Abstract
The booming consciousness of the problems associated with biodegradable solid waste generation has driven the globe for the reutilization of organic waste wherever it is generated, lowering trash volumes and transportation costs. The present study deals with the zero waste approach that endorses the fostering of sustainable long-term socio-economic and environmental benefits by transforming biodegradable solid waste into biofertilizers. A novel consolidated bioprocessing approach is adopted which in particular relates to the hydrolysis of biodegradable municipal solid waste by hydrolytic enzymes produced by Aspergillus niger S-30 and the growth of biofertilizer non-pathogenic strain of Klebsiella pneumoniae AP-407 in nutrients present in the hydrolysate to prepare liquid biofertilizer and carrier-based solid biofertilizer formulations with 1.03 × 1012 cfu/ml and 1.03 × 1012 cfu/g, respectively. Furthermore, the formulations significantly enhanced the % oxidizable organic carbon, available phosphate (P2O5), available potassium (K2O), ammonical nitrogen (NH3-N), and nitrate nitrogen (NO3-N) in soil and chlorophyll content of plant leaves of Brassica juncea. It is further appreciated that the process is economically attractive as no exogenous enzyme loadings are required, no exogenous carrier substance is added, and the entire process is carried out in a single fermenter vessel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The organic residue of biodegradable solid waste from human, agricultural, and industrial establishments is a serious threat not only to the environment or natural resources but also to the economy. All over the globe, various approaches to mitigate the obstacles associated with biodegradable solid waste disposal have been instigated and commercialized. A prevailing priority is to energize the transition toward a circular economy. The “zero waste approach” endorses the fostering of sustainable long-term socio-economic and environmental benefits [1]. Rising urbanization, population growth, and increased rates of food consumption have caused significant increases in daily kitchen waste output. Several countries, notably India, Australia, Mexico, the USA, and Korea, have followed suit, creating between 624 and 3500 tonnes of kitchen waste every year [2]. Presently, garbage disposal technologies separate kitchen waste (KW) from municipal solid waste (MSW) before it is disposed of in landfills or burned in open fields. Since KW is mostly composed of organic material contained inside MSW, it has been processed using some of the biomass recycling methods [3]. The zero-value and predominant organic nature of kitchen waste makes it a better contender to endorse the zero-waste approach.
Heightened awareness of the problems associated with the management of biodegradable solid waste, research on the transformation of food and agricultural residue into biofuel, biochemical, and biopolymers has received growing deliberation. The significance of developing biofertilizers from biodegradable solid waste has been overlooked in contrast to biochemical and biofuel production. Substituting synthetic chemical fertilizer with biofertilizer derived from biodegradable solid waste would axe the use of synthetic chemical fertilizers which not only reduce the environmental impact of solid waste and directly benefit agronomy [4].
In 2019, India was the world’s second-largest consumer and producer of synthetic chemical fertilizers. Chemical fertilizer consumption in India during 2020–2021, excluding single super phosphate (SSP), was 62.98 million tons, representing an increase of more than 82.5% since 2000–2001. Fertilizer consumption per hectare for 2020–2021 is 161 kg, representing a 75% increase since 2000–2001 [5]. The massive cost of fertilizer production, as well as the environmental pollution caused by the use of chemical fertilizers, necessitates the use of alternative sources, particularly biofertilizers. The increasing cost and uneven use of chemically formulated fertilizers stress the call to explore the potential of bioinoculants for saving fertilizer nitrogen (N) and phosphorus (P). Irrespective of formulation and doses, applications of biofertilizers in the soil are competitive with chemical formulations and thus improve various biological properties. In this context during the past few years, the number of plant growth-promoting bacteria (PGPB) that have been identified has seen a substantial increase. Species of bacteria like Alcaligenes, Arthrobacter, Aspergillus, Azospirillum, Azotobacter, Bacillus, Burkholderia, Enterobacter, Klebsiella, Pseudomonas, and Serratia have been reported to significantly enhance plant growth [6–14].
The incorporation of compost, an organic fertilizer, and nitrogen: phosphorus: potassium (NPK) (20:10:10) which is a chemical fertilizer, and using them as a blend not only enhances the growth of plants but also reduces our dependability on chemical fertilizers by some proportion [9]. In 2020–2021, India generated around a total of 134,323 tonnes of carrier-based biofertilizers, and liquid biofertilizer production in India was approximately 26,442 kilolitre (kl) [5]. Keeping in view the need for scientific management of solid waste residues and the significance of biofertilizers, the current study is designed to resolve the environmental complications, resulting from the dumping and burning of various solid waste residues and the indiscriminate use of chemical fertilizers. The key focus of the study is to explore the organic fraction of solid waste as a potential feedstock for its transformation into liquid biofertilizer by simultaneous depolymerization of organic residue for the release of sugars and production of liquid biofertilizer. Another approach is the utilization of left-out compost as an efficient support for the preparation of carrier-based biofertilizer consortium. Integrating microorganisms and organic fertilizers into soil-plant systems is considered an essential strategy for solving challenges connected with the overuse of chemical fertilizers and pesticides. Traditional microorganism-bearing fertilizers like compost or animal manure should be recognized separately from formulated biofertilizers. Traditional microorganism-bearing fertilizer includes a diverse variety of well-studied and classified microorganisms, including harmful bacteria that can be difficult to manage in some situations. Biofertilizer products/formulations, on the other hand, often comprise one or more microbial cultures with verified quality and cell number and, in some cases, regulated release following introduction into the soil-plant system making them environmentally/crop friendly [15, 16]. Continuation to the preceding statement in the present study, Aspergillus niger S-30 has the potential to secrete multiple carbohydrases in addition to various plant growth-promoting traits including phosphate solubilization, siderophore production, and IAA production [9, 10] and non-pathogenic strain of Klebsiella pneumoniae AP-407 producing multiple plant growth promoting traits was selected for biofertilizer production [11–14].
Consolidated bioprocessing (CBP) incorporates the generation of enzymes, saccharification, and fermentation of released sugars into a single process. The primary motive for employing this strategy is to reduce costs while increasing efficiency. The savings from employing CBP can be enormous because the enzyme costs in other procedures might be very expensive. CBP is a potential technology that can minimize the number of unit operations while simultaneously lowering the process’s overall capital cost. In conventional technologies, the ultimate simple sugars often inhibit the saccharification process, however, in CBP; fermentation converts these products to value-added compounds before they become inhibitive to hydrolysis. CBP bacteria do not require foreign saccharifying enzymes because they produce cellulolytic and hemicellulolytic enzymes for lignocellulose degradation, resulting in significant cost savings. CBP systems cut down the number of unit operations (i.e., fewer reactor vessels), lowering maintenance and capital expenses [17–20]. Starting with the production of the inoculum and continuing through the fermentation phase, all downstream stages, including product formulation and packing, are performed under sterile circumstances, and as a result, these biotechnological goods ought to be contaminant-free [16].
A rapid and efficient consolidated bioprocess has been developed for the disintegration of various polymeric organic compounds in the waste for the release of simple sugars and amino acids to support the growth of the natural variants of microbial strains capable of atmospheric N-fixation, mobilizing P and K besides producing plant growth promoting hormones. This yielded a liquid supernatant and a solid residue with both containing the novel biofertilizer organism having all the above-cited traits thus yielding the liquid and carrier-based biofertilizer formulations. Since, the expenses of exogenous enzymes necessary for composite kitchen waste hydrolysis are eliminated, consolidated bioprocessing offers less energy-demanding technology and a potentially low-cost path for the synthesis of biofertilizers. Furthermore, feedstocks for CBP do not need to be completely saccharified with complicated and expensive pretreatment technologies, massive quantities of exogenous enzymes, which is a key bottleneck in lowering the net cost of hydrolysis of biodegradable vegetable and composite kitchen waste for biofertilizer preparation is kept low. The process, when used at an industrial scale, will not only reduce the burden of the cost of nutrients for the preparation of different types of biofertilizer formulations but will also provide a scientific solution for the management of biodegradable municipal solid waste (BMSW) residues primarily kitchen waste in addition to reducing the dependency on synthetic chemical fertilizers.
2 Materials and methods
2.1 Experimental site
The entire experiment was carried out at the Department of Microbiology, South Campus, Panjab University, Chandigarh. The latitude of Chandigarh, India is 30° 44′14 N and the longitude is 76° 47′ 14 E. The Department of Microbiology has DBT, a Government of India-funded Pilot Scale Fermentation Facility in Laboratory number 407. The plant growth experiment was performed from November 2022 till the end of December 2022. In Chandigarh, November is the warmest month of the post-monsoon fall, with temperatures averaging spanning from 13.5 to 25.8 °C. In November, the mean high temperature in Chandigarh is 25.8 °C, while the average minimum temperature is 13.5 °C. Temperatures in Chandigarh in December are pleasant, with lows of 10 °C and highs of 21 °C. The weather in Chandigarh in December can range from cool, pleasant days to a few wet days, but rarely more.
2.2 Microorganisms
The fungal strain Aspergillus niger S-30 (MTCC 25569) was selected by assessing its multiple carbohydrase-producing potentials comprising of cellulase, hemicellulase, pectinase, and amylase by solid-state fermentation on composite kitchen waste as reported earlier by. The non-pathogenic bacterial strain of Klebsiella pneumoniae AP-407 (MTCC 25568) isolated from the natural biodiversity of Panjab University, Chandigarh for its ability of nitrogen fixation, HCN production, phosphate solubilization, potassium mobilization, siderophore production, ammonia, and IAA production.
2.3 Quantitative estimation of multiple carbohydrases on composite kitchen waste
Five grams of crushed composite kitchen waste was moistened with 97.5 ml of distilled water in 250 ml Erlenmeyer flasks (in triplicate). The flasks were sterilized by autoclaving, cooled to room temperature, and then inoculated with 2.5 ml liquid suspensions of the bacterial isolates and fungal strains, respectively. Under submerged shaking conditions, fungal flasks were incubated at 28 °C for 96 h.
The enzyme(s) were extracted by keeping the flasks on a rotary shaker (Remi Scientific Instruments, India) at 150 rpm for 30 min at room temperature. The flask contents were minced in a laboratory blender before being filtered through a metallic sieve of the 200-micron mesh size. The remaining solid left extract was pressed through muslin cloth with as much liquid as possible. For 10 min, the filtrate obtained was centrifuged at 10,000 rpm and 4 °C (Thermoscientific). At 50 °C and pH 4.0, supernatants from submerged-state cultures were tested for cellulases (exo-1,4-glucanase, endo-1,4-glucanase, and β-1,4-glucosidase), hemicellulases (xylanase, mannanase), pectinase, and glucoamylase. The yields were expressed in terms of IU/ml.
Cellulase enzyme complex was measured in terms of endo-β-1,4-glucanase, exo-β-1,4- glucanase, and β-glucosidase activities as described by Mandels et al. [21] using CMC, Whatman filter paper number 1 strips (1× 6 cm), salicin, respectively, as their substrates and the enzyme yields were expressed in terms of CMCase, FPase, and salicinase, respectively, by determining the μmoles of glucose liberated/min; hemicellulose complex in terms of endo-β-1,4-xylanase and endo-β-1,4- mannanase using xylan [22] and guar gum [23], respectively, by μmoles of xylose and mannose liberated/min; pectinase activity by using pectin as the substrate [24] for μmoles of galacturonic acid liberated/min; glucoamylase [25] activity as the amount of glucose liberated (for glucoamylase), using dinitrosalicylic acid reagent as described by Miller [26].
2.4 Bacterial characterization for plant growth-promoting traits
The bacterial strain capable of Klebsiella pneumoniae AP-407 producing multiple plant growth-promoting traits comprising nitrogen fixation, phosphate solubilization, potassium mobilization, HCN production, siderophore production, and IAA production was also isolated from the botanical garden of Panjab University, Chandigarh, India. To analyze plant growth-promoting traits, 24 h freshly cultivated bacterial culture with 106 CFU/ml was used.
The nitrogen-fixing ability of bacterial strain was identified by growing on nitrogen free medium (Jensen medium) [27]. For indole acetic acid (IAA) production, K. pneumoniae was grown in Luria-Bertani broth supplemented with 1 g L-tryptophan. The test tubes were inoculated by K. pneumoniae and incubated in a shaker incubator at 120 rpm for 3–4 days at 28 °C. Each test tube received 5 drops of Salowski’s reagent after incubation. The appearance of the cherry-red color confirms the production of IAA [11]. The level of IAA production was estimated as described by Bhardwaj et al. [12] for which 24 h grown K. pneumoniae was inoculated into 10 ml of sterile Luria Broth supplemented with 0.1% tryptophan. After 96 h of incubation at 37 °C in 1 ml culture supernatant 2 ml Salowski’s reagent was added. The amount of IAA produced was inferred from the standard curve by measuring optical density at 530 nm. For phosphate solubilization, Pikovskaya’s agar plates were streaked with K. pneumoniae followed by incubation at 28 °C for 4 days and observed for the formation of a clear halo zone around the bacterial colony [28].
To analyze the siderophore production, chrome azurol S agar plates were prepared by dissolving 60.5 mg chrome azurol S in 50 ml of water followed by the addition of 10 ml iron (III) solution (1 mM FeCl3. 6H2O, 10 mM HCl). This solution was slowly mixed with 0.073 g of hexadecyl trimethyl ammonium dissolved in 40 ml of water. K. pneumoniae was spot inoculated on the blue agar plates and incubated for 48–72 h at 28 °C. The change in color around bacterial colonies indicated the production of the siderophore [29]. For potassium mobilization, GYCaA medium was used as suggested by Bhattacharyya et al. [30], and bacterial culture was spot inoculated at the center of GYCaA medium plate. Inoculated plates were then incubated at 28 °C for 4 days and observed for the formation of a clear halo zone around the bacterial colony.
The hydrogen cyanide (HCN) production was analyzed by amending Luria Bertani agar plates with 4.4 g glycine L−1. Whatman filter paper 1 was soaked in 0.5% picric acid and 2% sodium carbonate solution followed by placing paper on the upper lid of Petri plates. K. pneumoniae streaked plates were wrapped with parafilm and incubated at 28 °C for 5 d. The change in filter paper color from yellow to orangish brown had excellent effects on HCN generation [31]. Ammonia production was observed following Amna et al. [32]. In each tube, a freshly grown 24-h culture of K. pneumoniae was inoculated in 10 ml of peptone water (15 g peptone water in 1000 ml). The test tubes were shaken at 28 °C for 2–3 days. Following incubation, each tube was supplemented with 0.5 ml of Nessler’s reagent. The change in the color of the medium from brown to yellow indicated that the bacteria were responding positively to ammonia generation.
2.5 Biocompatibility between Aspergillus niger S-30 and Klebsiella pneumoniae AP-407
The Aspergillus niger S-30 and Klebsiella pneumoniae AP-407 were checked for their biocompatibility with each other. The Klebsiella pneumoniae AP-407 was streaked on a nutrient agar plate and Aspergillus niger S-30 was point inoculated in the middle of the plate which was analyzed for the emergence of the inhibition zone. The presence of an inhibition zone indicates antagonistic behavior.
2.6 Biodegradable solid waste
The City Beautiful, Chandigarh with a lot of vegetation and diverse flora is lagging in biodegradable solid waste management. Site visits were made to various localities near the Panjab University campus, Chandigarh to get a brief assessment of biodegradable solid waste generation and disposal methods adopted by locals and authorities. The biodegradable solid waste sample comprising composite kitchen waste was collected from these sites and kept in cold storage facility (Blue Star Storekool, India) present in the Department of Microbiology, Panjab University, Chandigarh for further action.
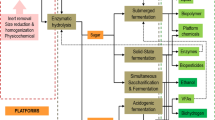
2.7 Consolidated bioprocessing for preparation of biofertilizer formulations
A natural variant of fungal strain Aspergillus niger S-30 capable of producing multiple hydrolytic enzymes including cellulases, hemicellulases, amylases, and pectinase was inoculated in thermally treated composite kitchen waste comprising of crushed vegetable, fruit, and other kitchen residues along with another bacterial strain of Klebsiella pneumoniae AP-407 having a broad range of plant growth promoting traits including N-fixation, P solubilization, and K mobilization in addition to the production of plant growth promoting hormones and incubated at 30 °C for 4 days. The whole process was carried out in a 100 l fermenter to which 20 kg of crushed biomass was added along with 55 l of tap water. The contents were steamed at 15 psi for 30 min and were inoculated with the 1 l spore suspension of Aspergillus niger S-30 and the 1 l liquid culture of Klebsiella pneumoniae AP-407 having 1 × 108 cfu/ml to make the total solid to liquid ratio 1:2.75 (between 1:2 to 1:3) after lowering the temperature of the biomass to 30 °C. The contents in the fermenter were continuously stirred at 200 rpm with temperature and pH kept at 30 °C and 6.5, respectively, for 4 days.
2.8 Physico-chemical and biological analysis during consolidated bioprocessing
Every 24 h, the physical variables for consolidated bioprocessing were assessed. After every 24 h, the biological and chemical analysis, which included enzymatic activity, organic carbon content, and microbial count, were performed on the residue which was collected each morning in a container. The chemical and biological parameters which were analyzed during consolidated bioprocessing are described with the standard method used in Table 1.
2.9 Separation of carrier and liquid biofertilizer formulations
After 96 h, the stable carrier and liquid biofertilizer formulation were separated. The content from the filtrate was filtered by passing through the sieve of 200-micron mesh size and the liquid filtrate was stored in glass bottles in a cold room facility available in the Department of Microbiology, Panjab University, Chandigarh till further use. The solid residue left out from liquid filtrate was taken in enamel plates followed by crushing in a laboratory blender after which carrier biofertilizer was packed in air-tight polythene bags and stored in a cold room facility. The contents from the 100 l fermenter led to the generation of a liquid component and a solid residue both containing a high count of biofertilizer organisms. For better comprehension of shelf life, the log CFU of biofertilizer formulation was examined for 10 months, with regular samples obtained from the stored carrier and liquid biofertilizer formulations. The additional purpose for analysis was to notice any form of contamination throughout their storage time.
2.10 Plant development assay
A plant growth system experiment was conducted for assessing the effect of the carrier and liquid biofertilizer formulations on the development of plants. The plant growth experiment was performed from November 2022 till the end of December 2022. The seeds of plants Brassica juncea were surface sterilized using 70% ethanol and further rinsed three times using sterile distilled water. Furthermore, the seeds were shade dried for 30 min, and later, all the respective seeds were sowed in separate pots having a diameter of 28 cm and depth of 20 cm filled with 2500 g of soil sterilized by autoclaving at 15 psi for 1 h. The biofertilizer was applied by soil treatment method for which seeds were initially sowed and after 2 h, the 2 g of carrier-based biofertilizer was blended in soil and 2 ml of liquid-based biofertilizer initially suspended in 100 ml of water followed by sprinkling on soil containing seeds. The same treatment was repeated on the 15th day after taking soil and plant samples. For each treatment, three replicate pots were maintained with a natural photoperiod (12 h) and watered with tap water for 45 days. Morphometric analyses of the host plant for the different treatments were assessed after 15, 30, and 45 days of sowing and on maturity for plant height (cm), shoot height (cm), root length (cm), plant fresh weight (g), plant dry weight (g), shoot fresh weight (g), root fresh weight (g), shoot dry weight (g), and root dry weight (g).
2.11 Estimation of chlorophyll
The chlorophyll content of leaves was analyzed after 45 days. One gram of finely chopped fresh leaves was suspended in 20 ml of 80% acetone. The supernatant was separated after the centrifugation for 5 min at 10,000 rpm. The process was repeated until the residue was colorless. The absorbance of the solution was taken at 645 nm and 663 nm against acetone. The concentrations of total chlorophyll (t), chlorophyll a, and chlorophyll b were calculated using the equation as described by Arnon [34]:
Chlorophyll t: 20.2(A645) + 8.02(A663)
Chlorophyll a: 12.7(A663) – 2.69(A645)
Chlorophyll b: 22.9(A645) – 4.68(A663)
2.12 Quantitative analysis of soil
The soil was tested for macro and micro-nutrients using a soil testing kit procured from Himedia, India for determining organic carbon in the soil in terms of % oxidizable organic carbon, available phosphate (P2O5), available potassium (K2O), ammonical nitrogen (NH3-N), and nitrate nitrogen (NO3-N) in the soil in terms of kg per hectare (kg ha−1).
3 Results
3.1 Time course of Aspergillus niger S-30 for production of multiple carbohydrases
The fungal strain Aspergillus niger S-30 capable of producing multiple carbohydrases on cheap substrate, i.e., mixed kitchen waste was selected as a strong contender for consolidated bioprocessing. The evolution of multiple hydrolytic enzyme activities of Aspergillus niger S-30 is depicted in Table 2. The enzyme activity is presented in terms of IU/ml which is defined as the amount of enzyme required to catalyze the conversion of 1 μmole of substrate per minute under specified enzyme assay conditions. Aspergillus niger S-30 showed that the maximum peak of CMCase was 2.35 ± 0.092 IU/ml, maximum FPase (0.82 ± 0.015 IU/ml) h and β-glucosidase (1.20 ± 0.065 IU /ml) after 48 h, respectively. Xylanase production showed maximum production (10.20 ± 1.12 IU/ml) after 48 h and mannanase production was maximum (4.50 ± 0.045 IU/ml) after 24 h. Pectinase peaked (3.15 ± 0.046 IU/ml) after 24 h. The production of glucoamylase gave the highest yield (3 ± 0.020 IU/ml) after 24 h.
3.2 Assessment of Klebsiella pneumoniae AP-407 for biofertilizer formulation preparation
The bacterial strain, Klebsiella pneumoniae AP-407, was isolated from the rhizospheric soil of healthy plants on the Panjab University campus. The Klebsiella pneumoniae AP-407 showed positive results for its nitrogen-fixing ability, HCN production, phosphate (P) solubilization, siderophore production, potassium (K) mobilization, ammonia production, and IAA production all depicted in Fig. 1. The nitrogen-fixing ability of Klebsiella pneumoniae AP-407 was identified by growth on nitrogen free medium (Jensen medium) indicating a positive result. The color of the filter paper was changed from yellowish to orangish brown, which resulted in increased HCN production. The formation of a halo zone observed around the bacterial colony revealed a favorable result for phosphate solubilization. The change in color of blue agar plates containing chrome azurol S and hexadecyl trimethyl ammonium to the yellowish confirmed presence of a carboxylate type of siderophore. The presence of a distinct halo zone around the bacterial colony indicates the existence of positive potassium mobilization activity. The shift in color of the broth from brownish to yellowish indicated that the bacterium was responding positively to ammonia generation. In the presence of 0.1% tryptophan, the creation of a cherry-reddish hue confirmed the generation of IAA, which was determined to be 82.45 g/ml.
3.3 Biocompatibility between Aspergillus niger S-30 and Klebsiella pneumoniae AP-407
The Aspergillus niger S-30 and Klebsiella pneumoniae AP-407 were streaked and incubated for 96 h. The Klebsiella pneumoniae AP-407 colonies manage to grow in the vicinity of Aspergillus niger S-30 with no inhibition zone which indicated the negative antagonistic behavior depicted in Fig. 2.
3.4 Physico-chemical and biological analysis during consolidated bioprocessing
The physicochemical properties of the altered dramatically as the consolidated bioprocess continued, and specific analogies could be formed seen between biological and physicochemical aspects. Table 3 indicates the evolution of enzyme levels during 96 h consolidated bioprocessing. CMCase (1.20± 0.07 IU/ml) and xylanase (4.85± 0.32 IU/ml) peaked after 72 h, whereas FPase (2.47± 0.13 IU/ml), β-glucosidase (1.15± 0.07 IU/ml), mannanase (1.70± 0.08 IU/ml), pectinase (1.20± 0.09 IU/ml), and glucoamylase peaked to 5.34± 0.38 IU/ml after 48 h of consolidated bioprocessing under specified temperature and pH conditions.
A positive correlation could be drawn between the utilization of sugars and the cell count of Klebsiella pneumoniae AP 407. After 24 h of consolidated bioprocessing, the total reducing sugar was reduced from 6.76 to 0.75 mg/ml, and the glucose level was reduced from 1.3 mg/ml to nil. Furthermore, the cell count of Klebsiella pneumoniae AP 407 increased from 3.46 × 106 to 3.46 × 108 cfu/ml within 24 h of consolidated bioprocessing which increase to 1.03 × 1012 cfu/ml after 96 h of consolidated bioprocessing depicted in Table 4. The liquid and carrier biofertilizer were prepared after 96 h of consolidated bioprocessing which was packed, sealed, and stored later on in a cold room facility available in the Department of Microbiology, Panjab University, Chandigarh.
3.5 Shelf life of biofertilizer formulations
The log CFU of biofertilizer formulation was observed throughout 10 months for a better estimation of shelf life. The final cell count of both liquid biofertilizer and carrier biofertilizer was observed to be 1.03 × 1012 cfu/ml and 1.03 × 1012 cfu/g before storage. The cell count of liquid biofertilizer reduces to 3.10 × 1011 cfu/ml while cell count of carrier biofertilizer reduces to 1.13 × 109 cfu/g after 6 months of storage. After 10 months of storage, the cell count of carrier biofertilizer showed some significant drop and was observed to be 1.5 × 106 cfu/g, whereas liquid biofertilizer showed a good shelf life and was carrying 2.2 × 109 cfu/ml. Figure 3 shows the trend of the shelf life of carrier and liquid biofertilizers throughout 10 months. There was no unwanted contamination observed during shelf life analysis throughout 10 months as the prepared formulations were packed and stored aseptically in a cold storage facility which validates the biofertilizer preparation to be contaminated free up to 10 months.
3.6 Plant development assay for validation of biofertilizer formulations
The liquid and carrier biofertilizer showed a significant positive response in enhancing various morphometric characteristics and yield of Brassica juncea. In the present experiment as depicted in Table 5, the plant height in carrier-treated biofertilizer was +4 cm more than in control and plant height was +8 cm more in liquid biofertilizer-treated Brassica juncea after 45 days. The shoot height was observed to be similar in both control and carrier biofertilizer-treated Brassica juncea, whereas it was +4 cm more in the case of liquid biofertilizer-treated Brassica juncea. Brassica juncea showed +4 cm more root length in the case of carrier biofertilizer and + 8 cm more root length in liquid biofertilizer-treated Brassica juncea as compared to the control set of Brassica juncea after 45 days.
After 45 days, the carrier biofertilizer-treated Brassica juncea showed +4 g more weight and liquid biofertilizer treated showed + 5 g more weight as compared to the control set. Similarly, plant dry weight was 0.90 ± 0.071 g, 1.90 ± 0.175 g, and 1.80 ± 0.175 g in control, carrier biofertilizer, and liquid biofertilizer treatment, respectively. Shoot fresh weight was 9.00 ± 0.890 g, 12.00 ± 0.955 g, and 13.50 ± 1.115 g in control, carrier biofertilizer, and liquid biofertilizer treatment, respectively. Shoot dry weight was 0.70 ± 0.065 g, 1.20 ± 0.105 g, and 1.20 ± 0.115 g in control, carrier biofertilizer, and liquid biofertilizer treatment, respectively. Root fresh weight was 1.00 ± 0.100 g, 2.00 ± 0.200 g, and 1.50 ± 0.140 g in control, carrier biofertilizer, and liquid biofertilizer treatment, respectively. Root dry weight was 0.20 ± 0.018 g, 0.70 ± 0.065 g, and 0.30 ± 0.025 g in control, carrier biofertilizer, and liquid biofertilizer treatment, respectively, as depicted in Table 6.
3.7 Influence of biofertilizer formulations on soil
The organic carbon in soil was determined in terms of % oxidizable organic carbon. The available phosphate (P2O5), available potassium (K2O), ammonical nitrogen (NH3-N), and nitrate nitrogen (NO3-N) in soil were determined in terms of kg per hectare (kg ha−1) depicted in Table 7. The % oxidizable organic carbon was 0.300–0.500, available phosphate (P2O5) was 22 to 56 kg ha−1, available potassium (K2O) was 112 to 280 kg ha−1, ammonical nitrogen (NH3-N) was low about 15 kg ha−1, and nitrate nitrogen (NO3-N) was nil on 0th day of sowing of Brassica juncea in soil.
The % oxidizable organic carbon reduced in the control set was in the range of 0.100–0.300 during the whole trial, available phosphate reduces after 30 days and was nil after 45 days, available potassium reduces after 45 days, ammonical nitrogen was maintained low about 15 kg ha−1 during whole experiment, and nitrate nitrogen was observed only after 30 days.
The % oxidizable organic carbon reduced in liquid biofertilizer treatment was in the range of 1.00–1.50 during the whole trial, available phosphate was above 73 kg ha−1 which only reduced after 45 days, available potassium was also maintained in the range of 112 to 280 kg ha−1, ammonical nitrogen was maintained about 73 kg ha−1, and nitrate nitrogen was available in medium to high range of 20 and 50 kg ha−1 during the whole trial. The % oxidizable organic carbon reduced in carrier biofertilizer treatment was in the range of 1.00–1.50 during the whole trial, available phosphate started reducing after 30 days and was observed to be between 56 and 73 kg ha−1, and available potassium was also maintained in the range of 112 to 280 kg ha−1, ammonical nitrogen was maintained about 73 kg ha−1, and nitrate nitrogen was available mostly in the high range of 50 kg ha−1 during the trial.
3.8 Influence of biofertilizer formulations on chlorophyll content
The highest chlorophyll content (a+b) of 83.7 ± 3.2 μg/ml was observed in liquid biofertilizer-treated Brassica juncea, followed by carrier-based biofertilizer 66.1 ± 2.3 μg/ml and control set of Brassica juncea 54.8 ± 2.6 μg/ml as depicted in Fig. 4.
4 Discussion
Among most underdeveloped or developing countries, biodegradable wastes from kitchens, vegetable and fruit markets, gardens and public parks, schools, institutes, and societies are disposed of by open dumping, burning, or landfilling. Esteban-Lustres et al. [35] emphasized improving the management of these steadily produced and globally available resources, and their commodification into novel and profitably fascinating product lines that will help the bioeconomy. The effective implementation of such an approach necessitates collaboration between academic institutions, industrial stakeholders, and government entities, and is dependent on the numerous aspects involved, including social, economic, environmental, and technological.
The significant expense of producing fertilizer, along with the pollution produced by the use of chemical fertilizers, necessitates the adoption of alternate sources, notably biofertilizers. Biofertilizer application in the soil, irrespective of formulation or dose, is competitive with chemical formulations and so enhances a wide variety of biological traits [8, 14]. CBP consolidates the production of enzymes, saccharification, and fermentation into a single process. The primary motivation for employing this method is to reduce costs while increasing efficiency. CBP systems minimize the number of unit operations in addition to lowering maintenance and capital costs [8, 17–19].
Based on the preceding, the present work proposes a novel method of transformation of biodegradable solid waste in its natural state into biofertilizer formulations by a natural variant of the fungal and bacterial strain. The fungal strain capable of producing multiple carbohydrases converts complex polysaccharides present in biodegradable solid waste into simple sugars which are utilized by bacterial strains having plant growth-promoting traits.
Researchers suggested that Aspergillus niger has immense potential that could co-produce multiple kitchen waste, rice straw, and de-oiled rice bran, respectively. Fungi due to their capacity to produce considerable amounts of non-complex cellulases and hemicellulases are more focused upon [36–38]. Furthermore, in the present study, the in-house formulation has a greater spectrum of enzymes on composite kitchen waste, including the whole cellulase system as well as xylanases, mannanases, pectinases, and amylases, making it unique.
The rhizosphere is the microbial hotspot, and so many rhizosphere microorganisms have favorable effects on plant development and health. Regarding their stress resistance and metal tolerance in soil, bacterial strains from the genera Klebsiella, Stenotrophomonas, Bacillus, and Serratia have a high potential for usage as plant growth promoters [39]. In the present study, K. pneumoniae exhibited a broad range of plant growth-promoting traits comprising its nitrogen fixation, HCN production, phosphate (P) solubilization, siderophore production, potassium (K) mobilization, ammonia production, and IAA production. K. pneumoniae showed positive attributes in the fixing of nitrogen, solubilization of phosphate, and mobilization of potassium from unavailable to available form to plants. In addition to these, K. pneumoniae also showed significant potential to produce phytohormone IAA which primarily governs plant cell proliferation, differentiation, and promotes root length [40]. Additionally, K. pneumoniae also produced siderophores which are responsible to sustain stress conditions as it was also demonstrated by Ahmad et al. [39]. K. pneumoniae promotes plant growth which is also associated with HCN and ammonia synthesis. The synthesis of HCN and ammonia is regarded to be an indirect plant growth enhancer. The generation of ammonia can assist the host plant to achieve its nitrogen demands while also limiting pathogen invasion. The HCN is extensively utilized as a biocontrol agent in agricultural settings, because of its great toxicity against phytopathogens [41].
Considering CBP is a one-step process approach using a single reactor, it saves a lot of money in the microbial industry. Since researchers began working on the valorization of biodegradable solid biomass utilizing several bioprocessing technologies such as simultaneous saccharification and fermentation, and separate hydrolysis and fermentation, CBP evolved to make a significant contribution to the environment and economy [22, 42], although researchers emphasized that the CBP microbe should have certain characteristics, such as extremely effective cellulase synthesis and secretion for fast lignocellulose solubilization [17, 43]. In continuation to these key points in the present work, Aspergillus niger S-30 was able to produce a wide range of multiple hydrolytic enzymes on composite kitchen waste and transform them into simple sugars. Furthermore, the CBP process does not require any additional carbon, nitrogen, potassium, or micronutrient source. Thus, present strategy shows more feasibility towards its practical approach and adaptation to be upscaled further at industrial setup. Both Aspergillus niger S-30 showed a better enzymatic yield at 30 °C and Klebsiella pneumoniae showed an increasing trend in its cell count at 30 °C during CBP. The current method provides excellent conditions for both saccharification and fermentation processes. The present CBP technique has demonstrated significant cost savings in enzyme manufacturing and biofertilizer formulation. The downstream process was also oversimplified and flexible which makes the operational cost cheap to generate carrier-based and liquid biofertilizers.
The carrier materials employed to ensure the future of the cell suspension before its distribution into the soil govern the shelf-life of any inoculant. Carrier materials act as protective habitats, promoting inoculum success while guarding against predators. Particles of powder, granules, and common carrier material such as peat moss are popular biofertilizer carriers, although several other materials, including vermiculite, lignite, and sodium alginate encapsulation, have been investigated as possibilities [44]. Contamination of the biofertilizer formulations could be seen both during the production/formulation stage and during storage. Contamination has been identified as one of the primary reasons for failed field application of plant-beneficial microorganisms. Herrmann et al. [45] found that 37% of the tested formulated products could be considered “pure,” while 63% were contaminated with bacteria and 40% contained only contaminants. The current work employs a consolidated bioprocessing approach in which a closed liquid submerged fermenter is employed for inoculum preparation, fermentation, and downstream stages, including product formulation and packaging, all of which are carried out in sterile and contaminant-free conditions.
As a result, biofertilizers with an extended shelf life and convenient and controlled dispersion of the studied microorganisms are the need of the hour. Kumar et al. [44] recently utilized biochar from agricultural waste, fly ash from coal power plants, and a blend of both as a carrier to sustain the plant growth promoter bacterial species in a recent attempt. The current process used the zero-cost composite kitchen waste as the carrier for inoculum adsorption as well as liquid biofertilizer formulation from the said process. Composite kitchen waste is a rich source of carbon and other micronutrients, thereby acting as a stabilizing source of carbon and other micronutrients. The present work also adheres to the guidelines and specifications of FCO (India), according to which the minimum CFU should be 5 × 107 cells per gram of powder, granules, or carrier material; or 1 × 108 cells per ml of liquid biofertilizer [5]. The effect of compost on plant growth is well studied as both a fertilizer and plant growth promoter [46]. The use of compost as the carrier showed a better shelf life as compared to the study by Kumar et al. [44], in which they used biochar from agriculture waste, fly ash from a coal power station, and a mixture of both as a carrier. The fact that prepared biofertilizer formulations have a shelf life of up to 10 months suggests that they are contaminant free and are suitable for use in fields. The consolidated bioprocessing approach thus provides a safe and scientific approach to the preparation of contaminant-free biofertilizers with an extended shelf life which is otherwise difficult to be maintained by conventional agricultural activities or traditional microorganism-bearing fertilizers which follows Vassileva et al. [16].
The present work was designed to provide the economic importance of biodegradable solid waste to transform it into a carrier and liquid biofertilizer. To further validate our viewpoint on the safety level of biofertilizers on environment and crop production, a plant growth development assay was also employed. The result of carrier and liquid biofertilizer on plant growth as well as the soil was as per the hypothesis behind it. The present study explored the ability of Aspergillus niger for the production of multiple carbohydrasaes and Klebsiella pneumoniae to exhibit numerous plant growth-promoting traits. Some studies have also explored the potential of Aspergillus niger for the production of biofertilizer formulations owing to its IAA production, siderophore production, and ammonia production. Studies explored the potential of Aspergillus niger to be used as a phosphate solubilizer fungus [9, 10]. Aspergillus niger S-30 used in the present study also had the potential to solubilize phosphorus. The prepared biofertilizer formulations thus have properties of two biofertilizer microorganisms. The carrier and liquid biofertilizer prepared from composite kitchen waste significantly improved the plant yield and soil fertility. Both carrier and liquid biofertilizers significantly improved the plant height, root height, plant fresh weight, shoot fresh weight, plant dry weight, root fresh weight, shoot dry weight, and root dry weight in 45 days. The carrier-based biofertilizer did not enhance the shoot height in comparison to the control plant set although the overall plant height was significantly enhanced in the carrier-based biofertilizer. However, liquid biofertilizer significantly enhanced the shoot height. The better plant growth yield can be attributed to IAA [44, 47], phosphate solubilization [44], and ammonia excretion [48, 49]. Bhardwaj et al. [12] also observed similar kind of results while working with Klebsiella pneumoniae VRE36 as a PGPR and attributed the better seed germination to IAA. The better yield can also be attributed to HCN and siderophore which act as protecting agents for plants in stress conditions [50]. El Komy et al. [13] employed a mixture of Azotobacter, Azospirillum, and Klebsiella strains in a trial that enhance root-rot disease complex management; boost growth in sunflower due to N2 fixation and phosphate solubilization; and produces indoleacetic acid (IAA), siderophore, and hydrogen cyanide (HCN). In a nutshell, it is possible to deduce that better nitrogen, phosphorus, and potassium uptake, as well as IAA biosynthesis, ammonia production, siderophore production, and HCN production, all contribute to greater Brassica juncea growth in the present study. Hang et al. [10] recently attributed the cucumber plant growth promotion and growth promotion of biofertilizer strain Arabidopsis to the fungal community of Aspergillus spp. which itself did not attribute the plant growth but helps the biofertilizer strain.
Nitrogen, phosphorus, and potassium are the three most important nutrients for plants, and their levels in the soil have a direct impact on plant growth and development. The accessibility of nitrogen, phosphorus, and potassium nutrients to plants is low even in the presence of an excess of fertilizer; thus, increasing the availability of these nitrogen, phosphorus, and potassium nutrients in the soil is an important way to promote plant growth [51, 52]. The present work following the preceding point as Klebsiella pneumoniae not only solubilized the phosphate and mobilized the potassium to soil but also the significant amount of nitrate nitrogen was available to plants in soil in both carrier and liquid biofertilizer in the high range of 50 kg ha−1. Govindarajan et al. [14] also emphasized the potential of Klebsiella sp. GR9 in improving rice production and attributed it to the N-fixing efficiency of Klebsiella sp. GR9. Nitrogen and potassium are both key requirements in chlorophyll synthesis [53]. The carrier and liquid biofertilizer also significantly improved the chlorophyll content of Brassica juncea which can be attributed to better nitrogen and phosphorus availability. The findings of Zafar-ul-Hye et al. [54] support our findings who also found similar kind of results while working with cadmium-resistant rhizobacteria for nitrogen and phosphorus availability. Environmental issues associated with the application of high doses of chemical fertilizers, which include nitrate deposition in surface water and low utilization effectiveness of nitrogenous and phosphate fertilizers, compelled farmers to seek credible replacement irrespective of formulation and dose of biofertilizers [55, 56]. The present study in the context of the current global trend adheres to the zero-waste approach and sustainable principles of agriculture by utilizing biodegradable solid waste for biofertilizer preparation.
5 Conclusion
The current study provides a highly appealing alternative for the management of biodegradable solid waste by transforming it into a carrier and liquid biofertilizer formulations with 1.03 × 1012 cfu/ml and 1.03 × 1012 cfu/g, respectively. The process provides a cost-effective method of solid waste management and an economical method of biofertilizer formulations with an improved shelf life. At a time when the globe is facing two problems at a time that are fulfilling the food requirement of expanding population and managing biodegradable solid waste, the consolidated bioprocessing approach by transforming solid waste into biofertilizer will be a major milestone in sustainable agriculture. To summarize, the current study is technically possible and, if carried out on a commercial scale, can lead to sustainable management of municipal solid waste with low-cost manufacture of biofertilizers, which is currently in demand in the agricultural market.
References
Aghbashlo M, Tabatabaei M, Soltanian S, Ghanavati H (2019) Biopower and biofertilizer producton from organic municipal solid waste: an exergo enviornmental analysis. Renew Energy 143:64–76
Bhatt SM (2022) Biogas from kitchen waste. In: Food waste to green fuel: trend & development. Springer Nature, Singapore, pp 153–164
Wang H, Qin Y, Xin L, Zhao C, Ma Z, Hu J, Wu W (2023) Preliminary techno-economic analysis of three typical decentralized composting technologies treating rural kitchen waste: a case study in China. Front Environ Sci Eng 17(4):47
de Sousa MH, da Silva AS, Correia RC, Leite NP, Bueno CE, dos Santos Pinheiro RL, de Santana JS, da Silva JL, Sales AT, de Souza CC, da Silva Aquino KA (2022) Valorizing municipal organic waste to produce biodiesel, biogas, organic fertilizer, and value-added chemicals: an integrated biorefinery approach. Biomass Convers Biorefin 12:827–841
Khurana A, Kumar V (2020) State of organic and natural farming: challenges and possibilities Centre for Science and Environment, New Delhi. https://www.cseindia.org/state-of-organic-andnatural-farming-in-india-10346. Accessed 5 Jan 2023
Ji SH, Gururani MA, Chun SC (2014) Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol Res 169(1):83–98
Kaushal M, Devi S, Kumawat KC, Kumar A (2023) Microbial consortium: a boon for a sustainable agriculture. In: Climate change and microbiome dynamics: carbon cycle feedbacks. Springer International Publishing, Cham, pp 15–31
Rai A, Belkacem M, Assadi I, Bollinger JC, Elfalleh W, Assadi AA, Mouni L (2022) Bacteria in soil: promising bioremediation agents in arid and semi-arid environments for cereal growth enhancement. Appl Sci 12(22):11567
Jyothi V, Basaiah T (2022) Isolation and characterization of phosphofungi–Aspergillus niger from rhizosphere soil to supplement phospho-biofertilizer. https://doi.org/10.21203/rs.3.rs-2295676/v1
Hang X, Meng L, Ou Y, Shao C, Xiong W, Zhang N, Kowalchuk GA (2022) Trichoderma-amended biofertilizer stimulates soil resident Aspergillus population for joint plant growth promotion. Npj Biofilms Microbiomes 8(1):57
Gang S, Sharma S, Saraf M, Buck M, Schumacher J (2019) Analysis of indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio-protoc 9(9):e3230
Bhardwaj G, Shah R, Joshi B, Patel P (2017) Klebsiella pneumoniae VRE36 as a PGPR isolated from Saccharum officinarum cultivar Co99004. J Appl Biol Biotechnol 5(1):47–52
El Komy MH, Hassouna MG, Abou-Taleb EM, Al-Sarar AS, Abobakr Y (2020) A mixture of Azotobacter, Azospirillum, and Klebsiella strains improves root-rot disease complex management and promotes growth in sunflowers in calcareous soil. Eur J Plant Pathol 156(3):713–726
Govindarajan M, Kwon SW, Weon HY (2007) Isolation, molecular characterization and growth-promoting activities of endophytic sugarcane diazotroph Klebsiella sp. GR9. World J Microbiol Biotechnol 23(7):997–1006
Shaji H, Chandran V, Mathew L (2021) Organic fertilizers as a route to controlled release of nutrients. In: Controlled release fertilizers for sustainable agriculture. Academic Press, pp 231–245
Vassileva M, Mocali S, Canfora L, Malusá E, Del Moral LFG, Martos V, Vassilev N (2022) Safety level of microorganism-bearing products applied in soil-plant systems. Front Plant Sci 13:862875
Parisutham V, Kim TH, Lee SK (2014) Feasibilities of consolidated bioprocessing microbes: from pretreatment to biofuel production. Bioresour Technol 161:431–440
Olguin-Maciel E, Singh A, Chable-Villacis R, Tapia-Tussell R, Ruiz HA (2020) Consolidated bioprocessing, an innovative strategy towards sustainability for biofuels production from crop residues: an overview. Agronomy 10(11):1834
Singhania RR, Patel AK, Singh A, Haldar D, Soam S, Chen CW, Dong CD (2022) Consolidated bioprocessing of lignocellulosic biomass: technological advances and challenges. Bioresour Technol 127153
García-Solares SM, Mena-Cervantes VY, Sosa-Rodríguez FS, Hernández-Altamirano R, Vazquez-Arenas J (2023) Circular economy involving microbial consortia in consolidated bioprocesses to produce biofuels. In: Biofuels in Circular Economy. Singapore, Springer Nature Singapore, pp 279–301
Mandels M, Andreotti RE, Roche C (1976) Measurements of saccharifying cellulases. Biotechnol Biophy Symp 6:21–23
Bailey MJ, Biley P, Poutanen K (1992) Inter laboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270
Stålbrand H, Siika-aho M, Viikari L (1993) Purification and characterization of two β-mannanases from Trichoderma reesei. J Biotechnol 29:229–242
Minjares-Carranco A, Trejo-Aguilar BA, Guillermo A, Viniegra-Gonzalez G (1997) Physiological comparision between pectinase producing mutants of Aspergillus niger adopted either to solid state fermentation or submerged fermentation. Enzyme Microb Technol 21:25–31
Cori GT (1955) Amylo-1,6-glucosidase. Methods Enzymol 1:211–214
Miller GL (1959) Use of DNS reagent for determination of reducing sugars. Anal Chem 31:426–428
Kayasth M, Gera R, Dudeja SS, Sharma PK, Kumar V (2014) Studies on salinization in Haryana soils on free-living nitrogen-fixing bacterial populations and their activity. J Basic Microbiol 54(3):170–179
Gupta M, Kiran S, Gulati A, Singh B, Tewari R (2012) Isolation and identification of phosphate solubilizing bacteria able to enhance the growth and aloin-A biosynthesis of Aloe barbadensis Miller. Microbiol Res 167(6):358–363
Louden BC, Haarmann D, Lynne AM (2011) Use of blue agar CAS assay for siderophore detection. J Microbiol Biol Educ 12(1):51–53
Bhattacharyya PN, Dutta P, Madhab M, Phukan IK, Sarmah SR, Pathak SK (2016) Isolation of potash mobilizing microorganisms in tea soil and evaluation of their efficiency in potash nutrition in tea: a novel approach. Two Bud 63(1):8–12
Dinesh R, Anandaraj M, Kumar A, Bini YK, Subila KP, Aravind R (2015) Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol Res 173:34–43
Amna Din BU, Sarfraz S, Xia Y, Kamran MA, Javed MT, Sultan T, Chaudhary HJ (2019) Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol Environ Saf 183:109466
James G (1978) Native Sherman Rockland Community College, State University of New York. Benjamin/Cummins Publishing Co, pp 75–80
Arnon DI (1949) Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Esteban-Lustres R, Torres MD, Piñeiro B, Enjamio C, Domínguez H (2022) Intensification and biorefinery approaches for the valorization of kitchen wastes–a review. Bioresour Technol 127652
Fan W, Huang X, Liu K, Xu Y, Chi Z (2023) Advanced upcycling of agro-industrial co-products of corn via different microorganisms. Biomass Bioenergy 168:106669
Fasim A, More VS, More SS (2021) Large-scale production of enzymes for biotechnology uses. Curr Opin Biotechnol 69:68–76
Mlaik N, Sayadi S, Hamza M, Khoufi S (2020) Production and characterization of β-glucosidase from Aspergillus niger fermentation: application for organic fraction of municipal solid waste hydrolysis and methane enhancement. Biotechnol Prog 36(1):e2902
Ahmad I, Akhtar MJ, Zahir ZA, Naveed M, Mitter B, Sessitsch A (2014) Cadmium-tolerant bacteria induce metal stress tolerance in cereals. Environ Sci Pollut Res 21(18):11054–11065
Santner A, Calderon-Villalobos LI, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5:301–307
Rijavec T, Lapanje A (2016) Hydrogen cyanide in the rhizosphere: not suppressing plant pathogens, but rather regulating availability of phosphate. Front Microbiol 7:1785
Liu YJ, Li B, Feng Y, Cui Q (2020) Consolidated bio-saccharification: leading lignocellulose bioconversion into the real world. Biotechnol Adv 40:107535
Liu CG, Xiao Y, Xia XX, Zhao XQ, Peng L, Srinophakun P, Bai FW (2019) Cellulosic ethanol production: progress, challenges and strategies for solutions. Biotechnol Adv 37(3):491–504
Kumar A, Kumar V, Bruno LB, Rajkumar M (2022) Synergism of industrial and agricultural waste as a suitable carrier material for developing potential biofertilizer for sustainable agricultural production of eggplant. Horticulturae 8(5):444
Herrmann L, Atieno M, Brau L, Lesueur D (2015) Microbial quality of commercial inoculants to increase BNF and nutrient use efficiency. In: Biological nitrogen fixation. Wiley Online Library, pp 1031–1040
Rasool M, Akhter A, Soja G, Haider MS (2021) Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease. Sci Rep 11(1):1–16
Xu S, Bai Z, Jin B, Xiao R, Zhuang G (2014) Bioconversion of wastewater from sweet potato starch production to Paenibacillus polymyxa biofertilizer for tea plants. Sci Rep 4(1):1–7
Babalola OO (2010) Beneficial bacteria of agricultural importance. Biotechnol Lett 32:1559–1570
Jimtha John C, Jishma P, Karthika NR, Nidheesh KS, Ray JG, Mathew J, Radhakrishnan EK (2017) Pseudomonas fluorescens R68 assisted enhancement in growth and fertilizer utilization of Amaranthus tricolor (L.). 3 Biotech 7(4):1–6
Wickramasinghe WRKDWKV, Girija D, Gopal KS, Kesevan S (2021) Multi-phasic nitrogen fixing plant growth promoting rhizobacteria as biofertilizer for rice cultivation. Res J Agric Sci 12(2):399–404
Enebe MC, Babalola OO (2018) The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: a survival strategy. Appl Microbiol Biotechnol 102(18):7821–7835
Shi JW, Lu LX, Shi HM, Ye JR (2022) Effects of plant growth-promoting rhizobacteria on the growth and soil microbial community of Carya illinoinensis. Curr Microbiol 79(11):1–12
Muniswami DM, Chinnadurai S, Sachin M, Jithin H, Ajithkumar K, Narayanan GS, Dineshkumar R (2021) Comparative study of biofertilizer/biostimulant from seaweeds and seagrass in Abelmoschus esculentus crop. Biomass Convers Biorefin 1–18
Zafar-ul-Hye M, Naeem M, Danish S, Khan MJ, Fahad S, Datta R, El-Esawi MA (2020) Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants 9(10):1386
Khandare RN, Chandra R, Pareek N, Raverkar KP (2019) Carrier-based and liquid bioinoculants of Azotobacter and PSB saved chemical fertilizers in wheat (Triticum aestivum L.) and enhanced soil biological properties in Mollisols. J Plant Nutr 43(1):36–50
Akinmutimi AL, Uluocha N (2020) The effect of compost manure and NPK (20:10:10) on performance growth, yield and nutrient content of Amaranthus sp. in soils of Umudike, a Ultisol in southeastern Nigeria. IJARD 23(2):5425–5430
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
Funding
The authors are highly thankful to the Panjab University, Chandigarh, India for the award of Research Fellowship and Contingency grant to Apurav Sharma.
Author information
Authors and Affiliations
Contributions
Apurav Sharma: methodology, validation, investigation, formal analysis, and writing—original draft; Himani Saini: methodology, validation, investigation, and formal analysis; Bishakha Thakur: methodology, validation, investigation, and formal analysis; Raman Soni: supervision and writing—review and editing; Sanjeev Kumar Soni: conceptualization, investigation, supervision, and writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, A., Saini, H., Thakur, B. et al. Consolidated bioprocessing of biodegradable municipal solid waste for transformation into biofertilizer formulations. Biomass Conv. Bioref. 14, 20923–20937 (2024). https://doi.org/10.1007/s13399-023-04110-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04110-2