Abstract
Evolutionary changes in microorganisms, in response to the current environment, can contribute generically to adaptations that have harmful effects on human welfare. Antibacterial resistance is one of the results of genetic and evolutionary changes in microorganisms. Many bacteria have developed resistance to antibiotics. Recently, most researchers have been focused on overcoming this problem, relying on nanoparticle-based drug delivery. In this study, our aim is to synthesize silver-doped fucoidan nanoparticle using Tridax procumbens plant leaf aqueous extract and examine their biological efficacy. We synthesized silver-doped fucoidan with the aqueous extraction of Tridax procumbens using the titration method. The synthesized nanoparticles were characterized, including UV-spectroscopy, SEM, FT-IR, EDX, and XRD. Furthermore, we assessed the efficacy of the nanoparticles in terms of antioxidant activity using the DHHP assay, anti-inflammatory activity using the protein degradation assay, and antibacterial activity using well diffusion method. Our result revealed that the synthesized nanoparticles were doped with Ag2+ in fucoidan using the plant extract. We observed a peak at 390 nm in the UV-spectra analysis, indicating the presence of silver nanoparticles. Further analysis, like SEM, FT-IR, EDX, and XRD showed the nanoparticle characterization. Moreover, these nanoparticles demonstrate good antioxidant and anti-inflammatory activity. Additionally, the nanoparticles exhibited antimicrobial activity against antibiotic-resistance bacteria. Finally, our synthesized silver-doped nanoparticles mediated by an aqueous extract of Tridax procumbens show potential in therapeutic aspects for bacterial infection.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology and its allied disciplines are expanding fields of research, due to its myriad applications in engineering, medicine, and sensors technologies. The surface morphology, shape, and size of the materials govern their electrical, photonic, catalytic, photocatalytic, and physicochemical capabilities [1]. Moreover, metal ions (Ag, AU, Zn, Mn, Ti) and their oxide nanoparticles have demonstrated excellent mechanistic action in various fields. Conventional methods for synthesizing silver nanoparticles are highly expensive and pose risks to the both human health and the environment. Notably, the green chemistry procedure for synthesizing metal and metal oxide nanoparticles was specifically developed to minimize environmental toxicity and eliminate pollution [2]. The plant-based synthesis of silver nanoparticles is eco-friendly and demonstrates potential biological activity [3]. The utilization of plants as bio reductants for the green synthesis of silver nanoparticles has gained more attention compared to other biological sources, primarily due to their incorporation of bioactive compounds in nanoparticles acting as capping agents [4]. Nanomedicine is an application for diagnostic and therapeutic purpose, as nanoparticles often serve as carriers for visualization and therapeutic compounds, enabling more targeted administration [5]. This capability expands the potential uses of these particles in various scientific and technological domains, such as catalysis, sensing, and medicine. In recent years, silver nanoparticles have gained recognition for their broad spectrum and significant biological assessments, including anti-oxidant, anti-inflammatory, antimicrobial, and anticancer activities [6].
Tridax procumbens a perennial plant belonging to the Asteraceae family, possesses multiple healing properties, particularly in the treatment of injury wounds, owing to its dose-dependent prohealing properties [7]. This plant is commonly found in the tropical areas of the Americas [8]. T. procumbens is a beneficial source for making foods and drinks that can be used to treat bacterial infections, oxidative stress, and antihyperuricemia [9]. Abundant classified secondary metabolites present in this plant exhibit high pharmacological activities, commonly employed in traditional treatment [10]. The phytocompounds of the plant may possess metal reducing properties, making it suitable for synthesizing silver nanoparticles to enhance biological efficacy.
Additionally, to improve the antioxidant and anti-inflammatory properties of silver nanoparticles, polysaccharides are doped into them. Due to their biological features, macroalgal polysaccharides such as fucoidan found in several species of brown seaweed have garnered interest. Despite the chemical richness and diverse range of compounds with promising anti-oxidant, anticancer, anti-inflammatory, and anti-microbial activities found in brown sea weed (marine algae) [11]. Fucoidan, a polysaccharide predominantly made up of l-fucose and sulfate groups, has potent antioxidant activities, aiding in the neutralization of damaging free radicals and protecting cells and tissues from oxidative damage caused by oxidative stress, which has been associated with a variety of chronic diseases [12]. Furthermore, fucoidan has anti-inflammatory properties by hindering the generation of pro-inflammatory substances and modifying immune cell activity, making it a promising therapeutic agent for the treatment of inflammatory illnesses and diseases [13]. In this work, we approach the one pot synthesis of fucoidan doped with silver nanoparticles using a Tridax procumbens and evaluate their antioxidant, anti-inflammatory, and antibacterial assessment.
2 Materials and methods
2.1 Materials
Silver nitrate (AgNo3) and fucoidan (Fu) were purchased from Loba Chemie Pvt. Ltd. Nutrient agar, Mueller–Hinton agar (MHA) and diclofenac sodium was purchased from Hi-Media (Mumbai, India). Four bacterial cultures (methicillin-resistant Staphylococcus aureus (MRSA), E. coli, Pseudomonas aeruginosa, and Enterococcus faecalis) were obtained from the Department of Microbiology, Saveetha Medical College.
2.2 Sampling
Tridax procumbens leaves were collected in Poonamallee, Chennai, Tamil Nadu, and Dr. N. Siva, Assistant Professor, Department of Botany, Raja Doraisingam Government Arts College Sivagangai, Tamil Nadu, authenticated the sample’s taxonomic identification.
2.3 Extraction
The Tridax procumbens leaves were rinsed three times using distilled water and air-dried. The dried sample was ground to powder 10 g of Tridax procumbens powder were added to 200 mL of distilled water and solution was boiled for 30 to 1 h. The solution was filtered using Whatman No. 1 filter paper and the extract was stored at 4 °C for further use.
2.4 Synthesis of Ag-Fu NPs
One pot synthesis was performed by the following procedure, an aqueous extract of Tridax procumbens was taken in a conical flask, 25 mM of silver nitrate (AgNO3) was taken in one burette and another burette was taken 25 mM of fucoidan using the titration method. The silver nitrate and fucoidan were added dropwise to the plant extract. The mixture was kept in an orbital shaker for overnight incubation. Color changes were observed from dark black to brown after incubation. Further, the solution was centrifuged at 4500 rpm for 30 min, and the pellet was separated and washed with distilled water and centrifuged at 4500 rpm for 30 min. After centrifugation, the pellet was collected and kept in hot air oven at 60 °C for 24 h. Finally, it was stored in an airtight container at room temperature for further studies.
2.5 Characterization
The physicochemical properties of the nanoparticles determine their efficacy, bio-distribution and mechanism; thus, it is important to characterize the Tridax procumbens-mediated Ag-Fu NPs to assess the functional aspects of the synthesized nanoparticles and utilize of various analytical techniques, such as a UV–vis spectrophotometer (Thermo Scientific Evolution 600), to obtain optical characteristics nanoparticle production with 1 cm quartz cuvettes in the 200–800 nm range. A Bruker FT-IR spectrophotometer was used to conduct a Fourier transform infrared (FTIR) spectroscopic investigation in the range of 4000–400 cm−1 to examine the function of several biomolecules that act as stabilizing agents in the production of Tridax procumbens–mediated Ag-Fu NPs. The abundance of diffraction peaks resulting from the reflection of X-ray radiation on particles represents the phytochemicals characteristics of the crystal lattice. The analytical technique called X-ray diffraction (XRD is utilized to qualitatively identify active chemicals; resolve different molecules, determine isomorphous substitution; and assess particle size. The surface morphology of the synthesized Tridax procumbens–mediated Ag-Fu NPs was examined using scanning electron microscopy (SEM) (JSM-7001F, JEOL, Tokyo, Japan) operating at an accelerating voltage of 20 keV. Energy dispersive X-ray (EDX) spectrometer (JSM-7001F, JEOL, Tokyo, Japan) was used for the analysis of the elements present in the silver-doped fucoidan nanoparticles.
2.6 Biological assessment
2.6.1 Agar well diffusion method
Antibacterial activity was performed by the well diffusion technique and inhibitory zone of Tridax procumbens–mediated Ag-Fu NPs against four bacterial strains namely, Escherichia coli, Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, and Enterococcus faecalis was observed. The respective bacterial strains were grown in MH broth for 18 h at ambient temperature and turbidity was adjusted to 0.5 McFarland standard. MHA plates were prepared by dissolving media in 300 mL of distilled water and autoclaving. Later, the plate was swabbed with the strains. The well was punched with the sterile tips and filled with the Tridax procumbens–mediated Ag-Fu NPs at various concentrations (10–50 μg/mL) and streptomycin as a control. The plates were incubated for 24 h, and the inhibitory zone was measured.
2.6.2 Antioxidant activity
To analyze the antioxidant activity of Tridax procumbens–mediated Ag-Fu NPs using a DPPH radical scavenging assay. The procedure was carried out on a 96-wells microtiter plate. Add a DPPH solution to each well. Then each well was added with Ag-Fu NPs at (10–50 μg/mL) at different concentrations and without nanoparticle served as the blank. Preparation of the of standard was carried out by using ascorbic acid (10–50 μg/mL). The plate was incubated in a dark room for 30 min. Record the absorbance at the 517-nm wavelength using a microplate reader.
2.6.3 Anti-inflammatory activity
To analyze the anti-inflammatory activity of Tridax procumbens–mediated Ag-Fu NPs an albumin denaturation assay was performed on the microtiter plate. Add a 1% BSA to each well. 10 to 50 μg/mL concentrations of Tridax procumbens–mediated Ag-Fu NPs were added to the well, without nanoparticle serving as a blank. Further, another well incubates the 1% BSA and diclofenac sodium serves as a standard. The solution was kept in the incubator for 15 min at room temperature and incubated for another 20 min at 55 °C. After complete incubation, the absorbance was measured at 660 nm, and the percentage of inhibition was calculated.
3 Result
3.1 Synthesis of Ag-Fu NPs
The one pot synthesis of the silver nanoparticles with the aqueous extract of Tridax procumbens. The black to brown color changes in solution indicate the formation of Ag-Fu nanoparticles using the Tridax procumbens shown in Fig. 1.
3.2 Characterization of Tridax procumbens–mediated Ag-Fu NPs
3.2.1 UV–Vis spectroscopy
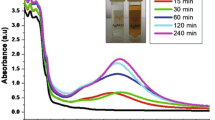
Using UV–Vis Spectroscopy the plasma resonance peak level of absorbance wavelength was noted, and the highest peak level of absorbance was noted to be 390 nm, as shown in Fig. 2. Thus, the presence of Ag-Fu NPs was confirmed.
3.2.2 FT-IR
The functional groups of the Tridax procumbens–mediated Ag-Fu NPs were identified using a Bruker FTIR spectrophotometer. The analysis showed more than four functional groups in the synthesized Tridax procumbens–mediated Ag-Fu NPs in the wavelength range of 1000–3500 cm−1. The functional groups and the chemical bonding were represented in the following: the most significant values are 1049.47 cm−1, 1211.53 cm−1, 1633.44 cm−1, and 2341.86 cm−1. Functional groups like C–O–C, C–C, (-OH), and nitrile groups correspond to these peaks (Fig. 3).
3.2.3 X-ray diffraction analysis
X-ray diffraction technique is used to find out the crystalline and amorphous nature of the respective nanoparticles. Synthesized Tridax procumbens–mediated Ag-Fu NPs demonstrate more crystalline (72.1%) and less amorphous (27.9%) characteristics as a result of our study (Fig. 4). Thus, the crystalline structure of the Tridax procumbens–mediated Ag-Fu NPs we generated is highly stable.
3.2.4 Scanning electron microscopy
Two different magnifications (1 μm and 100 μm) of the SEM showed the spherical to irregular shape of the Tridax procumbens–mediated Ag-Fu NPs, average particle size of the agglomerated nanoparticles was found to be 80–150 nm (Fig. 5), which confirmed the presence of the Tridax procumbens–mediated Ag-Fu NPs.
3.2.5 Energy dispersive X-ray analysis
EDX analysis determined to identify the composition of the elements present in Tridax procumbens–mediated Ag-Fu NPs. The results of the EDX spectra for the Tridax procumbens–mediated Ag-Fu NPs show that Ag, O, and C signals were observed at 58.7, 12.9 and 5.9 keV respectively. This confirms the anchoring of Ag on the surface (Fig. 6).
3.2.6 Antibacterial efficacy of Tridax procumbens–mediated Ag-Fu NPs
Antibacterial activity of the Tridax procumbens–mediated Ag-Fu NPs was performed against methicillin-resistant Staphylococcus aureus (MRSA), E. coli, Pseudomonas aeruginosa, and Enterococcus faecalis, and the inhibition zone was measured, respectively (Fig. 7). The zone of inhibition was given in detail in Table 1. When compared with the positive control for 80-μL concentrations, 15 mm, 13 mm, 23 mm, and 20 mm of inhibition against methicillin-resistant Staphylococcus aureus, E. coli, Pseudomonas aeruginosa, and Enterococcus faecalis respectively.
3.2.7 Antioxidant activity
The graphical representation of synthesized Ag-Fu NPs showed 97.28% of maximal antioxidant activity at 50 μg/mL of concentrations, when compared with the standard ascorbic acid (93.01%), 53% of moderate activity at 30 μg/mL, and 49.6% of minimal activity at 10 μg/mL (Fig. 8).
3.2.8 Anti-inflammatory activity
The synthesized Tridax procumbens–mediated Ag-Fu NPs exhibited 85% of anti-inflammatory activity at 50 μg/mL concentration when compared to the standard drug (72.63%). At a concentration of 30 μg/mL, the Ag-Fu NPs showed 50% and minimum activity was found in 10 μg/mL with 48.39% activity (Fig. 9).
4 Discussion
In our present study, we synthesized Ag-Fu NPs using Tridax procumbens as reducing agent. The color changes of nanoparticles are, due to the surface plasmon resonance (SPR). Previous studies showed the change from green to yellowish brown color, in which trisodium citrate is used as a reducing agent and silver nitrate as the initiator for the synthesis process [14]. Another study found, evidence that synthesizing the silver nanoparticles using carob leaf extract (Ceratonia siliqua) showed the color change from watery to yellow [15]. Similarly, in our observations, we noted black to brown color changes in Tridax procumbens–mediated Ag-Fu nanoparticles. These changes can be attributed to phytochemicals such as terpenoids, polyphenols, and peptides, which play a significant role as capping agents in binding silver nanoparticles. Specifically, dipeptides are involved in the binding of silver nanoparticles and also responsible for the various pharmacological activities [14].
In UV–Vis spectroscopy, the absorption of the nanoparticles depends on the particle size and chemical surroundings [16]. It is also having the capability to identify the quality of the nanoparticles [17]. Another similar study showed the recorded peak absorbance value at 485 nm [18]. When compared with our study, which showed 390 nm. However, in comparison with the peak of absorbance confirmed the presence of Ag-Fu NPs.
The application of FT-IR is used to investigate the surface chemistry of synthesized metal nanoparticles and observe the presence of biomolecules, and the chemical bonding of the various functional groups in the process of nanoparticle synthesis [19]. A similar study performed for the synthesis of silver nanoparticles showed peaks at 2919.48 cm−1, 1693.57 cm−1, 1547.31 cm−1, 1387.63 cm−1, and 1179.82 cm−1. The absorption peaks observed at 1002.73 cm−1 in the spectrum of Ag NPs are attributes of the stretching vibrations of aliphatic amine (C-N) bonds. These peaks indicate the presence of Tridax procumbens, which is responsible for the reduction and stabilization of silver nanoparticles. Additionally, the spectrum also shows prominent peaks at 3309.03 cm−1 and 1637.3. cm−1, which are associated with the functioning of reduction and stabilization processes [20]. When compared with this study similar functional groups (C = O) were identified. The crystalline and amorphous nature of the nanoparticle determines the binding and penetration of the nanoparticle on the surface of the pathogen. This technique is primarily used for analyzing atomic spacing and crystal structure of nanoparticles [21]. A similar study showed that XRD peaks observed in the crystalline structures of the synthesized silver nanocrystals specifically several Braggs peaks at 20, 38.06, 44.32, 64.58, and 77.3 correspond to the (111), (200), (220), and (311) planes of the face-cantered cubic structure of silver, indicating the signature peaks for the biosynthesized Ag NPs [22]. Through this overall comparison, the crystalline and amorphous characteristics of the Ag-Fu NPs are confirmed.
The SEM is used to analyze the nanoparticle size, shape, and distribution characteristics which confirm the presence of the specific nanoparticle. A similar study for the green synthesis of silver nanoparticles showed the same spherical to irregular-shaped nanoparticles; the average diameter of the NPs is 3.8 ± 1.1 and 9.1 ± 2.9 nm. It also indicates that the higher concentration of AgNO3 is responsible for the larger quantity of nanoparticles of silver [23]. Thus, comparing these studies, it can be concluded that the volume of nanoparticles production indirectly depends on the initiative material concentration. The existence of the Tridax procumbens–mediated Ag-Fu NPs can be confirmed by these EDX spectra. Similar study on the synthesis of silver nanoparticles with the natural extracts of various plants Viscum album, Salvia officinalis, and Aegopodium podagraria showed the peaks of elemental composition in which the presence of Ag nanoparticles (2.62, 2.98, 3.14, 3.34, and 3.50 keV) along with some impure compounds (Na, K, Ca, Al, Si) [24]. Thus in our study, the Tridax procumbens–mediated Ag-Fu NPs are present with less amount of impure components.
In relevant to the study of bio-synthesis of silver nanoparticles from leaf extracts of Cleistanthus collinus showed potential activity and exhibited zone of inhibition, 17 mm against Staphylococcus aureus and 21 mm Pseudomonas aeruginosa [25]. Similar study of antibacterial activity revealed that green synthesis of Ag NPs with T. procumbens showed antibacterial activity against both Gram-negative and Gram-positive bacterial strains. and its results shows that the zone of inhibition was found to be 8.78–10.31 mm at 50–100 μg/mL concentration for Bacillus subtilis and Klebsiella pneumoniae, respectively [18]. Another antibacterial study of silver nanoparticle against E. coli showed 11 mm zone of inhibition [26]. One more similar green synthesis study of silver nanoparticles leaf extract of Trigonila foenum-graecum showed 16.8 mm zone of inhibition at 50 μg/mL concentration against Pseudomonas aeruginosa [17]. Similarly, our studies revealed that Tridax procumbens mediated Ag-Fu NPs showed significant antibacterial activity against the multidrug-resistant bacterial pathogens. Antioxidant compound has the ability to decrease the quantity of reactive oxygen species (ROS). The anti-oxidant activity of Fucoidan is determined by the sulfate polysaccharides structural modification and over sulfation because of strong positive correlation between their sulfate content and biological activity [27]. Similar studies have shown Ag NPs exhibited significant radical scavenging activity and demonstrated does dependant inhibition properties. The inhibition rate showed an increase with higher concentration [28]. Comparing with other similar study of F. vesiculosus with silver and gold nanoparticles showed the antioxidant activity of 10 ± 0.001 for 95 μg/mL [29]. Another study of biosynthesized silver nanoparticle showed antioxidant activity of 50.66–98.53% in a dose-dependent manner at concentrations of 10–450 μg/mL [30]. This is in a line with our study which showed inhibition of highest concentration 500 μg/mL of the A. tribuloides root extract was 47% and greenly synthesis of Ag NPs was 64% [31]. Comparison with all these, our study has significant antioxidant activity of 97.28% at 50 μg/mL concentration. The inflammatory response plays a major role in the wound healing process [30]. When compared with the study of silver nanoparticles synthesis with A. tribuloides root extract showed 69% at highest concentration of 500 μg/mL. Thus, when compared with this study, our study showed significant anti-inflammatory activity of 85% at 50 μg/mL concentration. Our synthesized one spot synthesized silver doped with fucoidan using Tridax procumbens has high biological efficacy.
5 Conclusion
We concluded that the eco-friendly synthesized Tridax procumbens Ag-Fu NPs have antioxidant and anti-inflammatory activity. Moreover, we suggest that silver-doped fucoidan nanoparticle mediate Tridax procumbens has potential to inhibit the growth of antibiotic-resistance bacteria, including methicillin-resistant Staphylococcus aureus, E. coli, Pseudomonas aeruginosa, and Enterococcus faecalis. We need further analysis and clinical trials to better understand the mode of action of nanoparticles against pathogenic bacteria.
Data availability
Data available on request from first and corresponding author.
References
Bukhary HA, Zaman U, Ur Rehman K, Alissa M, Rizg WY, Khan D et al (2023) Acid protease functionalized novel silver nanoparticles (APTs-AgNPs): a new approach towards photocatalytic and biological applications. Int J Biol Macromol 242:124809
Saravanan M, Gopinath V, Chaurasia MK, Syed A, Ameen F, Purushothaman N (2018) Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb Pathog 115:57–63
Kim D-Y, Saratale RG, Shinde S, Syed A, Ameen F, Ghodake G (2018) Green synthesis of silver nanoparticles using Laminaria japonica extract: characterization and seedling growth assessment. J Clean Prod 172:2910–2918
Ameen F, Srinivasan P, Selvankumar T, Kamala-Kannan S, Al Nadhari S, Almansob A et al (2019) Phytosynthesis of silver nanoparticles using Mangifera indica flower extract as bioreductant and their broad-spectrum antibacterial activity. Bioorg Chem 88:102970
Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D et al (2015) Safety of nanoparticles in medicine. Curr Drug Targets 16:1671–1681
Ameen F, Abdullah MMS, Al-Homaidan AA, Al-Lohedan HA, Al-Ghanayem AA, Almansob A (2020) Fabrication of silver nanoparticles employing the cyanobacterium Spirulina platensis and its bactericidal effect against opportunistic nosocomial pathogens of the respiratory tract. J Mol Struct 1217:128392
Preveena J, Bhore SJ (2013) Identification of bacterial endophytes associated with traditional medicinal plant Tridax procumbens Linn. Anc Sci Life 32:173–177
Xu R, Zhang J, Yuan K (2010) Two new flavones from Tridax procumbens Linn. Molecules 15:6357–6364
Andriana Y, Xuan TD, Quy TN, Minh TN, Van TM, Viet TD (2019) Antihyperuricemia, antioxidant, and antibacterial activities of L. Foods 8. https://doi.org/10.3390/foods8010021
Sathishkumar P, Preethi J, Vijayan R, Mohd Yusoff AR, Ameen F, Suresh S et al (2016) Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesised silver nanoparticles using Coriandrum sativum leaf extract. J Photochem Photobiol B 163:69–76
Sonbol H, Ameen F, AlYahya S, Almansob A, Alwakeel S (2021) Padina boryana mediated green synthesis of crystalline palladium nanoparticles as potential nanodrug against multidrug resistant bacteria and cancer cells. Sci Rep 11:5444
Anisha GS, Padmakumari S, Patel AK, Pandey A, Singhania RR (2022) Fucoidan from marine macroalgae: biological actions and applications in regenerative medicine, drug delivery systems and food industry. Bioengineering (Basel) 9. https://doi.org/10.3390/bioengineering9090472
Sanjeewa KKA, Herath KHINM, Yang H-W, Choi CS, Jeon Y-J (2021) Anti-inflammatory mechanisms of fucoidans to treat inflammatory diseases: a review. Mar Drugs 19. https://doi.org/10.3390/md19120678
Pungle R, Nile SH, Makwana N, Singh R, Singh RP, Kharat AS (2022) Green synthesis of silver nanoparticles using the plant extract and screening of its antimicrobial and anticancer activities. Oxid Med Cell Longev 2022:9671594
Awwad AM, Salem NM, Abdeen AO (2013) Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Food Chem Toxicol 4:29
Zhang X-F, Liu Z-G, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17. https://doi.org/10.3390/ijms17091534
Asif M, Yasmin R, Asif R, Ambreen A, Mustafa M, Umbreen S (2022) Green synthesis of silver nanoparticles (AgNPs), structural characterization, and their antibacterial potential. Dose Response 20:15593258221088708
Das G, Patra JK, Debnath T, Ansari A, Shin H-S (2019) Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS One. 14:e0220950
Almatroudi A (2020) Silver nanoparticles: synthesis, characterisation and biomedical applications. Open Life Sci 15:819–839
Fatima F, Aldawsari MF, Ahmed MM, Anwer MK, Naz M, Ansari MJ et al (2021) Green synthesized silver nanoparticles using tridax procumbens for topical application: excision wound model and histopathological studies. Pharmaceutics 13. https://doi.org/10.3390/pharmaceutics13111754
Jain AS, Pawar PS, Sarkar A, Junnuthula V, Dyawanapelly S (2021) Bionanofactories for green synthesis of silver nanoparticles: toward antimicrobial applications. Int J Mol Sci 22. https://doi.org/10.3390/ijms222111993
Shameli K, Bin Ahmad M, Jazayeri SD, Sedaghat S, Shabanzadeh P, Jahangirian H et al (2012) Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Int J Mol Sci 13:6639–6650
Li P-J, Pan J-J, Tao L-J, Li X, Su D-L, Shan Y et al (2021) Green synthesis of silver nanoparticles by extracellular extracts from PJ01. Molecules 26. https://doi.org/10.3390/molecules26154479
Flieger J, Franus W, Panek R, Szymańska-Chargot M, Flieger W, Flieger M et al (2021) Green synthesis of silver nanoparticles using natural extracts with proven antioxidant activity. Molecules 26. https://doi.org/10.3390/molecules26164986
Mohanta YK, Panda SK, Syed A, Ameen F, Bastia AK, Mohanta TK (2018) Bio-inspired synthesis of silver nanoparticles from leaf extracts of Cleistanthus collinus (Roxb.): its potential antibacterial and anticancer activities. IET Nanobiotechnol. 12:343–348
Egodawaththa NM, Knight AL, Ma J, Knight DA, Guisbert E, Nesnas N (2022) Synthesis and characterization of ligand-stabilized silver nanoparticles and comparative antibacterial activity against. Int J Mol Sci 23. https://doi.org/10.3390/ijms232315251
Jiao G, Yu G, Zhang J, Ewart HS (2011) Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs 9:196–223
Ameen F, Al-Homaidan AA, Al-Sabri A, Almansob A, AlNAdhari S (2023) Anti-oxidant, anti-fungal and cytotoxic effects of silver nanoparticles synthesized using marine fungus Cladosporium halotolerans. Appl Nanosci 13:623–631
Mmola M, Roes-Hill ML, Durrell K, Bolton JJ, Sibuyi N, Meyer ME et al (2016) Enhanced antimicrobial and anticancer activity of silver and gold nanoparticles synthesised using Sargassum incisifolium aqueous extracts. Molecules 21. https://doi.org/10.3390/molecules21121633
Bold B-E, Urnukhsaikhan E, Mishig-Ochir T (2022) Biosynthesis of silver nanoparticles with antibacterial, antioxidant, anti-inflammatory properties and their burn wound healing efficacy. Front Chem 10:972534
Sharifi-Rad M, Pohl P, Epifano F, Álvarez-Suarez JM (2020) Green synthesis of silver nanoparticles using Delile. Root extract: characterization, antioxidant, antibacterial, and anti-inflammatory activities. Nanomaterials (Basel) 10. https://doi.org/10.3390/nano10122383
Acknowledgements
The corresponding author is grateful for the financial support provided by the ICMR research grant reference number IIRP-2023-8109 and all authors would like to acknowledge to the Saveetha Institute of Medical and Technical Sciences for providing research facilities encouragement successfully completion of this research.
Author information
Authors and Affiliations
Contributions
Conceptualization: MS; literature search: MA, PGS, RS, and DCS; methodology: MS, PGS, and RS; formal analysis: DSC and MS; original draft preparation: RS, DSC, and MS; manuscript review and editing: MS, AM, and PAM.; supervision: MS. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mathesh, A., Carmelin, D.S., Mohanprasanth, A. et al. Tridax procumbens–mediated one pot synthesis of silver-doped fucoidan nanoparticles and their antibacterial, antioxidant, and anti-inflammatory efficacy. Biomass Conv. Bioref. 14, 9887–9896 (2024). https://doi.org/10.1007/s13399-023-05265-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-05265-8