Abstract
Attention is called to numerous publications that have recently appeared in Biomass Conversion and Biorefinery and reported on the application of single heating rate methods to pyrolysis data. Emphasis is laid on the fact that these methods generally fail to determine trustworthy kinetics triplets, i.e., the reaction model, activation energy, and preexponential factor. The reasons and instances of the failure are briefly discussed and illustrated. It is stressed that the International Confederation for Thermal Analysis and Calorimetry recommends single heating rate methods to be avoided and the methods that use several heating rates simultaneously to be employed for reliable kinetic analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This note calls attention of the readership of Biomass Conversion and Biorefinery to multiple publications that employ single heating rate methods for kinetic analysis of pyrolysis data. Only in the years 2021 and 2022 at least 21 such publications [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21] have appeared in this journal. In these publications, the kinetic measurements are conducted by nonisothermal thermogravimetric analysis at a single heating rate. Then, the Coats-Redfern method [22] is typically applied to the obtained data for determining the kinetic parameters, which include the activation energy, E, preexponential factor, A, and reaction model, g(α). All three parameters are oftentimes called the kinetic triplet.
The Coats-Redfern method is the most popular among the single heating rate methods and rather representative of the problem characteristic of all such methods. This problem resides in the fact that these methods cannot generally produce trustworthy kinetic parameters. In actual practice, a single heating rate data set can normally be described by several statistically equivalent kinetic triplets [23, 24]. Unfortunately, this fact remains commonly unnoticed because of not performing a proper statistical analysis. To put it simply, one usually chooses a single kinetic triplet related to the largest value of the correlation coefficient. Such a choice is unjustified statistically without testing whether the difference between the largest value and the correlation coefficient values for other kinetic triplets is statistically significant. A proper statistical analysis typically discovers that there are at least 2 or 3 statistically equivalent triplets [23, 24]. Yet, the difference in the activation energies in the statistically equivalent triplets readily reaches 100%. This difference represents the actual level of uncertainty characteristic of the methods that employ a single heating rate [24]. That is why these methods generally fail in evaluating trustworthy kinetic parameters. This is a well-established fact that was officially recognized by the Kinetics Committee of the International Confederation for Thermal Analysis and Calorimetry (ICTAC). In its recommendations for kinetic analysis, the Committee states [25] that the “methods that use single heating rate program…should be avoided” and that “the methods that use multiple heating rate programs…are recommended for computation of reliable kinetic parameters.”
The ICTAC recommendations have been guiding kinetic studies by thermal analysis for over a decade and have been cited 3600 [26] times. Presently, the majority of kinetic computations on thermal analysis data are performed by simultaneously using the data collected at several heating rates. In this regard, the fact that some workers continue to choose single heating rate methods for their kinetic studies is quite alarming. Needless to say that this misfortunate situation is in dire need of rectification.
The relevance of the ICTAC recommendations is further enhanced by quickly examining the kinetic triplets found in the above referenced papers. A simple piece of evidence that the kinetic triplet is likely to be faulty is unrealistically large or small values of the activation energy. For example, the values higher than 400 [1, 2] or even 500 and 600 [2] kJ mol−1 are most likely to be faulty in the case of organic compounds that include carbon–carbon and carbon–oxygen chemical bonds because the dissociation enthalpy of these bonds is less than 400 kJ mol−1 [27].
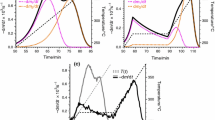
However, most of the above referenced papers report values that are much lower than 100 kJ mol−1. Such values are likely to be faulty because they are too low for decomposition of organic compounds that are known to be stable at ambient temperature. This can be exemplified by using one of the above referenced papers [9]. It reports the kinetic triplets for tea leaf brewing waste (TLBW) as well as for the cellulose (TLBW-C) and hemicellulose (TLBW-H) samples isolated from TLBW. The reaction model, g(α), for all three compounds is determined to be F3 (third-order reaction) and E (kJ mol−1) and ln(A/min−1) are reported as follows: 46.715 and 7.669 (TLBW), 23.838 and 11.916 (TLBW-C), and 14.424 and 13.407 (TLBW-H) [9]. These kinetic triplets can be used to predict the thermal stability of the samples at an ambient temperature of 27 °C. Such prediction is accomplished by inserting the reported kinetic triplets into Eq. (1) [23]:
where ta is time to reach the extent of decomposition a at temperature T.
According to this prediction (Fig. 1), the tea leaf brewing waste would decompose by ~ 50% in about 2 months. If this was true, this fact would be impossible to miss in practice. In turn, cellulose and hemicellulose would decompose completely on the scale of minutes and seconds, respectively, that would qualify both compounds as very unstable. This is most obviously incorrect based either on the common knowledge about cellulose and hemicellulose as well as on the fact that both compounds permitted their experimental examination without any evident problems associated with their stability [9].
The use of the kinetic triplets from Ref. [9] to predict decomposition at 27 °C for tea leaf brewing waste (TLBW) and the cellulose (TLBW-C) and hemicellulose (TLBW-H) samples isolated from TLBW
Even if a single heating rate method produces activation energy values that seem reasonable, it does not mean that the obtained kinetic triplets are not faulty. In this situation, the faulty nature of the kinetic triplets is easily discovered as their failure to correctly predict the isothermal kinetics. This issue is illustrated and discussed at length elsewhere [23].
On the other hand, reliable kinetic triplets are routinely obtained by various methods that simultaneously use data measured at multiple heating rates, as recommended by the ICTAC [25]. Hopefully, this note provides compelling arguments for abandoning single heating rate methods for the sake of the pyrolysis kinetics field.
Data availability
Data are derived from public domain resources. No materials are used.
References
Amer M, Nour M, Ahmed M et al (2021) Kinetics and physical analyses for pyrolyzed Egyptian agricultural and woody biomasses: effect of microwave drying. Biomass Convers Biorefinery 11:2855–2868. https://doi.org/10.1007/s13399-020-00684-3
Mu L, Wang R, Zhai Z et al (2021) Evaluation of thermokinetics methodology, parameters, and coke characterization of co-pyrolysis of bituminous coal with herbaceous and agricultural biomass. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01502-0
Lubwama M, Yiga VA, Wanambwa S et al (2021) Pyrolysis kinetics and combustion characteristics of local firewood species and charcoal produced by slow pyrolysis. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02050-3
Rivera JEG, Merencio DO, Vistín ASR et al (2022) Thermogravimetric characteristics and kinetic modeling of Piptocoma discolor pyrolysis and combustion processes to contribute to its use as a renewable energy source in the Ecuadorian Amazon region. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02178-2
Khan MA, Hameed BH, Siddiqui MR et al (2022) Physicochemical, structural and combustion analyses to estimate the solid fuel efficacy of hydrochar developed by co-hydrothermal carbonization of food and municipal wastes. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02875-6
Xu J, Yang L, Xu H et al (2022) Characteristics and kinetic analysis of the catalytic pyrolysis of oily sludge under new nickel-ore–based catalysts. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03069-w
Singh M, Gupta A, Yadav K et al (2021) Co-combustion properties of torrefied rice straw-sub-bituminous coal blend and its Hardgrove Grindability Index. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01696-3
Guo J, Ren X, Li S et al (2021) The impact of blending with poplar wood on the co-pyrolysis characteristics of waste particleboards. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01520-y
Taşar Ş (2022) Thermal conversion behavior of cellulose and hemicellulose fractions isolated from tea leaf brewing waste: kinetic and thermodynamic evaluation. Biomass Convers Biorefinery 12:2935–2947. https://doi.org/10.1007/s13399-021-01697-2
Aslam U, Aslam Z, Ashraf M, Kamal MS (2022) Influence of pretreatments on the fuel properties and pyrolytic kinetics of biomass. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02235-w
Li X, Zhao Q, Han M et al (2022) Pyrolysis characteristics and kinetic analysis of tobacco stem pretreated with different solvents. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02280-5
Elkhalifa S, Parthasarathy P, Mackey HR et al (2022) Biochar development from thermal TGA studies of individual food waste vegetables and their blended systems. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02441-0
Zhang J, Zhao R, Du Y et al (2022) Study on the co-pyrolysis characteristics of sewage sludge and wood powder and kinetic analysis. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02589-9
Wu J, Chen Z, Wang J et al (2022) Effect of glycerol addition on the pyrolysis characteristics and pyrolytic product distribution of cigar tobacco. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03175-9
Parthasarathy P, Al-Ansari T, Mackey HR, McKay G (2021) Effect of heating rate on the pyrolysis of camel manure. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01531-9
Demirpolat AB, Aydoğmuş E, Arslanoğlu H (2022) Drying behavior for Ocimum basilicum Lamiaceae with the new system: exergy analysis and RSM modeling. Biomass Convers Biorefinery 12:515–526. https://doi.org/10.1007/s13399-021-02010-x
Kumar P, Nandi BK (2021) Effect of rice husk and petroleum coke blending on combustion characteristics of high ash Indian coals. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02090-9
Jaguey-Hernández Y, Tapia-Ignacio C, Aguilar-Arteaga K et al (2022) Thermoplastic biofilms obtained from an arabinoxylan-rich fraction from brewers’ spent grain: physicochemical characterization and thermal analysis. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02288-x
Liu Y, Tan W, Liang S et al (2022) Comparative study on the co-combustion behavior of torrefied biomass blended with different rank coals. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02368-6
Shao F, Xu J, Jing Y et al (2022) Pyrolytic utilization of a typical halophyte: Suaeda glauca—the excellent adsorbent raw material for bisphenol S removal. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02859-6
Ashraf M, Aslam Z, Ramzan N et al (2021) Pyrolysis of cattle dung: model fitting and artificial neural network validation approach. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02051-2
Coats AW, Redfern JP (1964) Kinetic Parameters from Thermogravimetric Data. Nature 201:68–69. https://doi.org/10.1038/201068a0
Vyazovkin S, Wight CA (1999) Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta 340–341:53–68. https://doi.org/10.1016/S0040-6031(99)00253-1
Vyazovkin S (2001) Two types of uncertainty in the values of activation energy. J Therm Anal Calorim 64:829–835. https://doi.org/10.1023/A:1011573218107
Vyazovkin S, Burnham AK, Criado JM et al (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19. https://doi.org/10.1016/j.tca.2011.03.034
Scopus database. https://scopus.com. Accessed 01 Oct 2022
Atkins PW, De Paula J (2014) Physical chemistry: thermodynamics, structure, and change, 10th edn. Freeman, New York, W.H
Author information
Authors and Affiliations
Contributions
Sergey Vyazovkin: conceptualization, writing-original draft, review and editing. Nikita Muravyev: conceptualization, data analysis, writing-review and editing.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vyazovkin, S., Muravyev, N. Single heating rate methods are a faulty approach to pyrolysis kinetics. Biomass Conv. Bioref. 14, 16879–16881 (2024). https://doi.org/10.1007/s13399-022-03735-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03735-z