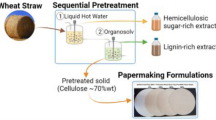
Abstract
Wheat straw and okra stalk were studied to evaluate their potential use for integrated lignocellulosic biorefining. Besides chemical pulp, a wide spectrum of value-added by-products were prepared by hot-water extraction of the feedstocks under varying conditions (140 °C for 60 and 240 min and 150 °C for 25 and 100 min) prior to sulfur-free soda-anthraquinone (AQ) pulping (NaOH charge 15 and 20% by weight on o.d. feedstock for wheat straw and okra stalk, respectively, with an AQ charge of 0.05% by weight on o.d. for both feedstocks). During the hot-water pre-treatment, the most significant mass removal, respectively, 12% (w/w) and 23% (w/w) of the initial wheat straw and okra stalk was obtained at 150 °C with a treatment time of 100 min. The hydrolysates were characterized in terms of pH and the content of carbohydrates (6–20% (w/w) of the initial amount), volatile acids (acetic and formic acids), and furans. The pre-treatment stage also facilitated the delignification stage, and, for example, the pulp yields (w/w), 57% (145 °C, 15 min, and kappa number 18) and 41% (165 °C, 180 min, and kappa number 32) were obtained for the pre-treated (150 °C, P200) wheat straw and okra stalk, respectively. Results clearly indicated that both non-wood materials were suitable for this kind of biorefining approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A modern forest biorefining approach includes the opportunity to produce, not only the main product (pulp fiber) but also the value-added green chemicals and various derivatives of cellulose and hemicelluloses; this integration results in an enhanced utilization of feedstocks and a decrease in greenhouse gas emissions [1,2,3]. One of the most promising biorefining techniques, mainly utilized for the partial recovery of carbohydrates, is based on the various pre-treatment processes [4, 5], such as hot-water extraction (HWE, “autohydrolysis”), conducted, for example, prior to delignification [6,7,8,9,10]. By pre-treatments, it is often possible to increase the reactivity of feedstock material leading to enhanced pulping performance and to recover potential by-streams (e.g., lignin and aliphatic carboxylic acids) [11]. However, in each case, a general prerequisite for finding a realizable design concept is that the presence of all the feedstock constituents is taken into account when planning target-oriented, economic processes for the manufacture of useful products [12].
Many studies have been carried out on a wide range of non-wood raw materials that could be used as alternative fibrous materials in many countries [12, 13]. Among the potential feedstocks, agricultural residues, such as wheat straw and okra stalk, offer great potential for lignocellulosic biorefineries due to their relatively high polysaccharide content and minor nutritional value for human consumption. Typically, the major problems in non-wood pulping include (i) silica-based depositions, which inhibit the recovery of chemicals from spent cooking liquor (black liquor) in alkaline pulping processes and (ii) a high amount of fines, which causes drainage problems. Many attempts have been made to solve these problems including HWE of feedstock prior to soda-anthraquinone (soda-AQ) pulping [14]. According to the base case designed by the National Renewable Energy Laboratory (NREL), pre-treatment has a great impact on overall process cost, accounting for up to 33% of the total [15].
HWE conditions are usually monitored by means of the “P-factor” (pre-hydrolysis factor), which is analogous to the “H-factor” used for similar purposes in chemical pulping [16]. It presents the combined overall effect of autohydrolysis time and temperature by a single numerical value calculated by the formula given in Eq. (1),
where t is time (h) and T is temperature (K). Creating a relationship between the materials removed from lignocellulosic feedstocks by autohydrolysis and the process variables, such as temperature and time, is essential to determine optimal operation conditions for the production of the desired lignocellulosic products and to allow a comparison of operations carried out under different conditions.
The aim of this work was to study the influence of HWE of wheat straw and okra stalk under varying conditions on the performance of soda-AQ pulping to clarify their potential for integrated biorefining. Huge amount of okra plant stem is discarded on the field annually after collecting vegetable, without proper utilization. So far, no studies have been conducted concerning HWE of okra stalk (after removing the pods), which actually is an important agricultural feedstock in certain countries. The total land area to produce okra has been reported to be almost 2 million hectares [17]. Recently, the maximum above ground biomass yield of okra whole plant is 120 t ha−1, and only stalk has been shown to be as high as 73 t ha−1 [18]. On the other hand, common wheat is widely cultivated for its seed (a cereal grain), and wheat straw was included in this study as a “reference feedstock.” The effect of different treatment conditions on the chemical composition of HWE hydrolysates was also investigated, and the potential utilization of these hydrolysates as feedstocks for biorefining was briefly discussed.
2 Materials and methods
2.1 Raw materials
Wheat straw (Triticum aestivum) of Finnish origin and okra stalk (Abelmoschus esculentus) of Pakistani origin were used in the laboratory-scale HWE experiments. The used wheat straw was manually screened, and straw samples having leaves, nodes, and other visible impurities were removed. In the case of okra stalk, only nodes were removed. The air-dried wheat straw and okra stalk were cut into a length range from 3 to 5 cm. The untreated and hot-water-extracted feedstocks were then used for the laboratory-scale alkaline cooking experiments.
2.2 Hot-water pre-treatments
HWEs were carried out in 1.25-L rotating stainless steel reactors (heated in a decene bath; CRS Autoclave System 420, Stenkullen, Sweden, Fig. 1S in supplementary files), each charged with 80 g of oven-dried (o.d.) raw material. Pre-treatments were performed at maximum temperatures of 140 and 150 °C having treatment times of 60 and 240, and 25 and 100 min, respectively (Table 1). A heating-up time of 30 min was added to the total treatment times. The liquor-to-raw material ratio was 5 L kg−1. At the end of each pre-treatment, reactors were removed from the oil bath and cooled rapidly in cold water. The hydrolysates were separated from the pre-treated feedstocks by filtration bags, and after measuring the pH, they were stored in a freezer for further analyses.
2.3 Soda-AQ cooks
Laboratory-scale cooking experiments (soda-AQ) were performed with the same reactors that were used for HWEs. One untreated sample from both wheat straw and okra stalk as a reference and two HWE samples treated at 140 °C (P-factor 50) and at 150 °C (P-factor 200) were pulped. Each reactor was loaded with 80 g of raw material, and an AQ charge of 0.05% (w/w) on o.d. feedstock was used. Effective alkali (EA) charge was 15% by weight on o.d. feedstock for wheat straw and okra stalk 1 and 20% by weight on o.d. feedstock for okra stalk 2, respectively, using liquor-to-feedstock ratio 5 L kg−1. At the end of each cook, reactors were removed from the oil bath and cooled rapidly in cold water. The black liquor (BL) was separated from the pulp by pressing and stored in a freezer. The employed cooking parameters and experimental design are presented in Table 2.
The pulp obtained was thoroughly washed with water, and the total pulp yield was calculated on the basis of o.d. initial feedstock. Pulp samples were screened with a 0.2-mm laboratory screening device (a Somerville-type apparatus from Serlachius Oy, Mänttä, Finland), and the amount of “process yield” (HWE + pulping) was determined by taking into account the material removed during HWE and the amount of rejects during pulp screening.
2.4 Analytical determinations
2.4.1 Raw materials
Sampling and preparation of raw materials for compositional analyses were performed according to TAPPI standards T257 cm-02 and T264 cm-07. Dry mass of feedstocks, pre-treated raw materials, and pulps were determined by drying a sample overnight in an oven at 105 °C. Extractives were analyzed using TAPPI standard 280 pm-99. The content of lignin in the extractive-free untreated feedstocks was gravimetrically determined as the sum of “acid-insoluble Klason lignin” and “acid-soluble lignin” according to TAPPI standard T222 om-98. Acid soluble lignin was measured by using a Beckman DU 640 UV/Vis spectrophotometer (Beckman Industries Inc., Fullerton, CA, USA) according to the TAPPI UM 250 method at wavelength 205 nm; the absorptivity value was 110 L g−1 cm−1 [19]. The content of different monosaccharides (i.e., arabinose, galactose, glucose, mannose, and xylose) in Klason hydrolysates was analyzed with a Dionex (Dionex Corp., Sunnyvale, CA, USA) high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) [20]. A Dionex CarboPac PA-1 column (250 mm × 4 mm inner diameter (i.d.)) was utilized for the separation at a flow rate of 1.0 mL min−1. Post-column alkali (300 mM NaOH) addition was employed at a flow rate of 0.2 mL min−1 to enhance the performance of PAD. The peak identification and the mass-based calibrations between an internal standard (l-fucose (Fluka Chemical Corp., Seeltze, Germany)) and each monosaccharide were based on separate runs with model monosaccharides (all from Fluka Chemical Corp., Seeltze, Germany). The content of the carbohydrates in acid hydrolysates was calculated and reported as anhydro forms of the measured monosaccharides. In addition, the yield decrease in the monosaccharides (determined separately) during acid hydrolysis caused by different side reactions (e.g., formation of furans) was taken into account. The chemical composition of the non-wood materials is presented in Table 3.
2.4.2 Hot-water extractions
The total content (after sulfuric acid hydrolysis) of different monosaccharides (i.e., arabinose, galactose, glucose, mannose, and xylose) and free monosaccharides in HWE hydrolysates were analyzed with a Dionex (Dionex Corp., Sunnyvale, CA, USA) HPAEC-PAD as described in Sect. 2.4.1.
Volatile acids (i.e., acetic and formic acids) in the HWE hydrolysates were determined using a Dionex IC25 ion chromatograph (IC) [21]. A Dionex IonPac AS 11-HC analytical column (250 mm × 4 mm i.d.) was used at a flow rate of 1.0 mL min−1. Gradient elution was performed by an EG40 eluent generator (KOH/ultra-high purity water). The chromatographic peaks were identified based on the model substances sodium acetate and sodium formate (Sigma-Aldrich, St. Louis, MO, USA).
Furanoic compounds (furfural and 5-(hydroxymethyl)furfural (HMF)) formed during HWE were analyzed by a Hewlett Packard (Palo Alto, CA, USA) 1100 series HPLC equipped with a Phenomenex (Phenomenex, Torrance, CA, USA) Gemini C18 column (100 mm × 4.6 mm i.d., with a particle size of 3 μm). The injection volume was 25 μL, and the detection wavelength of the diode array detector (DAD) was 280 nm. Ultra-high quality water/acetonitrile (ACN from J.T. Baker, Deventer, The Netherlands) and pure ACN were chosen as the eluents. The eluent flow rate was 0.8 mL min−1, and a gradient program according to ACN from 5 to 80% in 12 min was used for the elution. The chromatographic peaks were determined based on the model substances furfural and HMF (both from Sigma-Aldrich, St. Louis, MO, USA).
2.4.3 Pulps and soda-AQ black liquors
Screened pulp yields were measured according to TAPPI standard T274 sp-04. The kappa number from the pulps was determined according to TAPPI standard 236 cm-85. The residual alkali of BLs was determined by titrating the diluted and carbonated BL sample to pH 11.5 with 1 M hydrochloric acid solution according to the KCL procedure (number 67a:87). The amount of lignin dissolved during the cooks was calculated based on the difference of lignin contents of the original feedstock and cooked pulp. Volatile acids (see above) in the BLs were analyzed by means of the anion exchange method (Dionex IC) described in Sect. 2.4.2 [21].
3 Results and discussion
3.1 Removal of material during HWEs
The comparison of the effect of HWE on the dry mass yield of wheat straw and okra stalk is presented in Table 1. As can be seen, the higher the P-factor of the pre-extraction process, the higher the mass loss. This material loss in wheat straw during pre-treatment varied from 5.8% (w/w) in the mildest condition (at 140 °C for 60 min) to 12% (w/w) in the harshest condition (at 150 °C for 100 min) and for okra stalk from 14.4% (w/w) (at 140 °C for 60 min) to 23.3% (w/w) (at 150 °C for 100 min). Thus, these results indicated that an increase in the P-factor was proportional to the loss in mass and were in agreement with those reported earlier by Paredes et al. [22]. When aiming at reasonable pulp yields without significant losses in pulp quality properties, the targeted mass losses of 10–15% (w/w) in the HWE stage were obtained at higher P-factors for wheat straw than those for okra stalk. This difference in mass loss between wheat straw and okra stalk clearly suggested that okra is more sensitive to HWE. This increasing extraction efficiency was probably mainly due to the more porous structure of okra stalk than that of wheat straw since their chemical compositions were almost the same [23].
3.2 Hot-water extraction hydrolysates
3.2.1 Carbohydrates
As the HWE conditions became harsher, the formation of mono-, oligo-, and polymeric carbohydrates increased as presented in Tables 1S and 2S (see supplementary files). The amount of total carbohydrates in hydrolysates from okra stalk was almost four times higher than those in the corresponding hydrolysates from wheat straw. An increase in the content of carbohydrates of okra hydrolysates might be due to the partial dissolution of “fluffy material” in the inner stem of okra stalk, which was mainly composed of carbohydrate substances. Figure 1 shows the content of total carbohydrates in the hydrolysates compared to the content of carbohydrates in the initial raw materials. Typically, the highest content of total carbohydrates dissolved both from wheat straw and okra stalk was obtained with a P-factor of 200 (at 150 °C, P-factor 200) which accounted for 6 and 20%, respectively. In addition, it was noted that okra stalk behaved almost like softwood, and in these cases, the content of total carbohydrates in both hydrolysates was almost the same [24]. It could be concluded that the suitability of these carbohydrates, for example, after further enzymatic hydrolysis for the production of a wide-range of platform chemicals (i.e., alcohols, acids, and other products) by fermentation is high [12].
3.2.2 Volatile acids and liquor pHs
Figure 2 shows the content of volatile acids in the hydrolysates. The content of these acids increased along with temperature and P-factor; this was mainly due to the deacetylation of hemicelluloses, but partly also due to other enhanced degradation reactions [8, 25]. Thus, the HWE stage led to unwanted side reactions at elevated temperatures similarly to acid hydrolysis, which generally lowered the overall yield recovery of hemicelluloses [26, 27]. In general, higher amount of acetic acid was formed from okra than from corresponding wheat straw samples. These volatile acids are well known and widely utilized industrial chemicals which can be easily separated by distillation and utilized as well-known industrial chemicals.
The pH of the hydrolysates from the okra stalk (from 4.4 to 4.6) was lower when compared to that of the wheat straw liquors (from 5.0 to 5.6) (Table 1). The pH drop was mainly due to the self-catalyzed acidic hydrolysis of acetyl groups (deacetylation) present in hemicelluloses as well as to the formation of formic acid (i.e., the total amount of volatile acids). The formation of these organic acids also contributed to the cleavage of lignin-carbohydrate bonds, particularly typical for non-woods and promoted the further removal of hydrolysable hemicelluloses [6, 25].
3.2.3 Furans
Figure 3 shows the amount of furans in the wheat straw and okra stalk hydrolysates. The formation of organic acids and the decrease in pH contributed to the partial degradation and removal of hemicelluloses, which were further hydrolyzed to mono- and oligosaccharides [24]. In addition, due to the acidic pre-treatment conditions, some monosaccharides were further degraded into furans [8]. The results indicated that the amount of both HMF (formed from hexosans like glucomannan and cellulose) and furfural (formed from pentosans like xylan and arabinan) increased with the increase in P-factor; the highest content of furans formed from both feedstocks was obtained after harsher pre-treatments conducted at 150 °C with a P-factor of 200. It was also noted that the content of HMF in wheat straw hydrolysates was distinctly lower than that of furfural and that the formation behavior of these components was the opposite in the okra hydrolysates (i.e., HMF was the dominant furanoic compound in the okra hydrolysates). Different carbohydrates that were dissolved during the autohydrolysis from each feedstock could explain these differences; in okra hydrolysates, the main dissolved carbohydrates were hexoses (i.e., glucose, mannose, and galactose), whereas in the wheat straw hydrolysates, pentoses (i.e., xylose and arabinose) were the dominant ones. Additionally, the amount of furans in the hydrolysates from okra stalk was roughly ten times higher when compared to the corresponding wheat straw liquors. This was in agreement with the observation that also the content of dissolved carbohydrates (i.e., the source for furanoic components) was significantly higher in the okra hydrolysates. In some fermentation applications, furans may act as inhibitors and their selective removal should be carried out [24].
3.3 Soda-AQ pulping
The pre-treated and untreated wheat straw samples resulted in relatively good pulp yields (Fig. 4) during the soda-AQ pulping (EA 15% (w/w), 145 °C, and 15 min, see Table 2), while under these conditions, okra stalk 1 samples were not defibrated at all as indicated by a very low screened yield in Fig. 4. In the case of wheat straw, the total process yields (HWE + pulping) were about 50% (w/w). It should be pointed out that in the case of the pre-treated feedstocks, the pulp yield increased slightly with the increase in temperature and P-factor, but the total process yield decreased to some extent when the effect of HWE was taken into account.
In order to get the pulp yield for okra stalk comparable to that for wheat straw, the treatment conditions for okra stalk 2 had to be modified (EA 20% (w/w), 165 °C, and 180 min); the maximum yield obtained was 39% for the untreated okra as shown in Fig. 4. In general, the process yield of okra stalk pulp was lower when compared to that of wheat straw pulp because more material was lost from okra during HWE.
The difference in yields also indicated distinct morphological differences between wheat straw and okra stalk. The kappa number of wheat straw pulps was in the decreasing order (Fig. 5) W/140/P50 > W/150/P200 > W/Untreated/Ref and for okra stalk 2 pulps OK/150/P200 > OK/Untreated/Ref > OK/140/P50. It was noteworthy that under the same conditions (150 °C/P200), the lowest and the highest kappa number for the hot-water-extracted wheat and okra, respectively, was obtained. The difference in kappa number was probably due to differences in lignin structures and, on the other hand, due to the partial and selective redepositing of lignin on fiber surfaces during pre-treatment [28].
The residual alkali, mainly representing the total amount of neutralized aliphatic carboxylic acids (acetic and formic acids as well as various hydroxy acids) formed during delignification [12], was clearly lower in the case of okra stalk BLs indicating higher formation of organic carboxylic acids during the cooking when compared to the wheat straw cooks. Figure 6 shows the content of volatile acids (acetic and formic acids) in wheat straw and okra stalk 2 BLs. In the wheat straw BLs, the content of acetic acid increased with the increase in pre-treatment temperature, most likely indicating the enhanced removal of acetyl groups from hemicelluloses under harsher pre-treatments. The lowest content of formic acid formed by various degradation reactions of carbohydrates was determined from the BLs originating from the untreated feedstock. The increased formation of formic acid can be explained by the formation of new reducing end groups to the carbohydrate chains caused by the pre-treatments and hence, enhanced peeling reactions taking place in the subsequent pulping. In the okra case, the amount of volatile acids in BLs was almost the same for all feedstock materials but significantly higher than for the wheat straw feedstocks. The amount of lignin in BLs increased with the increase in pre-treatment temperature as shown in Fig. 7. The amount of lignin in wheat straw BL was more than that of okra stalk 2. The highest lignin content was 45 g L−1 at 150 °C/P200 for wheat straw and 35 g L−1 at 140 °C/P50 for okra stalk. The increase in BLs can be explained by the increase in porosity in the structure of feedstock materials during pre-treatments, and hence, an enhanced dissolution of lignin occurred with the increase in pre-treatment temperatures and subsequent pulping.
4 Conclusions
The effect of HWE on the soda-AQ pulping of wheat straw and okra stalk was studied. During the first phase (HWE), the mass removal increased with the increasing treatment temperature and time; besides the partial dissolution of hemicelluloses (in the forms of mono-, oligo-, and polysaccharides), the formation of some monomeric degradation products, volatile acids and furans, was observed. The highest mass removals from feedstocks were 12.0 (w/w) and 23.3% (w/w) for wheat straw and okra stalk, respectively.
In the second stage, the pre-treated feedstocks were pulped with soda-AQ method for producing pulp and soluble organic materials (sulfur-free lignin and various carbohydrate-derived degradation products). The highest cooking yields obtained were 57 (w/w) and 41% (w/w) for the pre-treated wheat straw and okra stalk, respectively. Results indicated that HWE stage prior to the delignification had a significant effect on the subsequent soda-AQ pulping. It can also be concluded that the biorefining approach described in this study creates promising possibilities for maximizing the utilization of the feedstocks and simultaneously offering a wide range of dissolved organic components (i.e., carbohydrates and organic acids) for manufacturing various value-added biomass-derived platform chemicals.
References
Mendes CVT, Carvalho MGVS, Baptista CMSG, Rocha JMS, Soares BIG, Sousa GDA (2009) Valorisation of hardwood hemicelluloses in the kraft pulping process by using an integrated biorefinery concept. Food Bioprod Process 87:197–207
Martin-Sampedro R, Eugenio ME, Moreno JA, Revilla E, Villar JC (2014) Integration of a kraft pulping mill into a forest biorefinery: pre-extraction of hemicellulose by steam explosion versus steam treatment. Bioresour Technol 153:236–244
Lehto JT, Alén RJ (2015) Chemical pretreatments of wood chips prior to alkaline pulping—a review of pretreatment alternatives, chemical aspects of the resulting liquors, and pulping outcomes. Bioresources 10:8604–8656
Kumar P, Barrett DM, Delwiche MJ (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729
Gong C, Goundalkar MJ, Bujanovic BM (2012) Evaluation of different sulfur-free delignification methods for hot-water extracted hardwood. J Wood Chem Technol 32:93–104
Carvalheiro F, Duarte LC, Gírio FM (2008) Hemicellulose biorefineries: a review on biomass pretreatments. J Sci Ind Res 67:849–864
Chirat C, Lachenal D, Sanglard M (2012) Extraction of xylans from hardwood chips prior to kraft cooking. Process Biochem 47:381–385
Jönsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16
Akhtar N, Gupta K, Goyal D, Goyal A (2015) Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ Prog Sustain Energy 35:489–511
Sánchez ÓJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol 99:5270–5295
Zhu JY, Pan X, Zalesny RS Jr (2010) Pretreatment of woody biomass for biofuel production: energy efficiency, technologies, and recalcitrance. Appl Microbiol Biotechnol 87:847–857
Alén R (2011) Principles of biorefining. In: Alén R (ed) Biorefining of forest resources. Paper Engineers’ Association, Helsinki, pp 55–114
Jahan MS, Rukhsana B, Baktash MM, Ahsan L, Fatehi P, Ni Y (2013) Pulping of non-wood and its related biorefinery potential in Bangladesh: a review. Curr Org Chem 17:1570–1576
Jahan MS, Shamsuzzaman M, Rahman MM, Moeiz SMI, Ni Y (2012) Effect of pre-extraction on soda-anthraquinone (AQ) pulping of rice straw. Ind Crop Prod 37:164–169
Lynd LR, Elamder RT, Wyman CE (1996) Likely features and costs of mature biomass ethanol technology. Appl Biochem Biotechnol 57:741–761
Tunc MS, van Heiningen ARP (2009) Autohydrolysis of mixed southern hardwoods: effect of P-factor. Nord Pulp Pap Res J 24:46–51
FAOSTAT (Food and Agricultural Organization of the United Nations Production Statistics), (http://fao.org), (2017) Accessed on 13 June 2017
Baw AO, Gedamu F, Dechassa N (2017) Effect of plant population and nitrogen rates on growth and yield of okra [Abelmoscus esculentus (L). Moench] in Gambella region, Western Ethiop. Afr J Agric Res 12:1395–1403
Swan B (1965) Isolation of acid-soluble lignin from the Klason lignin determination. Svensk Papperstidn 68:791–795
Pakkanen H, Alén R (2013) Alkali consumption of aliphatic carboxylic acids during alkaline pulping of wood and non-wood feedstocks. Holzforschung 67:643–650
Käkölä JM, Alén RJ, Isoaho JP, Matilainen RB (2008) Determination of low-molecular-mass aliphatic carboxylic acids and inorganic anions from kraft black liquors by ion chromatography. J Chromatogr A 1190:150–156
Paredes JJ, Jara R, Shaler SM, van Heiningen A (2008) Influence of hot water extraction on the physical and mechanical behavior of OSB. Forest Prod J 58:56–62
Kleen MA, Liitiä TM, Tehomaa MM (2011) The effect of the physical form and size of raw materials in pressurized hot water extraction of birch. In The proceedings of the 16th international symposium on wood, fiber and pulping chemistry (16th ISWFPC). Tianjin, China, pp 1013–1018
Lehto J (2015) Advanced biorefinery concept integrated to chemical pulping (doctoral thesis). Laboratory of Applied Chemistry, University of Jyväskylä, Jyväskylä
Zhang S, Yang H (2011) Effect of hot-water pre-extraction on alkaline pulping properties of wheat straw. Adv Mater Res 236:1174–1177
Yoon SH, van Heiningen A (2008) Kraft pulping and papermaking properties of hot-water pre-extracted loblolly pine in an integrated forest products biorefinery. TAPPI J 7:22–27
Tunc MS, Lawoko M, van Heiningen ARP (2010) Understanding the limitations of removal of hemicelluloses during autohydrolysis of a mixture of southern hardwoods. Bioresources 5:356–371
Arayaa F, Troncosob E, Mendoncab RT, Fareera J, Rencoretd J, Del Rio JC (2015) Structural characteristics and distribution of lignin in eucalyptus globulus pulps obtained by a combined autohydrolysis/alkaline extraction process for enzymatic saccharification of cellulose. J Chil Chem Soc 60:2954–2960
Acknowledgements
This study has been supported by the Doctoral Program in Chemistry, University of Jyväskylä. Additionally, financial support from the Finnish Cultural Foundation and Maj and Tor Nessling Foundation (Joni Lehto) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ullah, S., Pakkanen, H., Lehto, J. et al. A comparable study on the hot-water treatment of wheat straw and okra stalk prior to delignification. Biomass Conv. Bioref. 8, 413–421 (2018). https://doi.org/10.1007/s13399-018-0306-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-018-0306-x