Abstract
The content of xylan in sugarcane straw (culm top and leaves) is interesting to produce xylooligosaccharides (XOS), oligomers composed of xylose, which provide numerous health benefits. XOS were produced in this study by two types of treatment using sugarcane leaves: liquid hot water (LHW) and dilute acid (sulfuric and acetic acids), aiming to minimize sugar degradation production. A central composite design with axial points was performed to evaluate the effects of the independent variables on the hydrolysis production of XOS. Hydrolysis with acetic acid resulted in the conversion of xylan into XOS of 22.78% with 2% (%, m/v) of acid at 180 °C for 35 min. Hydrolysis with sulfuric acid resulted in XOS yield of 62.18% with 2% (%, m/v) of acid at 79.55 °C for 35 min. The LHW treatment using leaves resulted in XOS yield of 20.71% at 130 °C for 35 min. The LHW and dilute acid resulted in 0.018% and 0.195% (m/m) of furfural, respectively. For each ton of sugarcane leaves, an XOS production of 206.44 kg, 75.63 kg, and 68.69 kg can be estimated using sulfuric acid, acetic acid, and LHW, successively. The most effective treatment for XOS production was hydrolysis with dilute sulfuric acid; however, LHW generated lower degradation products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The importance of sugarcane for the economy of tropical countries is due to the numerous products that can be obtained such as sugar and ethanol. Byproducts such as vinasse are formed during ethanol production and can be used as fertilizer or as an animal feed ingredient, as well as bagasse, generated after grinding the culm, which is also reused for energy production, paper, second-generation ethanol, among other products [1,2,3]. Another byproduct obtained from sugarcane is straw [4], formed mainly by dry leaves, green leaves, and a culm pointer. Straw is usually left in the fields after harvesting, with a potential risk of combustion, producing ashes that pollute the atmosphere, destroying the soil microbiota, and causing environmental damage. The amount generated varies according to the productivity and the type of sugarcane cultivated, corresponding to approximately 15 t/ha. Recent studies suggested that around 50% of the straw could be recovered from the field without impairing the soil properties [5].
Straw has a large amount of xylan in its composition, which can be depolymerized into monomers and can be used for xylitol and ethanol production through fermentation; and into oligomers, called xylooligosaccharides (XOS) [6]. XOS acts as prebiotics because of their ability to stimulate the growth of beneficial bacteria in the gut, prevent infections by pathogenic bacteria; have antioxidant properties, prevent osteoporosis, improve intestinal functions, reduce blood glucose and cholesterol levels, among other benefits [7]. XOS are stable under various temperatures and pH conditions and have a sweet taste, which are characteristics that facilitate their incorporation in foods [8].
Treatments such as liquid hot water (autohydrolysis) and dilute acid hydrolysis are widely studied for the biomass conversion process. In autohydrolysis, only water at high temperatures is used, able to break the bonds of acetyl groups present in the xylan chain, acidifying the medium and enhancing the hydrolysis of polysaccharides [9, 10]. In the acid hydrolysis/treatment, diluted acid is usually applied, such as sulfuric acid, hydrochloric acid, nitric acid to promote breakdown of the glycosidic bond between the polysaccharides, resulting in the release of monomers. There is a greater tendency for the generation of monosaccharide degradation products. However, after adjusting the treatment condition (optimization), LHW and dilute acid can produce xylooligosaccharides minimizing monosaccharides and degradation product content [6].
XOS can be produced by enzymatic hydrolysis of xylan, requiring previous xylan isolation [11]. However, for XOS production purified enzyme is required to reach a high conversion yield [7]. Alternatively, this study aimed at using sugarcane leaves for XOS production evaluating acid and LHW treatment instead of xylan previous solubilization. The objective of this study was to use sugarcane leaves for XOS production, important components of food and pharmaceutical applications due to the prebiotic effect. A comparison was made between autohydrolysis and hydrolysis with sulfuric and acetic acid, under milder conditions than those frequently used to decrease the formation of xylose and other undesirable products.
Mineral acids such as sulfuric acid are difficult to be recycled and reused; therefore, acetic acid was chosen in the present study because it is a hemicellulose component and an important factor in autohydrolysis and has the potential to hydrolyze biomass in an effective and environmentally safer way. In addition, compared to sulfuric acid, it is expected to generate a smaller amount of furfural and hydroxy-methylfurfural from sugar degradation. Sulfuric acid is widely studied in biomass conversion, usually at low concentrations, as it is a strong acid. The same conditions were maintained for acetic acid (weak acid) because there are few comparative studies, and there is a need for statistical analysis to determine the ideal conditions and the most advantageous hydrolysis process. In this context, the present study aimed to compare the sulfuric and acetic acid according to XOS and degradation products, applying a central composite design. Furthermore, several parameters were compared to statistically analyze the most favorable conditions for an efficient process for XOS production with minimum xylose and degradation products.
2 Materials and methods
2.1 Sample preparation
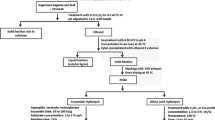
Sugarcane leaves were supplied by Usina São João, located in the city of Araras-SP, Brazil. Green leaves were dried in an oven at 60 °C for 48 h and ground in a knife mill, and the material was selected and sieved through a 20 mesh (825 μm) sieve [12].
2.2 Chemical characterization of biomass
The extractives were removed in Soxhlet with ethanol for a period of 8 h, followed by another extraction with distilled water for the same period. The filter paper bag was oven-dried at 105 °C for one night and cooled in a desiccator before mass determination. This procedure was repeated until constant mass. The extractive content was determined by the difference between the initial and final mass of filter paper bags and shown as a percentage.
Total ash content was determined with 1 g of sugarcane leaves burned in a muffle furnace at 575 °C for 4 h [13]. The crucible was then desiccated to room temperature (approximately 1 h) and weighed. The ash content was calculated based on the determined mass related to the leaf mass used.
Glucose, xylose, arabinose, acetic acid, and lignin contents were determined by the method reported elsewhere [14]. About 300 mg of extractive-free sugarcane leaves was hydrolyzed by adding 1.5 mL of 72% (m/m) sulfuric acid at 45 °C for 7 min.. After the reaction period, 45 mL of distilled water was added, and the flasks were autoclaved at 121 °C for 30 min.. After cooling, the hydrolysate was filtered through a previously tared crucible porous plate. The liquid fraction obtained was analyzed by high-performance liquid chromatography (HPLC) according to item 2.3. To determine the acid-soluble lignin content, the liquid fraction was diluted with 4% (m/m) sulfuric acid until the absorbance was between 0.2 and 1.0 and quantified by UV–Vis spectrophotometry at 215 and 280 nm (Eq. (1)). The solid residue was washed with distilled water and oven-dried at 105 °C to determine insoluble lignin (Eq. (2)):
where A215 is the absorbance at 215 nm; A280 is the absorbance at 280 nm.
where m2 = mass of crucible with lignin; m1 = mass of crucible; m3 = extractive-free sugarcane leaf mass.
2.3 Acid hydrolysis for XOS production
About 5 g of sugarcane leaves at 20 mesh was hydrolyzed with 50 mL of acetic or sulfuric acid in a stainless steel reactor of 0.08 L with 6.1-cm height and 4-cm diameter. The tests were performed with a variation of temperature (°C), time (min), and acid concentration (% m/v, mass acid/volume solution) according to a central composite design 23, star rotational, and triplicate at the central point (Table 1). The independent variables were divided into three levels: maximum, with a temperature of 160 °C, time of 55 min, and acid concentration of 3%; minimum, 100 °C, 15 min, and 1% (%, m/v) acid and the center point, 130 °C, 35 min, and 2% (%, m/v) acid. The heating process was performed in a thermostated bath. The acid hydrolysis experimental condition was selected based on literature reports [15,16,17].
After the reaction was completed, the reactor was cooled to room temperature using an ice bath. The liquid fraction containing XOS and xylose was separated from the solid fraction by vacuum filtration using filter paper, filtered with a syringe filter of 0.22 µm, and quantified by HPLC. XOS and xylose contents were calculated relative to the amount of xylan in the material. The results were analyzed by Statistica software, version 7.0.
2.4 Liquid hot water treatment for XOS production
In the LHW treatment, the assays were performed according to a central composite design 22, star rotational, and triplicate at the central point, varying temperature (°C) and reaction time (min). These independent variables were divided into three levels: maximum, with a temperature of 160 °C and time of 55 min; minimum, 100 °C and 15 min and the center point, 130 °C and 35 min (Table 2) [16].
About 5 g of sugarcane leaves at 20 mesh and 50 mL of distilled water were added to the reactor. After completion of the reaction time in a thermostated bath, the reactor was cooled in an ice bath, and the material was vacuum filtered using filter paper. The liquid fraction was filtered using a syringe filter with a 0.22-μm pore size and quantified by HPLC to determine the XOS and xylose contents according to Eqs. (3) and (4), respectively. XOS and xylose content was calculated relative to the amount of xylan in the material.
2.5 Determination of XOS, sugars, acetic acid, and degradation products
XOS contents were determined by HPLC in equipment (Shimadzu, model Nexera XR) under the following conditions: BIO-RAD Aminex HPX-87C (300 × 7.8 mm) column; temperature: 80 °C; eluent: ultrapure water with flow 0.6 mL/min; sample volume: 20 μL; detector: refractive index at 60 °C (Shimadzu, RID model) with an analysis time of 15 min. The pH of the samples was adjusted between 5.0 and 9.0 with 1 mol/L sodium hydroxide (NaOH). Xylose (Sigma), xylobiose (X2), xylotriose (X3), xylotetraose (X4), and xylopentaose/xylohexaose (X5/6) (Megazyme-Ireland) solutions were used as standards. Samples were pre-filtered on a syringe filter with a 0.22-μm pore size [18].
Glucose, xylose, arabinose, and acetic acid contents were determined by HPLC, using the Bio-Rad Aminex HPX-87H (300 × 7.8 mm) column maintained at 45 °C, Waters 2414 refractive index detector, a mobile phase of sulfuric acid 0.005 mol/L, a flow rate of 0.6 mL/min, the volume of the injected sample of 20 μL. Samples were pre-filtered on a syringe filter with a 0.22-μm pore size. The percentage of glucose, xylose, arabinose, and acetic acid were multiplied by the hydrolysis factor, 0.9, 0.88, 0.88, and 0.72, respectively, to determine the percentage of glucan/cellulose, xylan, arabinan, and acetyl groups.
Three samples from each treatment (acid hydrolysis and LHW) were analyzed: one with the highest yield of XOS, the second with the lowest yield, and one sample from the central point (intermediate experimental conditions). Hydroxymethyl-furfural (HMF) and furfural contents were determined by HPLC under the following conditions: C18 (150 × 4.6 mm) column maintained at 25 °C, a mobile phase of acetonitrile, water, and acetic acid, a flow rate of 0.8 mL/min, the volume of the injected sample of 10 μL, with an analysis time of 20 min. Samples were pre-filtered on a syringe filter with a 0.22-μm pore size.
3 Results and discussion
3.1 Leaf chemical composition
The leaf chemical composition was 37.9% of cellulose, 33.2% of hemicellulose, 25.2% of lignin, 3.5% of ashes, and 17.6% of extractives (Table 3). For the straw (top part of the culm plus leaves), data from the literature were in the range of 31.7–39.4% of cellulose, 27–29.9% of hemicellulose, 21.3–31.1% of lignin, and 1.5–6.2% of ash. This study used the leaves, excluding the top part of the culm. The chemical composition determined for the leaves showed to be in the range reported in the literature for straw (Table 3). The extractive content found in the leaves was 17.6%, higher than the content reported for straw [19, 20]. The advantage of performing a study with a material such as leaves is the lower heterogeneity compared to straw, which is a mix of materials (dry leaves, green leaves, and a culm pointer). Fractionation or isolation of biomass may be advantageous to evaluate the process or material influence on recalcitrance [11, 21].
3.2 Diluted acetic acid treatment for XOS production
The maximum XOS yield obtained from the treatment of the leaves with acetic acid was in assay 10, resulting in 6.20 g/L, with an acid concentration of 2%, 180 °C, for 35 min (Table 4). This condition resulted in higher xylotriose formation. The conversion of xylan into XOS was 22.78% (%, m/m), and into xylose it was only 4.28% (%, m/v). Oligomers larger than 6 units may have been formed during the reaction with acetic acid but could not be quantified by the chromatography method used.
Conversion of xylan into XOS equivalent to 55.8% was reported on the hydrolysis with two steps of acetic acid of poplar. The conditions used were 30 min of reaction time, 170 °C, and 5% (%, m/v) acid concentration [22]. For poplar hydrolysis, an acid concentration above that is used in the present study was necessary, showing the recalcitrance difference between poplar and sugarcane leaves. The hydrolysis of corncob with acetic acid resulted in a 45.91% conversion of xylan into XOS; however, it produced 24.53% (%, m/m) of xylose [23]. The authors used an acid solution with a pH adjusted to 2.7, a temperature of 150 °C, and a time of 30 min of reaction. High xylose production together with XOS is a disadvantage due to a requirement of the purification process. The present study showed the advantage of optimization to decrease the xylose production, avoiding, or minimizing purification needed.
Sugarcane bagasse was hydrolyzed with 10% acetic acid at 150 °C for 45 min resulting in an XOS yield of 39.1%, and a xylose yield of 8.95%, with degradation products of 0.09% of HMF and 0.46% of furfural [24]. In the present study, the highest yield of degradation products was obtained with 2% of acid concentration. A literature comparison suggested that processes with low severity (low acid concentration, temperature, or reaction time) tend to result in higher XOS formation and a lower concentration of xylose. On the other hand, high acid concentration tends to xylose production [25].
Table 5 shows the values of furfural and HMF formed in the hydrolysis with acetic acid of sugarcane leaves. The samples selected were those with higher and lower XOS yield and a center point sample. These samples were selected because they represent the best experimental condition for XOS production contrary to the lower yield. The central point sample represents the intermediate experimental condition. Analysis of variance (ANOVA) with 99% confidence (p < 0.01) showed that acid concentration and the temperature had a significant effect on the acetic acid hydrolysis for XOS production (Table 6). The polynomial model was significant as 72% of the experimental data adjusted to the acid hydrolysis model for sugarcane leaves.. The statistical model that describes xylan conversion into XOS with acetic acid was represented by Eq. (5).
where: X = temperature (°C); Y = reaction time (min); Z = CH3COOH concentration (m/v).
After the equation/model was obtained, it was possible to build the response surface. It was observed that the highest conversion of xylan into XOS occurred in the region with acid concentrations up to 2% and temperatures above 160 °C (Fig. 1). The increase in an acid concentration above 2% caused a decrease in XOS production according to the model (Fig. 1); therefore, this parameter had a significant negative effect. The temperature, however, had the opposite effect, as its increase potentiated the formation of XOS.
XOS was produced by hydrolysis of poplar with acetic acid, without xylan isolation. The experiment was carried out with the concentration of acetic acid up to 10% at 170 °C and time between 10 and 50 min. The optimized condition was with 5% acetic acid at 170 °C for 30 min, with xylan to XOS conversion of 31.6% [25]. The temperature and reaction time variables were similar to those of the present study. In the present study, 22.78% of XOS was obtained with 2% of acid at 180 °C/35 min. Considering the 2.5 times less acid used, 2/3 of XOS was obtained in relation to the poplar treatment. The acid is important as a catalyst and for acetic acid, a low concentration is suggested to work better at increasing temperature. The increase in the temperature is a limiting process for equipment; however, reaction time, on the other hand, is a parameter that can be altered for this process considering the constant temperature. The acetic acid treatment of sugarcane bagasse for XOS production [6] was in the same range of variables (acid concentration, reaction time) for the XOS production from sugarcane leaves in the present study. Acetic acid concentration should be used at a low level to improve XOS production with a temperature above 200 °C. Furoic acid applied to XOS production was identified as in the same region of the present study, in terms of acid concentration, reaction time, and temperature [26]. The need for the high temperature (> 160 °C) for acetic and furoic acidis possibly related to the acid dissociation to hydrolyze the xylan into XOS. For an improvement in the acetic acid treatment, a two-step hydrolysis was proposed at 140 °C and 190 °C [27]. A higher acid concentration in relation to the present study, the acid concentration of 5% (v/v) was applied in two-step wheat straw hydrolysis reaching 61.44% of XOS. The yield, chemicals used, and experimental conditions can influence the feasibility of the process [28]. Moreover, this process will be analyzed together with sulfuric acid and LHW treatment for XOS production.
3.3 Diluted sulfuric acid hydrolysis for XOS production
The maximum conversion of xylan into XOS was 62.18%, in a concentration of 16.91 g/L, and this occurred in the assay 9 conditions: a temperature of 79.55 °C, a reaction time of 35 min, and 2% sulfuric acid (m/v). There was a greater formation of xylotriose and conversion of xylan into xylose of 1.30% (Table 7). Under the conditions of the present study, there was a greater tendency for oligomer formation with 2 or 3 xylose units. The size of the xylan chain after hydrolysis depends on the method used and its severity [29]. XOS with a lower degree of polymerization probably act better as prebiotics because they are consumed faster by intestinal bacteria [30]. In hydrolysis with sulfuric acid of kraft liquor pulping process, XOS concentration was 11.63 g/L achieved with 0.3% acid at 120 °C for 2 h [31]. Acid concentrations and high temperatures are responsible for the formation of unwanted products such as furfural [32]. The acid hydrolysis success also depends on the material used, which is related to the recalcitrance of the material. Hemicellulose was solubilized from viscose and submitted to acid hydrolysis for XOS production. Using 0.70 mol/L sulfuric acid, for 30 min at 80 °C, the solubilized hemicellulose was hydrolyzed producing 67.9% of XOS and 29.2% of xylose [33]. Comparatively, in the present study only 1.30% of xylose was produced, probably due to the low acid applied. A 1% (m/m) sulfuric acid hydrolysis of hemicellulose for 60 min at 120 °C resulted in 45% of XOS, predominantly X6, and 20% of xylose [34]. The present study showed that an optimized condition (acid concentration, reaction time, and temperature) direct applied to the lignocellulosic material (sugarcane leaves) is better for a medium reach in XOS with a minimal content of xylose (Table 7).
Table 8 shows the values of furfural and HMF formed in the hydrolysis with sulfuric acid of sugarcane leaves. The samples selected for furfural and HMF determination were those with higher and lower XOS yield, and a center point sample (intermediate experimental conditions). Analysis of variance (ANOVA) with 99% confidence (p < 0.01) showed that the acid concentration and the interaction between acid and time presented a significant effect on XOS production (Table 9). The polynomial model showed significance as 84% of the experimental data adjusted to the hydrolysis model with sulfuric acid. The statistical model that describes xylan conversion into XOS using leaves was represented by Eq. (6).
where X = temperature (°C); Y = reaction time (min); Z = H2SO4 concentration (m/v).
By analyzing the response surface, it observed that the highest conversion of xylan into XOS was in the region where the acid concentration was between 1.5 and 3.5%, and the temperature was higher than 160 °C (Fig. 2).
An optimization study produced 42% of XOS in the best conditions of the reaction time of 5.5 min, sulfuric acid concentration up to 0.9%, and temperature between 160 °C and 190 °C [35]. The acid concentration and reaction time had little significance in the yield, except when associated with the temperature variable, which had more impact on the process. Probably, the low acid concentration and short reaction time required for high temperature justify the influence of this variable. In the present study, 1.5 times more XOS was produced with 2% of sulfuric acid, at 79.55 °C/35 min. The decreasing temperature required a higher acid concentration (2 times), and higher reaction time (6 times). Furthermore, it is important to understand the contribution of each variable to the XOS production cost.
3.4 Liquid hot water for XOS production
The highest values were obtained under the experimental conditions of the central point, with a temperature of 130 °C for 35 min, corresponding to 1.63% xylobiose, 6.68% xylotriose, 12.40% xylopentaose/xylohexaose. The total XOS concentration was 5.59 g/L. Xylan conversion into XOS was 20.71% and into xylose was 0.60% (Table 10). LHW of various lignocellulosic materials resulted in the maximum yield of XOS at the temperature and time in the range of 220 °C and 35 min. Conversion based on the material (mass XOS/mass raw material) was 27.1% for barley husks, 24.8% for corncobs, 18% for rice husks, and 15.4% for Eucalyptus globulus wood [34]. In the literature, yields are reported in the same range obtained in the present study and probably influenced the type of raw material used. Wheat straw was LHW treated in a microwave reactor for 30 min at 180 °C, producing 24% of XOS with DP 2–6 [36]. LHW treatment of hazelnut shells at a temperature of 190 °C and a reaction time of 5 min, resulted in 10 g/L of XOS [37]. The XOS concentration in the present study was 5.59 g/L with the central point conditions (130 °C/35 min). At 160 °C/15 min, the concentration was 5.22 g/L and at 172.43 °C/35 min, the concentration decreased to 3.93 g/L (Table 10). The XOS concentration in a hydrothermal process can be related to the raw material charge, producing a hydrolysate with a high concentration.
Table 11 shows the values of furfural and HMF formed in the LHW treatment of sugarcane leaves. The samples used were from those conditions that produced higher and lower XOS yields and a sample from the center point. The high severity of the reaction, which is defined by the influence of temperature and reaction time, the better the hydrolysis of the acetyl groups of the xylan, causing an increase in the acidity of the medium, and consequently improving the breakdown of the xylan chain into XOS. However, the high severity can result in sugar degradation products such as furfural and HMF [32]. LHW with low severity can generate XOS containing more than 6 xylose units. For the production of a smaller chain of XOS, additional treatment with enzymes or chemicals may be required.
Analysis of variance (ANOVA) was performed showing the effect of the independent variables (temperature and time) on the conversion of xylan into XOS (dependent variable), with a confidence level of 99% (p < 0.01) (Table 12). For the sugarcane leaves, LHW treatment reaction time and temperature presented a significant effect. The model proved to be significant, as 95% of the experimental data was adjusted to the polynomial model. The statistical model that describes the conversion of xylan into XOS using the leaves was represented by Eq. (7).
where X = reaction time (min); Y = temperature (°C).
Figure 3 shows the response surface and the contour plot for the variable XOS obtained in the LHW treatment of the sugarcane leaves. The best yield can be obtained with a temperature above 160 °C and a reaction time interval between 25 and 50 min.
An optimization LHW study for XOS production using sugarcane straw obtained a conversion of xylan into XOS of 41.13% at a temperature between 170 and 180° C and a reaction time of 60 min to 80 min [38]. This conversion is higher than that obtained in the present study (20.71%) but with the greater formation of unwanted products, such as xylose (14.6%) and furfural (7%). The present study determined conditions where unwanted products, such as furfural and HMF were lower than 0.02% (Table 11). Furfural production could be related to biomass used, perhaps influenced by the recalcitrance of each material. LHW treatment of sugarcane bagasse resulted in 1.6% of furfural. This amount of furfural is 4 times lower compared to LHW treatment of sugarcane straw [38].
3.5 XOS production from sugarcane leaves on a large scale
XOS with a degree of polymerization between 2 and 9 are used as prebiotics. Xylobiose and xylotriose are sweeter; they have no calories and can be used in weight loss diets. A degree of polymerization between 2 and 4 offers greater thermal stability, which is desirable for the food industry. Xylobiose is not hydrolyzed in our body, and is used by intestinal bacteria, such as Lactobacilli and various types of Bifidobacteria. Harmful bacteria such as Escherichia coli and Clostridium spp. are not able to consume XOS [39].
The chemical reaction to produce XOS occurs when the hydronium cation from the acid binds to oxygen between the xylose monomers, breaking the ether bond, and producing xylooligosaccharides of varying sizes and xylose (Supplemental Fig. 1). In the case of autohydrolysis, the hydronium cation comes from acetic acid formed by the cleavage of the acetyl groups of hemicellulose, under high temperature and pressure [40].
The industrial production of XOS from sugarcane leaves using acetic acid, sulfuric acid hydrolysis, and LHW treatment can be predicted based on the results from the present study. A sustainable process is desirable for XOS production using biomass as raw material, an advantage of using sugarcane leaves. The industrial XOS production depends on the availability of the raw material and the process used. Sugarcane leaves are produced in high amounts in tropical countries, such as Brazil. Considering hydrothermal treatment (with or without acid as a catalyst), the production of monosaccharides and degradation products need to be considered. These factors need to be investigated and considered as they could require a purification step.
For each ton of sugarcane leaves hydrolyzed with acetic acid (2%, 180 °C for 35 min), 75.63 kg of XOS, 14.21 kg of xylose, and 0.35 kg of furfural can be produced. Using sulfuric acid hydrolysis (79.55 °C, 35 min, and 2% acid), 206.44 kg of XOS, 4.32 kg of xylose, and 0.40 kg of furfural can be produced. With sulfuric acid hydrolysis (79.55 °C, 35 min, and 2% acid) can be produced 206.44 kg of XOS, 4.32 kg of xylose, and 0.40 kg of furfural. LHW treatment can produce 68.69 kg of XOS, 5.41 kg of xylose, and 0.07 kg of furfural (Fig. 4). The important question here is how much xylose and furfural could be presented in a prebiotic medium with no negative effect on the growth of the probiotic microorganisms. Certainly, xylose stimulates probiotic microorganism growth [7]. On the other hand, degradation products are known to inhibit microorganism growth [30], and this effect needs to be determined for probiotic microorganisms.
4 Conclusion
Sulfuric acid hydrolysis was more efficient than acetic acid hydrolysis and LHW treatment for XOS production, with a conversion of 62.18% of xylan into XOS and formation of short-chain oligomers, with up to 3 xylose units. There was a formation of xylose, furfural, and HMF making the XOS purification process probably more difficult and expensive. In LHW treatment, the highest XOS yield was 20.71% and hydrolysis with acetic acid produced 22.78% of XOS. In both cases, the lower XOS yield could be explained by mild process conditions that break xylan into long-chain oligomers. The temperature increase has the potential to increase the concentration of short-chain XOS, between 2 and 6 xylose units. LHW treatment resulted in lower XOS production compared to acid hydrolysis but it was the treatment that generated the lower amount of degradation products. Perhaps, these low degradation products do not require a purification step depending on the final application.
Data availability
Not applicable.
References
Tapia Carpio L, Simone de Souza F (2019) Competition between second-generation ethanol and bioelectricity using the residual biomass of sugarcane: effects of uncertainty on the production mix. Molecules 24:369. https://doi.org/10.3390/molecules24020369
Melati RB, Schmatz AA, Pagnocca FC et al (2017) Sugarcane bagasse: production, composition, properties, and feedstock potential. In: Murphy R (ed) Sugarcane Prod Syst Uses Econ Importance. Nova Science Publishers, Hauppauge, pp 1–38 Available from: https://novapublishers.com/shop/sugarcane-production-systems-uses-and-economic-importance/
Melati RB, Shimizu FL, Oliveira G et al (2019) Key factors affecting the recalcitrance and conversion process of biomass. Bioenerg Res 12:1–20. https://doi.org/10.1007/s12155-018-9941-0
de Moraes LAA, de Conti Medina C, de Melo TR et al (2021) Does the Partial Raw Cane System Present Possibilities to Increase Sugarcane Field Longevity in Clayey Soil? Sugar Tech 23:999–1009. https://doi.org/10.1007/S12355-021-00993-5/TABLES/3
de Aquino GS, de Conti MC, Shahab M et al (2018) Does straw mulch partial-removal from soil interfere in yield and industrial quality sugarcane? A long term study. Ind Crops Prod 111:573–578. https://doi.org/10.1016/j.indcrop.2017.11.026
Forsan CF, de Freitas C, Masarin F, Brienzo M (2021) Xylooligosaccharide production from sugarcane bagasse and leaf using Aspergillus versicolor endoxylanase and diluted acid. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01403-2
de Freitas C, Terrone CC, Masarin F et al (2021) In vitro study of the effect of xylooligosaccharides obtained from banana pseudostem xylan by enzymatic hydrolysis on probiotic bacteria. Biocatal Agric Biotechnol 33:101973. https://doi.org/10.1016/j.bcab.2021.101973
Mhetras N, Mapre V, Gokhale D (2019) Xylooligosaccharides (XOS) as emerging prebiotics: its production from lignocellulosic material. AiM 09:14–20. https://doi.org/10.4236/aim.2019.91002
Zamora Zamora HD, de Freitas C, Bueno D et al (2020) Biomass fractionation based on enzymatic hydrolysis for biorefinery systems. In: Verma P (ed) Biorefineries: a step towards renewable and clean energy. Springer Singapore, Singapore, pp 217–254
Shimizu FL, Zamora HDZ, Schmatz AA et al (2020) Biofuels generation based on technical process and biomass quality. In: Srivastava N, Srivastava M, Mishra PK, Gupta VK (eds) Biofuel production technologies: critical analysis for sustainability. Springer Singapore, Singapore, pp 37–64
Melati RB, Sass DC, Pagnocca FC, Brienzo M (2021) Anatomic influence of sugarcane biomass on xylan solubilization. Ind Crops Prod 164:113357. https://doi.org/10.1016/j.indcrop.2021.113357
Fernandes ÉS, Bueno D, Pagnocca FC, Brienzo M (2020) Minor biomass particle size for an efficient cellulose accessibility and enzymatic hydrolysis. ChemistrySelect 5:7627–7631. https://doi.org/10.1002/slct.202001008
ABNT Catalogo. https://www.abntcatalogo.com.br/norma.aspx?ID=395361. Accessed 5 Oct 2021
Gouveia ER, do Nascimento RT, Souto-Maior AM, de Rocha GJM (2009) Validação de metodologia para a caracterização química de bagaço de cana-de-açúcar. Quím Nova 32:1500–1503. https://doi.org/10.1590/S0100-40422009000600026
Tsoutsos T (2010) Modelling hydrolysis and fermentation processes in lignocelluloses-to-bioalcohol production. In: Bioalcohol Production. Elsevier, 340–362
Vallejos ME, Felissia FE, Kruyeniski J, Area MC (2015) Kinetic study of the extraction of hemicellulosic carbohydrates from sugarcane bagasse by hot water treatment. Ind Crops Prod 67:1–6. https://doi.org/10.1016/j.indcrop.2014.12.058
Vena PF, Brienzo M, García-Aparicio M et al (2015) Dilute sulphuric acid extraction of hemicelluloses from eucalyptus grandis and its effect on kraft and soda-AQ pulp and handsheet properties. Cellu Chem Technol 49:1
Mafei TDT, Neto FSPP, Peixoto G et al (2020) Extraction and characterization of hemicellulose from eucalyptus by-product: assessment of enzymatic hydrolysis to produce xylooligosaccharides. Appl Biochem Biotechnol 190:197–217. https://doi.org/10.1007/s12010-019-03076-0
Szczerbowski D, Pitarelo AP, Zandoná Filho A, Ramos LP (2014) Sugarcane biomass for biorefineries: comparative composition of carbohydrate and non-carbohydrate components of bagasse and straw. Carbohyd Polym 114:95–101. https://doi.org/10.1016/j.carbpol.2014.07.052
Costa SM, Aguiar A, Luz SM et al (2015) Sugarcane straw and its cellulose fraction as raw materials for obtainment of textile fibers and other bioproducts. In: Ramawat KG, Mérillon J-M (eds) Polysaccharides. Springer International Publishing, Cham, pp 513–533
Brienzo M, Ferreira S, Vicentim MP et al (2014) Comparison study on the biomass recalcitrance of different tissue fractions of sugarcane Culm. Bioenerg Res 7:1454–1465. https://doi.org/10.1007/s12155-014-9487-8
Wen P, Zhang T, Wang J et al (2019) Production of xylooligosaccharides and monosaccharides from poplar by a two-step acetic acid and peroxide/acetic acid pretreatment. Biotechnol Biofuels 12:87. https://doi.org/10.1186/s13068-019-1423-x
Zhang H, Xu Y, Yu S (2017) Co-production of functional xylooligosaccharides and fermentable sugars from corncob with effective acetic acid prehydrolysis. Biores Technol 234:343–349. https://doi.org/10.1016/j.biortech.2017.02.094
Zhou X, Xu Y (2019) Integrative process for sugarcane bagasse biorefinery to co-produce xylooligosaccharides and gluconic acid. Biores Technol 282:81–87. https://doi.org/10.1016/j.biortech.2019.02.129
Ying W, Fang X, Xu Y, Zhang J (2022) Combined acetic acid and enzymatic hydrolysis for xylooligosaccharides and monosaccharides production from poplar. Biomass Bioenerg 158:106377. https://doi.org/10.1016/j.biombioe.2022.106377
Zhao J, Zhang X, Zhou X, Xu Y (2021) Selective production of xylooligosaccharides by Xylan Hydrolysis Using a Novel Recyclable and Separable Furoic Acid. Front Bioeng Biotechnol 9:240. https://doi.org/10.3389/FBIOE.2021.660266/BIBTEX
Guo J, Gu Y, Zhou X et al (2021) Cascade temperature-arising strategy for xylo-oligosaccharide production from lignocellulosic biomass with acetic acid catalyst recycling operation. Renew Energy 175:625–637. https://doi.org/10.1016/J.RENENE.2021.05.066
Martins RP, Schmatz AA, de Freita LA et al (2021) Solubilization of hemicellulose and fermentable sugars from bagasse, stalks, and leaves of sweet sorghum. Ind Crops Prod 170:113813. https://doi.org/10.1016/J.INDCROP.2021.113813
Riley GL, Brienzo M, Flavia A et al (2016) Sugarcane bagasse hemicellulose properties, extraction technologies and xylooligosaccharides production. The sugarcane has been used for centuries for sugar production and, in the recent decades, for ethanol fuel production through biotechnological routes. From sugar and ethanol industry a large amount
Surek E, Buyukkileci AO (2017) Production of xylooligosaccharides by autohydrolysis of hazelnut (Corylus avellana L.) shell. Carbohyd Polym 174:565–571. https://doi.org/10.1016/J.CARBPOL.2017.06.109
Chen X, Cao X, Sun S et al (2018) Evaluating the production of monosaccharides and xylooligosaccharides from the pre-hydrolysis liquor of kraft pulping process by acid and enzymatic hydrolysis. Ind Crops Prod 124:906–911. https://doi.org/10.1016/J.INDCROP.2018.08.071
Candido JP, Claro EMT, de Paula CBC et al (2020) Detoxification of sugarcane bagasse hydrolysate with different adsorbents to improve the fermentative process. World J Microbiol Biotechnol 36(3):1–12. https://doi.org/10.1007/S11274-020-02820-7
Wang T, Li C, Song M, Fan R (2019) Xylo-oligosaccharides preparation through acid hydrolysis of hemicelluloses isolated from press-lye. Grain & Oil Science and Technology 2:73–77. https://doi.org/10.1016/J.GAOST.2019.07.001
Wang Y, Cao X, Zhang R et al (2018) Evaluation of xylooligosaccharide production from residual hemicelluloses of dissolving pulp by acid and enzymatic hydrolysis. RSC Adv 8:35211–35217. https://doi.org/10.1039/C8RA07140C
Marcondes WF, Milagres AMF, Arantes V (2020) Co-production of xylo-oligosaccharides, xylose and cellulose nanofibrils from sugarcane bagasse. J Biotechnol 321:35–47. https://doi.org/10.1016/j.jbiotec.2020.07.001
Parajó JC, Garrote G, Cruz JM, Dominguez H (2004) Production of xylooligosaccharides by autohydrolysis of lignocellulosic materials. Trends Food Sci Technol 15:115–120. https://doi.org/10.1016/J.TIFS.2003.09.009
Xu J, Liu B, Wu L et al (2019) A waste-minimized biorefinery scenario for the hierarchical conversion of agricultural straw into prebiotic xylooligosaccharides, fermentable sugars and lithium-sulfur batteries. Ind Crops Prod 129:269–280. https://doi.org/10.1016/J.INDCROP.2018.12.002
Dias LM, Neto FSPP, Brienzo M et al (2022) Experimental design, modeling, and optimization of production of xylooligosaccharides by hydrothermal pretreatment of sugarcane bagasse and straw. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-02151-z
Forsan CF, Paz Cedeño FR, Masarin F, Brienzo M (2021) Xylooligosaccharides production by optimized autohydrolysis, sulfuric and acetic acid hydrolysis for minimum sugar degradation production. Bioactive Carbohydrates and Dietary Fibre 26:100268. https://doi.org/10.1016/j.bcdf.2021.100268
Kamireddy SR, Li J, Tucker M et al (2013) Effects and mechanism of metal chloride salts on pretreatment and enzymatic digestibility of corn stover. Ind Eng Chem Res 52:1775–1782. https://doi.org/10.1021/ie3019609
Miléo PC, Mulinari DR, Baptista CARP et al (2011) Mechanical behaviour of polyurethane from castor oil reinforced sugarcane straw cellulose composites. Procedia Eng 10:2068–2073. https://doi.org/10.1016/j.proeng.2011.04.342
Ávila PF, Forte MBS, Goldbeck R (2018) Evaluation of the chemical composition of a mixture of sugarcane bagasse and straw after different pretreatments and their effects on commercial enzyme combinations for the production of fermentable sugars. Biomass Bioenerg 116:180–188. https://doi.org/10.1016/j.biombioe.2018.06.015
Hernández-Pérez AF, de Arruda PV, Felipe MD (2016) Sugarcane straw as a feedstock for xylitol production by Candida guilliermondii FTI 20037. Brazilian J Microbiol 47:489–496. https://doi.org/10.1016/j.bjm.2016.01.019
Canilha L, de LacerdaBrambilla Rodrigues RC, Fernandes FA et al (2013) Bioconversion of hemicellulose from sugarcane biomass into sustainable products. In: Chandel A (ed) Sustainable degradation of lignocellulosic biomass - techniques, applications and commercialization. InTech
Funding
This research received the support of the Brazilian Council for Research and Development (CNPq, process 303239/2021–2) and São Paulo Research Foundation (FAPESP, process 2017/22401–8).
Author information
Authors and Affiliations
Contributions
Conceptualization: Michel Brienzo; formal analysis: Caroline Froes Forsan, Alison Schmatz; resources: Michel Brienzo, Fernando Masarin; writing original draft: Caroline Froes Forsan; manuscript revision: Michel Brienzo, Alison Schmatz, Fernando Masarin.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Forsan, C.F., Schmatz, A., Masarin, F. et al. Xylooligosaccharide production by optimized sulfuric, acetic acid, and liquid hot water treatment of sugarcane leaves. Biomass Conv. Bioref. 14, 11217–11228 (2024). https://doi.org/10.1007/s13399-022-03316-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03316-0