are in good agreement and predict that the multilayer adsorption process increases with an increase in adsorbent dosage.
A close examination of the isotherm parameters presented in Table 4 indicates that the best-fitted isotherms are the F-R, H–S, and E-L isotherms because less error and excellent coefficient of determination are associated with these isotherms. Consequently, the formation of a multimolecular layer and mechanism of physical adsorption is proposed for the adsorption of Pb2+ by CaO–NPs.
3.5 Thermodynamics models for the adsorption of Pb 2+
The relationship between the standard free energy change of adsorption (\({\Delta G}_{ads}^{*}\)), enthalpy (\({\Delta S}_{ads}^{*}\)) and entropy (\({\Delta H}_{ads}^{*}\)) changes are given by the Gibb equation (Eq. 17) and are related to the adsorption equilibrium constant according to Eq. 18. The combination of the two equations led to Eq. 19 [92, 93].
The thermodynamic significance of Eq. 19 is that the slope of the plot of lnkads versus 1/T (Fig. 7) is equal to the enthalpy change divided by the gas constant while the intercept is equal to the entropy change divided by the gas constant. Calculated thermodynamic variables are recorded in Table 5. The favouritism of the model expressed by Eq. 19 is strongly supported by the excellent values of R2 and the low values of SSE and MSE (Table 5). The listed enthalpy and entropy changes show a proportional dependency on the initial Pb2+ concentration. The adsorption is exothermic and displayed positive entropy changes, which may suggest less interaction between the adsorbate and the CaO–NPs and further confirm the operation of a physisorption mechanism.
3.6 Photoactivation of adsorption
The exposure of the batch adsorption experimental setup to a UV light source significantly enhances the adsorption capacity of the CaO–NPs for Pb2+. This may be attributed to the activation of the adsorbent by UV radiation since the calculated band edge for the CaO–NPs (4.12 eV) suggests that it can absorb in this region. It can be confirmed from Fig. 8 that there is a significant enhancement in efficiency with an optimum value approaching almost 100% compared to about 98% observed in the absence of UV light. Therefore, the adsorption of Pb (II) by CaO–NPs is strongly favoured under UV light.
The reusability of the CaO–NPs was also investigated using different dosages of the used adsorbent for the re-trial experiment. The used adsorbent was washed severally with distilled water, dried, and reused. The results (Fig. 9b) indicated that at initial [Pb2+] of 10 and 40 ppm, the adsorbent displayed efficiency above 98 and 99% respectively. The recovered adsorbent did not show any change in the major FT-IR peak at 1400 cm−1, although there was a slight drop (by 0.7 units) in the intensity. This confirms that the adsorbent did not witness a significant denature after the first reuse. The concentrations of Pb2+ removed from the surface of the adsorbent were also re-determined, and the results reflected an almost 100% recovery when added to the equilibrium concentrations in the solution.
In Table 6, some literature data concerning the application of CaO–NPs as an adsorbent for Pb2+ are presented. From the recorded information, it is evident that most adsorption data favours the PDSO kinetics and that the present adsorbent has some levels of comparable advantages, especially when the photoactivated adsorption process is involved.
3.7 Changes in the physicochemical quality of the water
Measurements of some physicochemical parameters such as pH, conductivity, dissolved oxygen (DO), and alkalinity (alk) for the respective solution containing different concentrations of lead (II) ions were conducted before and after adsorption. The results obtained are shown in Fig. 9a–d. The initial addition of lead ions led to a decrease in pH (tending towards the acidic range) and DO. However, the pH (pH(A)) and DO (DO(A)) after adsorption removal of Pb(II) were favourably adjusted compared to their initial pH (PH(B) and DO (DO(B)). However, the alkalinity (Alk(B)) and conductivity (Cond(B)) showed an increment with the [Pb(II)] but were also readjusted after adsorption. Also adjusted after adsorption were the turbidity and salinity of the aqueous solution (plots not shown).
3.8 Optimization of the adsorption process/interaction
The response surface analysis was used to optimize the adsorption process and to study the interaction between the associated variables. Figure 10a shows the response surface plots for the effect of concentration (A), temperature (B), adsorbent dosage (C), and time (D) on the adsorption removal of Pb2+ from aqueous solution. The results obtained indicated that the highest interaction was contributed by temperature (42.67%), followed by time (26.22%), while adsorbent dosage showed 5.95% and concentration showed the least contribution (0.00197%). The interactions between the following factors concentration/temperature, concentration/dosage, concentration/temperature/dosage, concentration/dosage/time, temperature/dosage/time, and concentration/temperature/dosage/time showed the following percentage contributions, 0.23, 0.82, 6.06, 2.22, 4.09, 4.09, and 0.17% respectively. These results indicated that the highest interaction is between concentration, temperature, and dosage, contributing 6.06%, while the least is between the four factors (0.17%) respectively. Based on the response surface analysis and the consideration of the four factors, optimum percentage removal approaching 70% can be achieved under the following conditions: concentration = 250 ppm, temperature = 318 K, adsorbent dosage = 0.5 g, and contact time = 75 min.
3.9 Recyclability of the adsorbent
The used adsorbents were washed thoroughly with distilled water, dried, and recalcined for further application in the removal of the CaO–NPs. The results obtained after three trial experiments are shown in Fig. 10b. The Figure reveals that after three trials, the adsorbent could still retain more than 88% of its capacity, although a slight drop in efficiency was observed for every successive trial experiment.
4 Conclusion
CaO–NPs have been successfully synthesized, characterized, and applied for the removal of lead (II) ions from an aqueous solution. The CaO–NPs is a mesoporous material with an average crystalline size of 18 nm and shows a significant FT-IR peak due to Ca–O vibration. The adsorbent absorbs maximally in the UV region and displays a zeta potential that favours alkaline conditions for optimum removal of leaf (II) ions from an aqueous solution. Other optimum conditions were a rise in the initial heavy metal ion concentration, dosages of the adsorbent, and a decline in temperature. A physical adsorption mechanism is operational for this system and is controlled by liquid film diffusion from the bulk solution to the CaO–NP surface through the interface. The adsorbent surface shows a stronger degree of heterogeneity rather than the homogenous counterpart. The CaO–NPs has functional stability and re-usability and favourably adjusted the quality of the water concerning conductivity, pH, dissolved oxygen, and alkalinity.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Aziz KHH, Mustafa E, Omer KM, Hama S, Hamarwi RF, Rahman KO (2023) Heavy metal pollution in the aquatic environment: efficient and low-cost removal approaches to eliminate their toxicity: a review. RSC Adv 13(26):17595–17610. https://doi.org/10.1039/D3RA00723E
Suami RB, Sivalingam P, Al Salah DM, Grandjean D, Mulaji CK, Mpiana PT, Breider F, Otamonga JP, Poté J (2020) Heavy metals and persistent organic pollutants contamination in river, estuary, and marine sediments from Atlantic Coast of Democratic Republic of the Congo. Environ Sci Pollut Res Int 27(16):20000–20013. https://doi.org/10.1007/s11356-020-08179-4
Nnaji ND, Onyeaka H, Miri T et al (2023) Bioaccumulation for heavy metal removal: a review. SN Appl Sci 5:125. https://doi.org/10.1007/s42452-023-05351-6
Ungureanu N, Vlăduț V, Voicu G (2023) Water scarcity and wastewater reuse in crop irrigation. Sustainability 2020(12):9055. https://doi.org/10.3390/su12219055
Akhtar S, Khan ZT, Ahmad K, Nadeem M, Ejaz A, Hussain MI, Ashraf MA (2022) Assessment of lead toxicity in diverse irrigation regimes and potential health implications of agriculturally grown crops in pakistan. Agric Water Manag 271(1):107743. https://doi.org/10.1016/j.agwat.2022.107743
Kumar VS, Pradhan JK, Sharma SK, Singh P, Sharma JK (2021) Structural, optical and antibacterial response of cao nanoparticles synthesized via direct precipitation technique. Nano Biomed Eng 13(2):172–178. https://doi.org/10.5101/nbe.v13i2.p172-178
Lehigh University (2022) Study confirms lead-in-water causes adverse fetal health outcomes. ScienceDaily. www.sciencedaily.com/releases/2022/07/220713114629.htm
Narayana PL, Maurya AK, Wang X, Harsha MR, Srikanth O, Alnuaim AA, Hatamleh WA, Hatamleh AA, Cho KK, Paturi UMR, Reddy BS (2021) Artificial neural networks modeling for lead removal from aqueous solutions using iron oxide nanocomposites from bio-waste mass. Environ Res 199:111370. https://doi.org/10.1016/j.envres.2021.111370
Qasem NAA, Mohammed RH, Lawal DU (2021) Removal of heavy metal ions from wastewater: a comprehensive and critical review. Npj Clean Water 4:36. https://doi.org/10.1038/s41545-021-00127-0
Ahmed SF, Mofijur M, Parisa TA, Islam N, Kusumo,F, Inayat A, Le VG, Badruddin IA, Khan TMY, Ong HC (2022) Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere 286(Pt 1):131656. https://doi.org/10.1016/j.chemosphere.2021.131656
Shi L, Wang N, Hu X, Yin D, Wu C, Liang H, Cao W, Cao H (2023) Acute toxic effects of lead (Pb2+) exposure to rare minnow (Gobiocypris rarus) revealed by histopathological examination and transcriptome analysis. Environ Toxicol Pharmacol 79. https://doi.org/10.1016/j.etap.2020.103385.
Eddy NO, Garg R, Garg R, Garg R, Ukpe RA, Abugu H (2024) Adsorption and photodegradation of organic contaminants by silver nanoparticles: isotherms, kinetics, and computational analysis. Environ Monit Assess 196:65. https://doi.org/10.1007/s10661-023-12194-6
Maftouh A, El Fatni O, El Hajjaji S, Jawish MW, Sillanpää M (2023) Comparative review of different adsorption techniques used in heavy metals removal in water. Biointerface Res App Chem 13(4):397
Chowdhury IR, Chowdhury S, Mazumder MAJ, Al-Ahmed A (2022) Removal of lead ions (Pb2+) from water and wastewater: a review on the low-cost adsorbents. Appl Water Sci 12(8):185. https://doi.org/10.1007/s13201-022-01703-6
Ngamsurach P, Namwongsa N, Praipipat P (2022) Synthesis of powdered and beaded chitosan materials modified with ZnO for removing lead (II) ions. Sci Rep 12:17184. https://doi.org/10.1038/s41598-022-22182-4
Ahmad K, Shah HR, Khan MS, Iqbal A, Potrich E, Amaral LS, Rasheed S, Nawaz H, Ayub A, Naseem K, Muhammad A, Yaqoob MR, Ashfaq M (2022) Lead In drinking water: Adsorption method and role of zeolitic imidazolate frameworks for its remediation: A review. J Cleaner Prod 368. https://doi.org/10.1016/j.jclepro.2022.133010
Garg R, Garg R, Eddy NO, Almohana AI, Fahad S, Khan MA, Hong SH (2022) Biosynthesized silica-based zinc oxide nanocomposites for the sequestration of heavy metal ions from aqueous solutions. J King Saud University-Sci. https://doi.org/10.1016/j.jksus.2022.101996
Mohanapriya V, Sakthivel R, Pham NDK, Cheng CK, Le HS, Dong TMH (2023) Nanotechnology- A ray of hope for heavy metals removal. Chemosphere 311(Pt 1):136989. https://doi.org/10.1016/j.chemosphere.2022.136989.
Baby R, Hussein MZ, Abdullah AH, Zainal Z (2022) Nanomaterials for the treatment of heavy metal contaminated water. Polymers 14:583. https://doi.org/10.3390/polym14030583
Eddy NO, Ukpe RA, Garg R, Garg R, Odionenyi AO, Ameh P, Akpet I, N. (2024) Enhancing water purification efficiency through adsorption and photocatalysis: models, application and challenges. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2023.2295934
Motitswe MG, Badmus KO, Khotseng L (2022) Development of adsorptive materials for selective removal of toxic metals in wastewater: a review. Catalysts 12:1057. https://doi.org/10.3390/catal12091057
Essien UI, Odiongenyi AO, Obadimu CO, Obdimu IS (2023) Investigation of snail shells as an adsorbent and precursor for the synthesis of calcium oxide nanoparticles for the removal of amoxicillin from aqueous solution. Comm Physical Sci 8(4):453–464
Eddy NO, Garg R, Garg R, Eze SI, Ogoko EC, Kelle HI, Ukpe RA, Ogbodo R, Chijoke, F (2023) Sol-gel synthesis, computational chemistry, and applications of CaO nanoparticles for the remediation of methyl orange contaminated water. Adv Nano Res 15(1):35–48. https://doi.org/10.12989/anr.2023.15.1.000.
Eddy NO, Odiongenyi AO, Garg R, Ukpe RA, Garg R, El Nemir A, Ngwu CM, Okop IJ (2023) Quantum and experimental investigation of the application of Crassostrea gasar (mangrove oyster) shell–based CaO nanoparticles as adsorbent and photocatalyst for the removal of procaine penicillin from aqueous solution. Environ Sci Pollut Res. 30(23):64036–64057. https://doi.org/10.1007/s11356-023-26868-8
Ogoko EC, Kelle HI, Akintola O, Eddy NO (2023) Experimental and theoretical investigation of Crassostrea gigas (gigas) shells based CaO nanoparticles as a photocatalyst for the degradation of bromocresol green dye (BCGD) in an aqueous solution. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-023-03742-8
Kelle HI, Ogoko EC, Akintola O, Eddy NO (2023) Quantum and experimental studies on the adsorption efficiency of oyster shell–based CaO nanoparticles (CaONPO) towards the removal of methylene blue dye (MBD) from aqueous solution. J Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-023-04947-7
Bayuseno AP, Prasetya AI, Ismail R, Setiyana B, Jamari J (2022) Reuse of waste crab shells for synthesis of calcium carbonate as a candidate biomaterial. Rasayan J Chem 15(1):523–528. https://doi.org/10.31788/RJC.2022.1516640
Ismail R, Cionita T, Shing WL, Fitriyana DF, Siregar JP, Bayuseno AP, Nugraha FW, Muhamadin RC, Junid R, Endot NA (2022) Synthesis and characterization of calcium carbonate obtained from green mussel and crab shells as a biomaterials candidate. Materials 15:5712. https://doi.org/10.3390/ma15165712
Alghamdi AA, Al-Odayni A-B, Saeed WS, Al-Kahtani A, Alharthi FA, Aouak T (2019) Efficient adsorption of lead (II) from aqueous phase solutions using polypyrrole-based activated carbon. Materials 12:2020. https://doi.org/10.3390/ma12122020
Oyekanmi AA, Latiff AAA, Daud Z, Mohamed RMSR, Ismail N, Aziz A, Rafatullah M, Hossain K, Ahmad A, Abiodun AK (2019) Adsorption of cadmium and lead from palm oil mill effluent using bone-composite: optimisation and isotherm studies. Int J Environ Anal Chem 99(8):707–725. https://doi.org/10.1080/03067319.2019.1607318
Abdelatif Y, Gaber A.-AM, Fouda AE-AS, Alsoukarry T (2020) Evaluation of calcium oxide nanoparticles from industrial waste on the performance of hardened cement pastes: physicochemical study. Processes 8:401. https://doi.org/10.3390/pr8040401
Sinha P, Datar A, Jeong C, Deng X, Chung YG, Lin L (2019) Surface area determination of porous materials using the Brunauer–Emmett–Teller (BET) method: limitations and improvements. The J Physical Chem C 123(33):20195–20209. https://doi.org/10.1021/acs.jpcc.9b02116
Odiongenyi AO, Essien UI, Ukpong EJ, Ekwere IO (2023) Adsorption Efficiency of scotch bonnet shells as a precursor for calcium oxide nanoparticles and an adsorbent for the removal of amoxicillin from aqueous solution. Communication in Physical Science 9(3):367–382
Mostafa FA, Gad AN, Gaber AAM et al (2023) Preparation, Characterization and application of calcium oxide nanoparticles from waste carbonation mud in clarification of raw sugar melt. Sugar Tech 25:331–338. https://doi.org/10.1007/s12355-022-01150-2
Jalu RG, Ayala T, Kasirajan R (2021) Calcium oxide nanoparticles synthesis from hen eggshells for removal of lead (Pb(II)) from aqueous solution. Environ Chem 4(25):100193. https://doi.org/10.1016/j.envc.2021.100193
Phromma S, Wutikhun T, Kasamechonchung P, Eksangsri T, Sapcharoenkun C (2020) Effect of calcination temperature on photocatalytic activity of Synthesized TiO2 nanoparticles via wet ball milling sol-gel method. Appl Sci 10:993. https://doi.org/10.3390/app10030993
Triphati A, Pirzadah TB (2023) Chapter 3 - Synthesis methods of nanoparticles and their key applications,Editor(s): Munir Ozturk, Arpita Roy, Rouf Ahmad Bhat, Fazilet Vardar-Sukan, Fernanda Maria Policarpo Tonelli,In Micro and Nano Technologies. Synth Bionanomaterials Biomed Appl Elsevier 57–76. https://doi.org/10.1016/B978-0-323-91195-5.00001-5.
Eddy NO, Garg R, Garg R, Aikoye A, Ita BI (2022) Waste to resource recovery: mesoporous adsorbent from orange peel for the removal of trypan blue dye from aqueous solution. Biomass Convers Biorefinery 13:13493–13511. https://doi.org/10.1007/s13399-022-02571-5
Eddy NO, Ukpe RA, Ameh P, Ogbodo R, Garg R, Garg R (2022) Theoretical and experimental studies on photocatalytic removal of methylene blue (MetB) from aqueous solution using oyster shell synthesized CaO nanoparticles (CaONP-O). Environ Sci Pollut Res 0(34):81417–81432. https://doi.org/10.1007/s11356-022-22747-w
Naik TSSK, Singh S, Narasimhappa P et al (2023) Green and sustainable synthesis of CaO nanoparticles: its solicitation as a sensor material and electrochemical detection of urea. Sci Rep 13:19995. https://doi.org/10.1038/s41598-023-46728-2
Nayeem A, Mizi F, Ali MF, Shariffuddin JH (2022) Utilization of cockle shell powder as an adsorbent to remove phosphorus-containing wastewater. Environ Res. 2023 Jan 1;216(Pt 2):114514. https://doi.org/10.1016/j.envres.2022.114514.
Habte L, Shiferaw N, Mulatu D, Thenepalli T, Chilakala R, Ahn JW (2019) Synthesis of nano-calcium oxide from waste eggshell by sol-gel Method. Sustainability 11:3196. https://doi.org/10.3390/su11113196
Mushtaq M, Hassan SM, Mughal SS (2022) Synthesis, characterization and biological approach of nano oxides of calcium by Piper nigrum. Am J Chem Eng 10(4):79–88
Al-Odayni A-B, Alsubaie FS, Abdu NAY, Al-Kahtani HMAND, Saeed WS (2023) Adsorption kinetics of methyl orange from model polluted water onto N-doped activated carbons prepared from N-containing Polymers. Polymers 15:1983. https://doi.org/10.3390/polym15091983
Atchudan R, Perumal S, Joo J, Lee YR (2022) Synthesis and characterization of monodispersed spherical calcium oxide and calcium carbonate nanoparticles via simple pyrolysis. Nanomaterials 12:2424. https://doi.org/10.3390/nano12142424
Bold B-E, Urnukhsaikhan E, Mishig-Ochir T (2022) Biosynthesis of silver nanoparticles with antibacterial, antioxidant, anti-inflammatory properties and their burn wound healing efficacy. Front Chem 10:972534. https://doi.org/10.3389/fchem.2022.972534
Jadhav VR, Bhagare AM, Lokhande DD, Vaidya C, Dhayagude A, Khalid M, Aher J, Mezni A, Dutta M (2022) Green synthesized calcium oxide nanoparticles (CaO NPs) using leaves aqueous extract of Moringa oleifera and evaluation of their antibacterial activities. J Nanomater 2:1–7. https://doi.org/10.1155/2022/9047507
Kumar A, Kumar A, M.M.S, C.-P., Chaturvedi AK, Shabnam AA, Subrahmanyam G, Mondal R, Gupta DK, Malyan SK, Kumar SS, A Khan, S, Yadav KK (2020) Lead toxicity: health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health, 17, 2179https://doi.org/10.3390/ijerph17072179
Maswanganye MW, Kabongo GL, Mathevula LE et al (2023) Unveiling the effect of strain engineering on the electrochemical properties of hydrothermally grown nanostructured indium doped ZnSeO3 for photoanode applications. Sci Rep 13:20131. https://doi.org/10.1038/s41598-023-47436-7
Ayesha HI,Haider A, Shahzadi I, Moeen S, Ul-Hamid A, Nabgan W, Shahzadi A, Alshahrani T.& Ikram H (2023) Polyvinylpyrrolidone and chitosan-coated magnetite (Fe3O4) nanoparticles for catalytic and antimicrobial activity with molecular docking analysis. J Environ Chem Eng 11(3). https://doi.org/10.1016/j.jece.2023.110088
Dompreh KA, Arthur DS, Mensah SY, Adu KM, Edziah R, Amekpewu M (2023) Effect of band-gap tuning on absorption of phonons and acoustoelectric current in graphene nanoribbon. Physica E 147:115516. https://doi.org/10.1016/j.physe.2022.115516
Gandhi N, Shruthi Y, Sinsha G, Anusha CR (2021) Facile and eco-friendly method for synthesis of calcium oxide (CaO) nanoparticles and its potential application in agriculture. Haya The Saudi J Life Sci 6(5):89–103. https://doi.org/10.36348/sjls.2021.v06i05.003
Thakur S, Singh S, Pal B (2021) Superior adsorption removal of dye and high catalytic activity for transesterification reaction displayed by crystalline CaO nanocubes extracted from mollusc shells. Fuel Process Technol 213. https://doi.org/10.1016/j.fuproc.2020.106707
de Menezes LB, Muraro PCL, Druzian DM, Ruiz YPM, Galembeck A, Pavoski G, Espinosa DCR, da Silva WL (2024) Calcium oxide nanoparticles: biosynthesis, characterization and photocatalytic activity for application in yellow tartrazine dye removal. J Photochem Photobiol A Chem 447. https://doi.org/10.1016/j.jphotochem.2023.115182
Kasirajan R, Bekele A, Girma E (2022) Adsorption of lead (Pb-II) using CaO-NPs synthesized by solgel process from hen eggshell: response surface methodology for modeling, optimization and kinetic studies. S Afr J Chem Eng 40:209–229. https://doi.org/10.1016/j.sajce.2022.03.008
Adebowale K, Egbedina A, Shonde B (2020) Adsorption of lead ions on magnetically separable Fe3O4 watermelon composite. Appl Water Sci 10:225. https://doi.org/10.1007/s13201-020-01307-y
Alsohaimi IH, Nassar AM, Elnasr TA, S Cheba BA (2020) A novel composite silver nanoparticles loaded calcium oxide stemming from egg shell recycling: a potent photocatalytic and antibacterial activities. J Cleaner Prod 248. https://doi.org/10.1016/j.jclepro.2019.119274
Dehghani MH, Afsari Sardari S, Afsharnia M et al (2023) Removal of toxic lead from aqueous solution using a low-cost adsorbent. Sci Rep 13:3278. https://doi.org/10.1038/s41598-023-29674-x
Tejada-Tovar C, Villabona-Ortíz A, González-Delgado ÁD (2023) High-efficiency removal of lead and nickel using four inert dry biomasses: insights into the adsorption mechanisms. Materials 16:4884. https://doi.org/10.3390/ma16134884
Odoemelam SA, Emeh NU, Eddy NO (2018) Experimental and computational Chemistry studies on the removal of methylene blue and malachite green dyes from aqueous solution by neem (Azadiractha indica) leaves. Journal of Taibah University of Science 12(3):255–265. https://doi.org/10.1080/16583655.2018.1465725
Revellame ED, Fortela DL, Sharp W, Hernandez R, Zappi ME (2020) Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: a review. J Cleaner Eng Technol 1. https://doi.org/10.1016/j.clet.2020.100032
Kostoglou M, Karapantsios TD (2022) Why is the linearized form of pseudo-second order adsorption kinetic model so successful in fitting batch adsorption experimental data? Colloids Interfaces 6:55. https://doi.org/10.3390/colloids6040055
Edet UA, Ifelebuegu AO (2020) Kinetics, isotherms, and thermodynamic modeling of the adsorption of phosphates from model wastewater using recycled brick waste. Processes 8:665. https://doi.org/10.3390/pr8060665
Wang J, Guo X (2022) Rethinking of the intraparticle diffusion adsorption kinetics model: interpretation, solving methods and applications. Chemosphere 309(2):136732. https://doi.org/10.1016/j.chemosphere.2022.136732
Hasani N, Selimi T, Mele A, Thaçi V, Halili J, Berisha A, Sadiku M (2022) Theoretical, Equilibrium, kinetics and thermodynamic investigations of methylene blue adsorption onto lignite coal. Molecules 2022(27):1856. https://doi.org/10.3390/molecules27061856
Vareda, JP. (2023). On validity, physical meaning, mechanism insights and regression of adsorption kinetic models. J Mol Liq 376. https://doi.org/10.1016/j.molliq.2023.121416
Tran HN (2023) Applying linear forms of pseudo-second-order kinetic model for feasibly identifying errors in the initial periods of time-dependent adsorption datasets. Water 15:1231. https://doi.org/10.3390/w15061231
Robati D (2023) Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J Nanostruct Chem 3:55. https://doi.org/10.1186/2193-8865-3-55
Taraba B, Bulavová P (2022) Second or pseudo-second-order model for adsorption kinetics? Sep Sci Technol 57(10):1558–1562. https://doi.org/10.1080/01496395.2021.1998124
Zand AD, Abyaneh MR (2020) Adsorption of Lead, manganese, and copper onto biochar in landfill leachate: implication of non-linear regression analysis. Sustain Environ Res 30:18. https://doi.org/10.1186/s42834-020-00061-9
Soldatkina L, Yanar M (2021) Equilibrium, kinetic, and thermodynamic studies of cationic dyes adsorption on corn stalks modified by citric acid. Colloids Interfaces 1(5):52. https://doi.org/10.3390/colloids5040052
Jayakumar S, Sudarsan S, Sridhar B, Parthiban F, Prabu AV, Jha S (2023) Synthesis, characterization, and performance of chitosan/nylon 6/polyurethane blend for the removal of chromium (VI) and lead (II) ions from aqueous solutions for enhanced kinetic adsorption studies. Water Reuse 13(3):319–328. https://doi.org/10.2166/wrd.2023.019
Manjuladevi M, Anitha R, Manonmani S (2018) Kinetic study on adsorption of Cr(VI), Ni(II), Cd(II) and Pb(II) ions from aqueous solutions using activated carbon prepared from Cucumis melo peel. Appl Water Sci 8:36. https://doi.org/10.1007/s13201-018-0674-1
Ton-That L, Huynh TNL, Duong BN et al (2023) Kinetic studies of the removal of methylene blue from aqueous solution by biochar derived from jackfruit peel. Environ Monit Assess 195:1266. https://doi.org/10.1007/s10661-023-11867-6
Weyrich JN, Mason JR, Bazilevskaya EA, Yang H (2023) Understanding the mechanism for adsorption of Pb(II) ions by Cu-BTC metal–organic frameworks. Molecules 28:5443. https://doi.org/10.3390/molecules28145443
Devi B, Goswami M, Rabha S, Kalita S, Sarma HP, Devi A (2023) Efficacious sorption capacities for Pb(II) from contaminated water: a comparative study using biowaste and its activated carbon as potential adsorbents. ACS Omega 8(17):15141–15151. https://doi.org/10.1021/acsomega.3c00142
Shikuku VO, Mishra T (2021) Adsorption isotherm modeling for methylene blue removal onto magnetic kaolinite clay: a comparison of two-parameter isotherms. Appl Water Sci 11:103. https://doi.org/10.1007/s13201-021-01440-2
Wong S, Ghafar NA, Ngadi N et al (2020) Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci Rep 10:2928. https://doi.org/10.1038/s41598-020-60021-6
Kuśmierek K, Fronczyk J, Świątkowski A (2023) Adsorptive removal of rhodamine B dye from aqueous solutions using mineral materials as low-cost adsorbents. Water Air Soil 234:53. https://doi.org/10.1007/s11270-023-06511-5
Amrutha J, Jeppu G, Girish CR et al (2023) Multi-component adsorption isotherms: review and modeling studies. Environ Process 10:38. https://doi.org/10.1007/s40710-023-00631-0
Abewaa M, Mengistu A, Takele T et al (2023) Adsorptive removal of malachite green dye from aqueous solution using Rumex abyssinicus derived activated carbon. Sci Rep 13:14701. https://doi.org/10.1038/s41598-023-41957-x
Chen H, Qu X, Liu N, Wang S, Chen X, Liu S (2018) Study of the adsorption process of heavy metals cations on Kraft lignin. Chem Eng Res Des 139:248–258. https://doi.org/10.1016/j.cherd.2018.09.028
Mahamadi C, Nharingo T (2010) Utilization of water hyacinth weed (Eichhornia crassipes) for the removal of Pb(II), Cd(II) and Zn(II) from aquatic environments: an adsorption isotherm study. Environ Technol 31(11):1221–1228. https://doi.org/10.1080/09593331003646604
Qi J, Li Y, Majeed H, Douglas Goff H, Rahman MRT, Zhong F (2019) Adsorption mechanism modeling using lead (Pb) sorption data on modified rice bran-insoluble fiber as universal approach to assess other metals toxicity. Int J Food Prop 22(1):1397–1410. https://doi.org/10.1080/10942912.2019.1650764
Mahanty B, Behera SK, Sahoo NK (2023) Misinterpretation of Dubinin-Radushkevich isotherm and its implications on adsorption parameter estimates. Sep Sci Technol 58(7):1275–1282. https://doi.org/10.1080/01496395.2023.2189050
El-Nemr MA, Yılmaz M, Ragab S et al (2023) Isotherm and kinetic studies of acid yellow 11 dye adsorption from wastewater using Pisum Sativum peels microporous activated carbon. Sci Rep 13:4268. https://doi.org/10.1038/s41598-023-31433-x
Neskoromnaya EA, Khamizov RK, Melezhyk AV, Memetova AE, Mkrtchan ES, Babkin, AV (2022) Adsorption of lead ions (Pb2+) from wastewater using effective nanocomposite GO/CMC/FeNPs: kinetic, isotherm, and desorption studies. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 655, https://doi.org/10.1016/j.colsurfa.2022.130224.
Perera GRY, Neetha Dayanthi WKC (2023) Low-cost treatment method for organic matter and nutrients in landfill leachate. J Water Health 21(6):789–814. https://doi.org/10.2166/wh.2023.014
Chu KH, Bashiri H, Hashim HA, Shukor HYA, Bollinger J (2023) The Halsey isotherm for water contaminant adsorption is fake. Separation and Purification Technology, 313, https://doi.org/10.1016/j.seppur.2023.123500.
Chu KH, Bashiri H, Hashim MA, Abd Shukor MY, Bollinger J (2023) The Halsey isotherm for water contaminant adsorption is fake. Separation and Purification Technology, 313, https://doi.org/10.1016/j.seppur.2023.123500.
Gedam VV, Raut P, Chahande A, Pathak P (2019) Kinetic, thermodynamics and equilibrium studies on the removal of Congo red dye using activated teak leaf powder. Appl Water Science 9:55. https://doi.org/10.1007/s13201-019-0933-9
Wu J, Xia A, Chen C, Feng L, Su X, Wang X (2019) Adsorption thermodynamics and dynamics of three typical dyes onto bio-adsorbent spent substrate of Pleurotus eryngii. Int J Environ Res Public Health 16(5):679. https://doi.org/10.3390/ijerph16050679
Zaid R, Khan SU, Azam A, Farooq IH (2021) A study on effective adsorption of lead from an aqueous solution using copper oxide nanoparticles. IOP Conf. Ser.: Mater. Sci. Eng. 1058 012074.
Bidhendi ME, Parandi E, Meymand MM, Sereshti H, Nodeh HR, Joo S, Vasseghian YV, Khatir NM, Rezania S (2022) Removal of lead ions from wastewater using magnesium sulfide nanoparticles caged alginate microbeads. Environ Res 216:1. https://doi.org/10.1016/j.envres.2022.114416
Ramos-Guivar JA, Checca-Huaman NR, Litterst FJ, Passamani EC (2023) Surface adsorption mechanism between lead(II, IV) and nanomaghemite studied on polluted water samples collected from the peruvian rivers Mantaro and Cumbaza. Nanomaterials 2023(13):1684. https://doi.org/10.3390/nano13101684
Tsague CF, Abbo HS, Yufanyi MY, Ondoh AM, Titinchi SJJ (2023) Recyclable functionalized polyethyleneimine-coated magnetic nanoparticles for efficient removal of lead from aqueous solutions. Chem Technol Biotechnol 98(8):2023–2038. https://doi.org/10.1002/jctb.7423
Jalu RG, Chamada TA, Ramachandran K (2021) Calcium oxide nanoparticles synthesis from hen eggshells for removal of lead (Pb(II)) from aqueous solution. Environmental Challenges, 4, https://doi.org/10.1016/j.envc.2021.100193
Bayo AB, Amibo TA, Beyan SM (2022) Adsorptive capacity of calcinated hen eggshell blended with silica gel for removal of lead ii ions from aqueous media: kinetics and equilibrium studies. J Environ Public Health. 2882546. https://doi.org/10.1155/2022/2882546
Funding
The research work that generated this publication was sponsored by the Tertiary Education Trust Fund of Nigeria (TETFUND) through the National Research grant (2020) with Prof. Nnabuk Okon Eddy as the Principal investigator (Grant number: TRTF/ES/DR&D-CE/NRF2020/SETI/98/VOL.1). The work was also supported by the Project Number (RSP2024R448), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
The research was conceived by Nnabuk Okon Eddy, Richard Alexis Ukpe, and Paul Ocheje Ameh. The characterization study was done by Nnabuk Okon Eddy, Rajni Garg, Rishav Garg, Saikh Mohammad Wabaidur, Masoom Raza Siddiqu, and Raphael Ogbodo. Musa Runde and Iliya Dimas Kwanchi carried out the adsorption study. All authors wrote the first draft, revised, and edited the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All authors approve the ethics and consent to participate in this research.
Consent to publish
All authors have consented to publish this paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eddy, N.O., Garg, R., Ukpe, R.A. et al. Application of periwinkle shell for the synthesis of calcium oxide nanoparticles and in the remediation of Pb2+-contaminated water. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05285-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05285-y