Abstract
Four typical antibiotics were added to human feces for aerobic composting using batch reactors with sawdust as the bulk matrix. Under three composting temperatures (room temperature, 35 ± 2 °C and 55 ± 2 °C), decreases in the extractable concentrations of antibiotics in the compost were monitored for 20 days. As a result, the removals of extractable tetracycline and chlortetracycline were found to be more temperature-dependent than the removals of sulfadiazine and ciprofloxacin. However, more than 90 % of all of the extractable antibiotics were removed at 55 ± 2 °C. Three specific experiments were further conducted to identify the possible actions for antibiotic removal, including self-degradation in aqueous solution, composting with a moist sterile sawdust matrix without adding feces and composting with human feces and moist sterile sawdust. As a result, it was found that the removal of tetracycline and chlortetracycline was mainly due to chemical degradation in water, whereas the removal of sulfadiazine was mainly attributed to adsorption onto sawdust particles. The microbial activity of compost varied with temperature to a certain extent, but the differences were insignificant among different antibiotics. Although microbial action is important for organic matter decomposition, its contribution to antibiotic degradation was small for the investigated antibiotics, except for ciprofloxacin, which was degraded by up to 20 % due to microbial action.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aerobic composting is a widely used process for organic waste treatment (Zorpas and Loizidou 2008). Hazard-free stable composting products containing rich nutrients such as nitrogen, phosphorus, and potassium can be used as fertilizers and soil conditioners for agricultural production. The use of aerobic composting for excrement treatment (the bio-toilet) has become a popular topic in recent studies (Lopez Zavala et al. 2004; Zavala et al. 2005).
The purpose of aerobic composting is to produce more stable and safer compost products, especially via the inactivation of pathogenic bacteria and the degradation of trace contaminants. Studies have shown that as much as 30 to 90 % of antibiotics are excreted in the forms of their parent compounds or metabolites via urine and feces, while retaining biological activity (Alcock et al. 1999). Thus, antibiotic residues in human feces should be considered in the context of composting. In Europe, two-thirds of all antibiotics are used in human medicine, and one-third is used for veterinary applications (Thiele-Bruhn 2003). In China, there are many antibiotics users and manufacturers, with an annual output of antibiotic material of approximately 210 thousand tons, including 30 thousand tons of exports. The remaining tonnage is destined for medical, agricultural, and other uses in China, with an annual consumption per capita of approximately 138 g (compared to 13 g in the United States). Tetracyclines, quinolones, and sulfonamides are the most commonly used antibiotics. Feces containing large amounts of antibiotics can be reutilized by the soil, resulting in accumulation of antibiotics in the soil and leading to the development of antibiotic resistant bacteria or resistance genes (Li et al. 2015; Mo et al. 2015). The spread of antibiotic residues in the environment poses a potential risk, and residues may enter the human body through the food chain, thus endangering human health.
Antibiotics are gradually removed from the environment through processes such as adsorption, migration, and degradation via hydrolyzation, photodegradation, and biodegradation. Many studies have indicated that the degradation of antibiotics is temperature-dependent. The higher the temperature, the higher the rate of degradation (Arikan et al. 2009; Kim et al. 2012; Wu et al. 2011). A study reported that the degradation efficiency of chlortetracycline during 30 days of beef manure composting was 99 % at a temperature of 55 °C and 49 % at room temperature (Arikan et al. 2009). Dolliver et al. (2008) reported that the concentration of chlortetracycline during manure composting declined by 99 %, whereas tylosin and monensin declined approximately by 54 and 76 %, respectively, during 35 days of turkey-litter composting. Using a bulky matrix such as sawdust for aerobic composting can not only improve the porosity of the compost and adjust the moisture content of the mixture but also provide a large number of binding sites (Gu et al. 2007; Kulshrestha et al. 2004) that can be used by antibiotics for decomposition. Kim et al. (2012) reported that decreases in tetracycline and sulfonamide concentrations were highly dependent on the presence of sawdust. Therefore, the removal of antibiotics during aerobic composting depends on the temperature, substrate, and antibiotic characteristics. Many recent studies have focused on the effect of aerobic composting on the removal efficiency of antibiotics in livestock and poultry manure (Ho et al. 2013; Kim et al. 2012; Selvam et al. 2012) rather than the specific degradation processes of antibiotics during aerobic composting.
In this analysis, a batch study was performed to investigate decreases in the extractable concentrations of tetracycline, chlortetracycline, sulfadiazine, and ciprofloxacin during composting at three typical temperatures, namely a thermophilic temperature of 55 ± 2 °C, mesophilic temperature of 35 ± 2 °C and room temperature (RTC). The objectives of the study were to determine the main actions that contribute to decreasing the extractable antibiotics concentrations during aerobic composting, including self-degradation (chemical degradation) of antibiotics in a moist environment, adsorption onto sawdust particles, and aerobic composting using human feces. The impact of composting temperature on these actions was then assessed.
Materials and methods
Experimental device
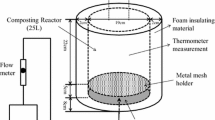
Three laboratory-scale composting reactors were constructed using plastic containers (25 L) with air pumps and flow meters (Fig. 1). An air chamber with a height of 5 cm was established at the bottom of the reactor for distributing the airflow. The top of the air chamber, which was composed of a metal mesh (pore size of 0.5 mm), was used to support the sawdust and prevent leakage from the reaction unit. Air was pumped into the air chamber at a flow rate of 1.5 L/min based on several experiments. After distribution by the air diffuser, the air entered the reaction unit to provide sufficient oxygen for organismal growth. To prevent moisture loss, the reactors were covered with plastic covers that contained small holes. A water bath was used to maintain the compost at set temperatures of 35 ± 2 °C (mesophilic condition) or 55 ± 2 °C (thermophilic condition).
Raw materials
Collection of human feces and sawdust
In this study, human feces were collected from a university campus. After being well-mixed to a homogeneous state, the feces were packed in batches and stored at −20 °C for later use. The bulky matrix comprised sawdust collected from a local timber processing plant and was screened to select for sawdust with a particle size within 1–2 mm. The sawdust was stored in a cool and dry place. The physicochemical properties of the feces and sawdust are shown in Table 1.
Antibiotics
The initial compost samples were simultaneously spiked with a combination of tetracycline (TC, 97.5 % purity), chlortetracycline (CTC, 99.0 % purity), sulfadiazine (SDZ, 99.3 % purity) and ciprofloxacin (CIP, 99.5 % purity). Sulfameter (99.9 % purity) and demeclocycline (98.3 % purity) were used as the internal standards in recovery and sample analyses. Detailed information for the individual antibiotics is summarized in Table 2.
Operation procedures
Aerobic composting operation
According to previous studies, 10–12 days were considered sufficient for organic matter decomposition and microbial community succession to reach a stable state of aerobic composting (Bai and Wang 2010; Zavala et al. 2005). To ensure the safety and maturity of the compost that was spiked with antibiotics, the composting period was set to 20 days in this study. Human feces were mixed with sawdust particles to a 1:4 dry weight ratio of feces to sawdust (Bai and Wang 2010). First, 0.2 kg of human feces and 0.8 kg of sawdust (both dry weights) were added to each composting reactor, totaling 1 kg of dry weight. The initial moisture content was adjusted to 60 % using deionized water. Then, 1 mg/kg of the target antibiotics (TC, CTC, SDZ, and CIP) was spiked into each mixture. The mixtures in reactors were stirred twice per day. The ambient temperature and the mixture temperature were recorded in the center of the composting material using a temperature meter twice a day. Composting samples were collected from the mixture every day after they were well-mixed and were then stored at −20 °C.
Composting without the addition of human feces
To elucidate the effect of the bulk matrix (sawdust) on the decrease of extractable antibiotics, composting experiments with sawdust (treated through radiation sterilization) containing the standard antibiotics without the addition of human feces were carried out at three temperatures. The effect of sterilization on sawdust was assessed based on bacterial plate counts, and no microorganisms were found. The composting procedure was the same as that explained in “Aerobic composting operation” section. First, 1.0 kg (dry weight) of sawdust was added to each composting reactor, with the moisture content adjusted to 60 %. Similarly, 1 mg/kg of the target antibiotics (TC, CTC, SDZ, and CIP) was spiked into each mixture. The composting samples were collected from the mixture 2–3 days later after they were well-mixed. They were then stored at −20 °C.
Degradation of antibiotics in aqueous solution
In nature, the degradation of antibiotics can be affected by temperature, moisture, and light conditions. Because antibiotics can degrade in deionized water, the moist conditions in the composting reactor can influence the various processes. Therefore, in this study, the degradation of four antibiotics in sterilized deionized water at three different temperatures (thermophilic temperature, mesophilic temperature, and room temperature) was defined as self-degradation (chemical degradation). Four compounds (TC, CTC, SDZ, and CIP) were accurately weighed and then dissolved in the sterilized deionized water to prepare 1 μg/mL antibiotic solution. Each antibiotic solution (1 L) was prepared in triplicate, and the reactions were conducted in beakers (1 L) with temperature controls set at 25 ± 2 °C, 35 ± 2 °C, and 55 ± 2 °C to simulate room temperature, mesophilic, and thermophilic conditions, respectively. All beakers were covered with a clear stretchable plastic food wrap and sealed with a rubber band to minimize evaporation. Aluminum foil was applied over the plastic wrap to entirely cover the beakers and prevent light exposure. The experimental period was 20 days. A 2-mL antibiotic aqueous solution sample was taken from each beaker daily. The antibiotic aqueous solution was directly injected into a UPLC-MS/MS system for analysis.
Analytical methods
Chemical analysis of the compost
The moisture content of the compost samples was determined by measuring the moisture loss after drying in an oven at 105 °C for 24 h. The total nitrogen (TN) of the compost samples was directly analyzed by a modified alkaline potassium persulfate digestion-spectrophotometric method (AWWA-APHA-WEF 1995). Using chemical oxygen demand (COD) as an indicator of the fecal organic content, total COD was measured using a modified closed reflux, colorimetric method by directly digesting the compost samples (Zavala et al. 2005). To evaluate the COD in the soluble fraction, termed as “soluble COD”, and pH of the compost sample, 3 g of compost sample was stirred vigorously in 30 mL distilled water for 30 min. After removing the suspended substances by a 0.45-μm filter, the soluble COD was analyzed by standard methods (AWWA-APHA-WEF 1995). The pH of the solution was taken as a measure of the acidity and/or alkalinity state in the composting reactor. The data were analyzed using triplicate samples after calculating the average and standard error.
Extraction of antibiotics from the compost
Prior to sample extraction, compost samples were defrosted to equilibrate to ambient temperature and then homogenized and filtered through a 2-mm sieve. Antibiotics in the compost samples were extracted using the methods documented by Białk-Bielińska et al. (2009), Capone et al. (1996) and Ho et al. (2012) with modifications. Briefly, 1 g wet weight of solids was accurately weighed in a 50 mL centrifuge tube and spiked with 0.5 mL of 0.5 μg/mL internal standard (IS) mixtures. Then, 10 mL of extraction buffer (MeOH:ACN:0.1 M Na2EDTA-McIlvaine buffer (pH 4) as 30:20:50) was used to extract the antibiotics. The mixture was vortex mixed for 30 s and then placed into a 100 W ultrasonic bath for 10 min. The tube was centrifuged at 5000 rpm for 10 min. The same extraction procedure was repeated twice more, and then all of the supernatants were mixed and transferred into a clean 50 mL plastic tube. Finally, the extract was filtered through 0.45-μm nylon membrane filter paper (Whatman, UK) to remove particulate matter.
An Oasis HLB (3 cc/60 mg) cartridge was conditioned using 6 mL of methanol followed by 6 mL of 0.1 M Na2EDTA-McIlvaine buffer. The sample extract was loaded into the cartridges at approximately 10 mL/min. The cartridge was then washed with 9 mL ultrapure water and dried under vacuum for 30 min. Finally, the analytes were eluted into a clean 10 mL centrifuge tube with 6 mL of methanol. The eluent was evaporated to near dryness under a gentle stream of N2 gas with the aid of a hot plate heated to 45 °C. The dry extract was then reconstituted in a 1 mL acetonitrile:water solution (1:9, v/v). The extract was filtered through a 0.22-μm nylon syringe filter and transferred to a 2-mL amber glass vial. Finally, the extract was automatically injected into the UPLC-MS/MS for analysis.
UPLC-MS/MS analysis of antibiotics
Ultra-performance liquid chromatography-electrospray ionization tandem mass spectrometry (Acquity TQD, Waters Corporation, Milford, MA, USA) was used to identify and quantify the target antibiotics. An ACQUITY UPLC shield RP18 (50 mm, 2.1 mm i.d., 1.7 μm) (Waters, MA, USA) was used to separate the antibiotics. The column temperature was 35 °C, the flow rate was 0.2 mL/min and the injection volume was 10 μL. The mobile phase compositions were as follows: A, acetonitrile; and B, 0.5 % formic acid in ultrapure water. The solvents were mixed as follows: 0 to 4 min 10 % A, 90 % B; 4 to 5 min linear increase to 30 % A, 70 % B; 5 to 7 min linear increase to 40 % A, 60 % B; 7.1 min back to 10 % A, 90 % B and kept until end of total runtime at 8 min. Acquisition was performed using the multiple-reaction monitoring mode (MRM) in ESI+ mode. The optimum MS parameters were as follows: capillary voltage, 3.3 kV; cone voltage, 32 V; extractor, 3 V; source temperature, 110 °C; desolvation temperature, 350 °C. The cone gas and desolvation flows were 50 and 450 L/h, respectively.
Antibiotic concentrations were calculated based on the internal standard method using sulfameter and demeclocycline as internal standards (Arikan et al. 2009). Recovery was evaluated by spiking four replicates of samples with native standard mixtures and IS mixtures. For this analysis, 1 mL of 0.5 mg/L native standard mixtures and 0.5 mL of 0.5 mg/L IS mixtures was spiked into each sample. The extraction and cleanup process for the compost samples was identical to the procedures described above. The method detection limit (MDL) and the method quantification limit (MQL) were evaluated by spiking samples with a mixture of all standards and calculated as the signal-to-noise (S/N) ratio. The S/N ratio was 3 or 10, based on the obtained peaks. The data were acquired through Masslynx 4.1. The basic parameters for UPLC-MS/MS analysis of four antibiotics and two internal standards are shown in Table 3.
Results and discussion
General characteristics of the aerobic composting processes
Changes in the composting temperature, pH, fecal COD, and total nitrogen were investigated. As shown in Fig. 2a, the initial temperatures of the three composts were slightly lower than ambient temperature. Later, due to recovery of microbial activity, the activity of microorganisms that metabolize organic compounds effectively resulted in a fast temperature rise in the compost under RTC and 35 ± 2 °C. The maximum temperature of 42 °C was achieved in the compost on the 3rd day. The maximum temperature in this study was slightly lower than those reported in other studies for composting under RTC (Dolliver et al. 2008; Ho et al. 2013), potentially due to the smaller compost volume, which may result in heat loss. Under 55 ± 2 °C, no significant temperature increase was observed in the compost because the set temperature was higher than the temperature caused by microbial metabolism.
In the initial stage of composting under RTC and 35 ± 2 °C, with the temperature rise due to microbial metabolism, noticeable “pH sags” appeared during the first 2 or 3 days (Fig. 2b), potentially indicating the formation of fatty acids as a result of organic decomposition (Sundberg et al. 2004). The pH variations were similar under RTC and 35 ± 2 °C, with maxima of 9.2 and 8.9, respectively. For composting under 55 ± 2 °C, a quick temperature rise and slight pH decrease were observed in the initial stage, but both became stable from the 2nd or 3rd day onward. The pH was approximately 5.7 through the end of the composting period. Similar results were reported in a previous study (Li et al. 2013), with a microbial community analysis showing varied microbes under mesophilic and thermophilic conditions, as well as pH variation. Similar results have been reported by Asano et al. (2010).
Because the organic substances in human feces might include biodegradable and biological inert material (Lopez Zavala et al. 2004), COD variations in its total content and soluble fraction can provide information regarding the biodegradability of organic substances under the influence of the composting temperature. Figure 2c shows the variations of fecal COD in the composting processes under the three temperatures of RTC, 35 ± 2 and 55 ± 2 °C. When total COD was considered, the organic substances were decomposed at a higher rate at 55 ± 2 °C than they were at RTC and 35 ± 2 °C at the beginning of the composting reaction. After 8–10 days, there was no apparent decrease in total COD under all conditions. Under RTC, soluble COD began to decrease on the first day. At 35 ± 2 °C and 55 ± 2 °C, apparent increases in soluble COD were observed during the first 2 days. This suggests that a higher temperature benefited the hydrolysis process, which converted non-soluble COD into soluble COD during the initial stage of composting. The gradual decrease in soluble COD under the three conditions is an indication that the readily degradable organic matter was utilized by microorganisms. If we compare the soluble COD variation with that of the compost temperature, it can be observed that higher concentrations of degradable organic matter in the compost during the first 7 days were accompanied by higher compost temperatures, indicating higher microbial activity. The compost temperature almost decreased to the ambient level after the 15th day, when soluble COD stabilized in the compost. This shows the close relationship between the availability of readily degradable organic matter and microbial activity. Figure 2c shows that the residual COD concentrations were at the same level under the three operation conditions. Additionally, aerobic composting resulted in a COD reduction of approximately 60 %.
As nitrogen is an important nutrient for crop growth, from the viewpoint of fertilizer utilization, it is desirable to retain nitrogen elements in the final composting product (Hua et al. 2009). As shown in Fig. 2d, in the process of aerobic composting, nitrogen loss is inevitable because organic nitrogen, which took up approximately 70 % of the total nitrogen in the feces (Table 1), may be easily decomposed into ammonia nitrogen that can be further gasified into ammonia gas. Compared with RTC and 35 ± 2 °C, thermophilic composting at 55 ± 2 °C retained a higher concentration of nitrogen in the final compost. The physicochemical and microbiological mechanisms for this were discussed in a previous study (Li et al. 2013).
Decrease of extractable antibiotics in the composting processes
A number of studies have demonstrated that composting can significantly decrease antibiotic concentrations (Aust et al. 2008; Dolliver et al. 2008; Kim et al. 2012). However, few studies have analyzed the removal characteristics of antibiotics with different molecular structures. As shown in Fig. 3, of the four antibiotics used in this study, extractable TC and CTC exhibited a strong temperature-dependent removal characteristic during the composting process, while the removal of SDZ and CIP was not significantly affected by the composting temperature. Under RTC, extractable concentrations of both TC and CTC decreased slowly and only 43.1 and 58.1 % were removed, respectively, at the end of the composting process. The temperature increase substantially improved the removals of TC and CTC to 66.4 and 82.1 %, respectively, under the mesophilic condition of 35 ± 2 °C and to 97.3 and 99.5 %, respectively, under the thermophilic condition of 55 ± 2 °C. As shown in Table 2, both TC and CTC are tetracyclic structures with the same range of molecular weights and common functional groups such as dimethylamino, phenolic hydroxyl, and tricarbonyl methane. The sole difference is the chloridion on the first benzene ring of CTC, which contributes to its higher log Kow compared to that of TC. This may be one of the reasons for the slightly higher removal of CTC compared to the removal of TC under the three conditions.
SDZ and CIP are much different from TC and CTC in their molecular structures. As a sulfonamide, SDZ has a sulfonyl group connected to an amine group, which is more easily degradable than the polycyclic structure (Loftin et al. 2008; Morrison and Boyd 1983). The azabicyclo structure of CIP is also easily degradable. The log Kow values of SDZ and CIP are much higher than those of TC and CTC. Although the removal efficiencies of extractable SDZ and CIP were different under different temperatures in the early stage of composting, the effect of temperature were minor after approximately 6 days. The final removals of extractable SDZ at RTC, 35 ± 2 °C and 55 ± 2 °C were 96.8, 97.5, and 97.6 %, respectively, while those for extractable CIP were 84.2, 87.4, and 91.8 %, respectively. The azabicyclo structure of CIP may be slightly harder to degrade than the sulfonamide structure of SDZ, and the effect of composting temperature cannot be ignored.
Actions that decrease extractable antibiotics
The decrease of extractable antibiotics during the composting process cannot be merely associated with aerobic composting of human feces, which may relate to the microbiological action (MA). Other actions, such as the self-degradation (SD) of antibiotics in moist environments and the decrease attributed to adsorption onto sawdust particles (SA), may also contribute to the removal of antibiotics depending on their physicochemical properties. In this section, each of these actions and their contributions to decreasing each antibiotic under different composting temperatures are discussed based on specific experimental results. If the removal efficiencies due to SD, SA, and MA are denoted as RSD, RSA, and RMA, respectively, the total removal efficiency can be written as RTF = RSD + RSA + RMA.
Degradation of antibiotics in aqueous solution
Figure 4 shows the decreased concentrations of antibiotics in aqueous solutions in a 20-day period. Generally, the influence of temperature was significant for all four antibiotics, and higher temperature apparently benefited self-degradation in aqueous solution. However, TC and CTC were unstable, especially under the thermophilic condition of 55 ± 2 °C. Abiotic degradation of antibiotics usually occurs through pathways such as dehydration, isomerization, and epimerization (Wu et al. 2011), of which dehydration is strongly temperature-dependent (Loftin et al. 2008). From Fig. 4a and b, the RSD values for TC and CTC reached 21.0 and 46.0 %, respectively, under RTC (set as 25 ± 2 °C for the experiment), increased to 51.3 and 76.7 %, respectively, under the mesophilic condition of 35 ± 2 °C and further increased to 90.8 and 95.3 %, respectively, under the thermophilic condition of 55 ± 2 °C. The difference between the RSD values of TC and CTC at each temperature is likely due to the slight difference in their structures. However, at higher temperatures, frequent molecular collisions may also result in a faster degradation rate (Loftin et al. 2008).
The self-degradation of SDZ was slightly temperature-dependent (Fig. 4c), and the RSD values under RTC, 35 ± 2 °C and 55 ± 2 °C were 15.8, 17.9, and 27.9 %, respectively. These values were lower than the RSD values of TC and CTC under each temperature because the sulfonamide functional group is usually more difficult to break than is the amide functional group due to steric and electronic effects (Morrison and Boyd 1983). For CIP, self-degradation was even less temperature-dependent, and the RSD was approximately 45 % under each temperature.
Decrease in extractable concentrations of antibiotics in a moist sawdust matrix without feces addition
Using a bulk matrix such as sawdust in aerobic composting can improve the porosity of the compost, adjust the moisture content, and provide a large number of binding sites (Gu et al. 2007; Kulshrestha et al. 2004). Figure 5 shows the decreased levels of extractable antibiotics in the moist sawdust matrix under different temperatures without the addition of human feces. The sawdust used for this experiment was sterilized, and no microorganisms could be detected by bacterial plate counts, indicating that no microbial action occurred in the mixture during the 20-day period. Therefore, the decreased levels of extractable antibiotics in such an experiment are due to two actions, namely, self-degradation (SD) in a moist environment and adsorption onto sawdust particles (SA). If the removal of antibiotics in this case is denoted as RTS, then RTS = RSD + RSA.
For TC, RTS values of 40.9, 60.9, and 95.8 % under RTC, 35 ± 2 °C and 55 ± 2 °C, respectively, were obtained from Fig. 5a, suggesting a strong temperature-dependent characteristic. These RTS values are higher than the corresponding RSD values of 21, 51.,% and 90.8 % obtained from Fig. 4a, indicating that the adsorption of TC onto the sawdust matrix provided a better condition for its removal than did the aqueous solution. Similar results were obtained from Fig. 5b for CTC, with RTS values of 52.7, 80.4, and 98.6 % under RTC, 35 ± 2 °C and 55 ± 2 °C, respectively. These values were also higher than the corresponding RSD values.
The enhancement of the decrease attributed to adsorption onto sawdust particles was more obvious for SDZ because its RTS values were obtained from Fig. 5c as 87.4, 95.8, and 96.5 %, respectively, under RTC, 35 ± 2 °C and 55 ± 2 °C in contrast to the corresponding RSD values as 15.8, 17.9, and 27.9 %. Regarding CIP, the influence of temperature on its RTS was insignificant (Fig. 5d). However, the RTS value obtained (approximately 69 %) was significantly higher than the RSD value (approximately 45 %).
The use of a solid matrix such as sawdust to improve antibiotic removal in a composting system has also been noted by other studies (Arikan et al. 2009; Zhang and Huang 2007). When temperature is increased during composting, the organic substances from sawdust, which potentially generate more antibiotics binding sites, may be readily absorbed, and transformed into non-extractable forms (Kim et al. 2012). Consequently, extractable concentrations of antibiotics and their metabolites decreased with time in high organic matrices.
Evaluation of various actions that decrease extractable antibiotics during composting processes
By monitoring the degradation of antibiotics in aqueous solution, self-degradation characteristics were investigated to obtain RSD. Then, using a composting experiment without the addition of human feces, the characteristics of the antibiotic decrease attributed to adsorption onto sawdust particles were revealed to obtain RTS (RTS = RSD + RSA). If we compare this with the result of composting operations (Fig. 3), from which the total removal efficiency for each antibiotic under each composting temperature can be obtained as RTF, the difference between RTF and RTS is due to the decrease associated with aerobic composting of human feces, which may relate to the microbiological process. The principle of aerobic composting involves the metabolism of microorganisms under appropriate conditions (Ding et al. 2014; Selvam et al. 2012). In most cases, microorganisms in compost systems originate from the feces (in this study, the human feces) that are decomposed. However, human feces contain many impurities, such as organic matters, inorganic substances, cations, and anions that affect the acid–base environment of the compost mixture. Therefore, in addition to microorganisms, other factors related to the feces may also affect the degradation of antibiotics (Bao et al. 2009). The efficiency of antibiotic removal, which is referred to as the decrease associated with the aerobic composting of human feces (MA) in this study, denoted RMA, can be obtained from Figs. 3 and 5 as RMA = RTF − RTS.
Based on the RSD values obtained from Fig. 4, RSA values calculated as RSA = RTS − RSD (RTS values were obtained from Fig. 5) and RMA values calculated as RMA = RTF − RTS, Fig. 6 was obtained to show the contribution of each action to the decrease of extractable antibiotics in the composting process.
Figure 6a shows that under RTC, only 43.1 % of the TC spiked into the compost mixture decreased after 20 days of aerobic composting. SD and SA played the major roles (approximately 20 % for each) in composting, while the role of MA was minor. Under mesophilic temperature of 35 ± 2 °C, 66.4 % of TC was removed, but compared with RTC, the increase in TC removal was mainly due to SD. When the composting temperature increased to 55 ± 2 °C, the TC removal further increased to 97.3 %, of which 90.8 % was due to SD, while the contributions from SA and MA were limited (collectively less than 7 %). Thus, dehydration in moist environments is the main action that removes TC with a tetracyclic structure. Such an action becomes stronger at higher temperatures partially due to increased reaction activity and partially due to the more acidic environment (Halling-Sørensen et al. 2002; Loke et al. 2003), as shown by the low pH at 55 ± 2 °C (Fig. 2b). Microbial action may not provide sufficient energy to break down the cyclic structure.
For CTC, the action due to SD became more dominant even under RTC (Fig. 6b). Of the 58.1 % total CTC removal at RTC, SD contributed to approximately 46 %. As the temperature increased, the total CTC removal increased to 82.1 and 99.5 % under 35 ± 2 °C and 55 ± 2 °C, respectively, for which the contributions of SD were 76.7 and 95.3 %, respectively. The existence of chloridion on the CTC molecule increased its potential for dehydration in the moist environment.
In contrast to TC and CTC, the dominant action for SDZ removal was SA, contributing to approximately 60 % at each composting temperature when the total removal was approximately 97 %, irrespective of the temperature change (Fig. 6c). MA contributed to a certain extent (approximately 11 %) under RTC and became insignificant under higher temperatures. This indicates that due to its sulfonamide structure, SDZ exhibits a stronger affinity with the solid surfaces such as the sawdust in this study. Additionally, its removal is mainly due to reactions at adsorption sites on the sawdust particles.
Regarding CIP (Fig. 6d), although the total removal slightly increased from 84.2 % under RTC to 87.4 and 91.8 % at increased temperatures of 35 ± 2 °C and 55 ± 2 °C, respectively, the contributions of SD, SA, and MA did not change much, as shown by the slight RSD (always approximately 46 %), RSA (20–25 %) and RMA (10–18 %) variations. Each action seemed to have received little influence from the composting temperature.
Conclusions
Decreased levels of extractable antibiotics during aerobic composting have been noted by many studies. However, little information is available regarding the details of the associated actions. This study analyzed three actions that potentially occur during the composting process, namely, self-degradation (SD), sawdust adsorption (SA), and microbial activity (MA). Although the principle of composting is based on the metabolism of microorganisms, MA did not play a major role, while the physicochemical actions of SD and SA contributed substantially to the removal of antibiotics. For temperature-dependent antibiotics such as TC and CTC, SD played the main role and the total removal decreased with the decreasing temperature. For SDZ and CIP, SA played the main role, and the removal was almost independent of the composting temperature.
References
Alcock RE, Sweetman A, Jones KC (1999) Assessment of organic contaminant fate in waste water treatment plants I: selected compounds and physicochemical properties. Chemosphere 38:2247–2262. doi:10.1016/S0045-6535(98)00444-5
Arikan OA, Mulbry W, Rice C (2009) Management of antibiotic residues from agricultural sources: use of composting to reduce chlortetracycline residues in beef manure from treated animals. J Hazard Mater 164:483–489. doi:10.1016/j.jhazmat.2008.08.019
Asano R, Otawa K, Ozutsumi Y, Yamamoto N, Abdel-Mohsein HS, Nakai Y (2010) Development and analysis of microbial characteristics of an acidulocomposting system for the treatment of garbage and cattle manure. J Biosci Bioeng 110:419–425. doi:10.1016/j.jbiosc.2010.04.006
Aust MO, Godlinski F, Travis GR, Hao X, McAllister TA, Leinweber P, Thiele-Bruhn S (2008) Distribution of sulfamethazine, chlortetracycline and tylosin in manure and soil of Canadian feedlots after subtherapeutic use in cattle. Environ Pollut 156:1243–1251. doi:10.1016/j.envpol.2008.03.011
AWWA-APHA-WEF (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington
Babić S, Horvat AJM, Mutavdžić Pavlović D, Kaštelan-Macan M (2007) Determination of pKa values of active pharmaceutical ingredients. TrAC Trend Anal Chem 26:1043–1061. doi:10.1016/j.trac.2007.09.004
Bai F, Wang X (2010) Nitrogen-retaining property of compost in an aerobic thermophilic composting reactor for the sanitary disposal of human feces. Front Environ Sci Eng 4:228–234. doi:10.1007/s11783-010-0022-7
Bao Y, Zhou Q, Guan L, Wang Y (2009) Depletion of chlortetracycline during composting of aged and spiked manures. Waste Manag 29:1416–1423. doi:10.1016/j.wasman.2008.08.022
Białk-Bielińska A, Kumirska J, Palavinskas R, Stepnowski P (2009) Optimization of multiple reaction monitoring mode for the trace analysis of veterinary sulfonamides by LC-MS/MS. Talanta 80:947–953. doi:10.1016/j.talanta.2009.08.023
Capone DG, Weston DP, Miller V, Shoemaker C (1996) Antibacterial residues in marine sediments and invertebrates following chemotherapy in aquaculture. Aquaculture 145:55–75. doi:10.1016/S0044-8486(96)01330-0
Chen H, Gao B, Li H, Ma LQ (2011) Effects of pH and ionic strength on sulfamethoxazole and ciprofloxacin transport in saturated porous media. J Contam Hydrol 126:29–36. doi:10.1016/j.jconhyd.2011.06.002
Ding N et al (2014) Decline in extractable kitasamycin during the composting of kitasamycin manufacturing waste with dairy manure and sawdust. J Environ Manag 134:39–46. doi:10.1016/j.jenvman.2013.12.030
Dolliver H, Gupta S, Noll S (2008) Antibiotic degradation during manure composting. J Environ Qual 37:1245–1253. doi:10.2134/jeq2007.0399
Gu C, Karthikeyan KG, Sibley SD, Pedersen JA (2007) Complexation of the antibiotic tetracycline with humic acid. Chemosphere 66:1494–1501. doi:10.1016/j.chemosphere.2006.08.028
Halling-Sørensen B, Sengeløv G, Tjørnelund J (2002) Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch Environ Contam Toxicol 42:263–271. doi:10.1007/s00244-001-0017-2
Ho YB, Zakaria MP, Latif PA, Saari N (2012) Simultaneous determination of veterinary antibiotics and hormone in broiler manure, soil and manure compost by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1262:160–168. doi:10.1016/j.chroma.2012.09.024
Ho YB, Zakaria MP, Latif PA, Saari N (2013) Degradation of veterinary antibiotics and hormone during broiler manure composting. Bioresour Technol 131:476–484. doi:10.1016/j.biortech.2012.12.194
Hua L, Wu W, Liu Y, McBride MB, Chen Y (2009) Reduction of nitrogen loss and Cu and Zn mobility during sludge composting with bamboo charcoal amendment. Environ Sci Pollut Res 16:1–9. doi:10.1007/s11356-008-0041-0
Kim KR, Owens G, Ok YS, Park WK, Lee DB, Kwon SI (2012) Decline in extractable antibiotics in manure-based composts during composting. Waste Manag 32:110–116. doi:10.1016/j.wasman.2011.07.026
Kulshrestha P, Giese RF, Aga DS (2004) Investigating the molecular interactions of oxytetracycline in clay and organic matter: insights on factors affecting its mobility in soil. Environ Sci Technol 38:4097–4105. doi:10.1021/es034856q
Li Q, Wang XC, Zhang HH, Shi HL, Hu T, Ngo HH (2013) Characteristics of nitrogen transformation and microbial community in an aerobic composting reactor under two typical temperatures. Bioresour Technol 137:270–277. doi:10.1016/j.biortech.2013.03.092
Li J, Cheng W, Xu L, Strong PJ, Chen H (2015) Antibiotic-resistant genes and antibiotic-resistant bacteria in the effluent of urban residential areas, hospitals, and a municipal wastewater treatment plant system. Environ Sci Pollut Res 22:4587–4596. doi:10.1007/s11356-014-3665-2
Loftin KA, Adams CD, Meyer MT, Surampalli R (2008) Effects of ionic strength, temperature, and pH on degradation of selected antibiotics. J Environ Qual 37:378–386. doi:10.2134/jeq2007.0230
Loke M-L, Jespersen S, Vreeken R, Halling-Sørensen B, Tjørnelund J (2003) Determination of oxytetracycline and its degradation products by high-performance liquid chromatography–tandem mass spectrometry in manure-containing anaerobic test systems. J Chromatogr B 783:11–23. doi:10.1016/S1570-0232(02)00468-3
Lopez Zavala MA, Funamizu N, Takakuwa T (2004) Temperature effect on aerobic biodegradation of feces using sawdust as a matrix. Water Res 38:2405–2415. doi:10.1016/j.watres.2004.02.026
Mo WY, Chen Z, Leung HM, Leung AO (2015) Application of veterinary antibiotics in China’s aquaculture industry and their potential human health risks. Environ Sci Pollut Res. doi:10.1007/s11356-015-5607-z
Morrison RT, Boyd RN (1983) Organic chemistry, 4th edn. Allyn and Bacon, Boston
Mutavdžić Pavlović D, Pinušić T, Periša M, Babić S (2012) Optimization of matrix solid-phase dispersion for liquid chromatography tandem mass spectrometry analysis of 12 pharmaceuticals in sediments. J Chromatogr A 1258:1–15. doi:10.1016/j.chroma.2012.08.025
Qiang Z, Adams C (2004) Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res 38:2874–2890. doi:10.1016/j.watres.2004.03.017
Sarmah AK, Meyer MT, Boxall AB (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759. doi:10.1016/j.chemosphere.2006.03.026
Selvam A, Zhao Z, Wong JW (2012) Composting of swine manure spiked with sulfadiazine, chlortetracycline and ciprofloxacin. Bioresour Technol 126:412–417. doi:10.1016/j.biortech.2011.12.073
Sundberg C, Smars S, Jonsson H (2004) Low pH as an inhibiting factor in the transition from mesophilic to thermophilic phase in composting. Bioresour Technol 95:145–150. doi:10.1016/j.biortech.2004.01.016
Thiele-Bruhn S (2003) Pharmaceutical antibiotic compounds in soils—a review. J Plant Nutr Soil Sci 166:145–167. doi:10.1002/jpln.200390023
Thiele-Bruhn S (2005) Microbial inhibition by pharmaceutical antibiotics in different soils—dose–response relations determined with the iron (III) reduction test. Environ Toxicol Chem 24:869–876
Tolls J (2001) Sorption of veterinary pharmaceuticals in soils: a review. Environ Sci Technol 35:3397–3406. doi:10.1021/es0003021
Wu X, Wei Y, Zheng J, Zhao X, Zhong W (2011) The behavior of tetracyclines and their degradation products during swine manure composting. Bioresour Technol 102:5924–5931. doi:10.1016/j.biortech.2011.03.007
Zavala MA, Funamizu N, Takakuwa T (2005) Biological activity in the composting reactor of the bio-toilet system. Bioresour Technol 96:805–812. doi:10.1016/j.biortech.2004.07.009
Zhang H, Huang CH (2007) Adsorption and oxidation of fluoroquinolone antibacterial agents and structurally related amines with goethite. Chemosphere 66:1502–1512. doi:10.1016/j.chemosphere.2006.08.024
Zorpas AA, Loizidou M (2008) Sawdust and natural zeolite as a bulking agent for improving quality of a composting product from anaerobically stabilized sewage sludge. Bioresour Technol 99:7545–7552. doi:10.1016/j.biortech.2008.02.014
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 50838005), and the Program for Innovative Research Teamin Shanxi Province (Grant No. 2013KCT-13).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Shi, H., Wang, X.C., Li, Q. et al. Degradation of typical antibiotics during human feces aerobic composting under different temperatures. Environ Sci Pollut Res 23, 15076–15087 (2016). https://doi.org/10.1007/s11356-016-6664-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6664-7