Abstract
The heart of an electrical system network is power transformers, and its livelihood is insulating oil, which provides insulation and cooling to the transformers. The elements of insulating oil determine the electrical properties of the oil, and the properties of the insulating oil can be optimized by bring in nanofluid. The chief aim of this work is to analyze the impact of Fe3O4, ZnO and TiO2 nanoparticles individually and its mixtures (Fe3O4 + ZnO) and (Fe3O4 + TiO2) at different volume concentration on dielectric strength of mineral oil and ester oil. Laboratory-oriented experimental results are statistically analyzed using normal and Weibull distribution technique. The results of the experiments reveal that conductive nanoparticles blended mineral oil and pongamia ester oil-based nanofluids samples S6 (MO + 0.4% Fe3O4) and S18 (EO + 0.4% Fe3O4) reported with highest enhancement percentage in BDV as 52% and 53%, respectively, compared to other nanoparticles. While looking over the performance of mixed nanoparticles, the samples (S27 MO + 75% Fe3O4 + 25% TiO2) and S33 (EO + 75% Fe3O4 + 25% TiO2) reported with highest enhancement percentage in Breakdown Voltage (BDV) as 65% and 69%, respectively. When comparing the BDV of individual nanofluid samples and mixed nanofluid samples, the mixed nanofluid samples have higher enhancement percentage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Most commonly utilized insulating liquid in oil-filled devices such as power transformers, tap changers, power capacitors, bushings, circuit breakers and cables is Mineral oil (MO). It has strong dielectric strength and thermal performance, which are two important qualities for this type of component [1]. There are many different types of transformer fluids available nowadays, but ecologically friendly liquids are the most recent breakthrough in dielectric fluids for electrical transformers, bushings, circuit breakers and others. These fluids, also known as natural esters or bio-based fluids, are produced spontaneously by living organisms and are derived from plant crops such as coconut, neem, palm, rapeseed, sunflower, and others. Because of its environmentally friendly characteristics and strong fire resistance, this novel "green" alternative has found a niche in the market [2,3,4].
Dielectric properties, heat transfer ability and physical properties are the important qualities of transformer fluid. It can be further enhanced by using specialized nanoparticles (NP), such as conductive and semi-conductive. Indeed, the resulting nanofluids (NF) have higher thermal characteristics [5] as well as dielectric and physical properties than MO and ester oil. Choi and Eastern developed the concept of NF in 1995 [6] and detailed its advantages over traditional fluids for cooling and systems in various engineering applications[7]. Water, deionized water, glycol/water solutions, fluorocarbons, hydrogenated poly-alpha-olefins were utilized as base fluids for heat transfer fluid. Solar absorption, nuclear power plants, transportation, civil engineering, aircrafts, space stations and other applications of NF come to mind. The volume fraction, shape, sizes, and qualities of the NP added, as well as the surface contact area between particles and liquid, all influence the cooling effectiveness of NF [8,9,10].
It was reported that by adding particular type of nanoparticles in mineral oil- and ester oil-based nanofluids can considerably increase their dielectric, thermal, and physical properties [11, 12]. In the previous two decades, numerous studies have been undertaken on this subject. Aluminum oxide (Al2O3), aluminum (Al), copper (Cu), copper oxide (CuO), titanium dioxide (TiO2), iron oxide (Fe3O4), zinc oxide (ZnO), silicon dioxide (SiO), fullerene (C60), carbon nanotubes (CNT) and calcium copper titanate (CCTO) are the most commonly studied NPs. According to the studies published in the literature, Fe3O4 NPs improve the properties of MO as well as ester oil the most. These advances in dielectric and thermal qualities enable transformer size, weight, and cost reductions, as well as the transmission of significantly higher power densities [8,9,10,11,12,13].
The findings reported are focused on analyzing the impact of each type of NP employed separately. The aim of this work is to analyze the impact of varying concentrations of Fe3O4, TiO2 and ZnO nanoparticles on the dielectric strength of mineral oil and pongamia ester oil (EO). Further, the results are examined using statistical analysis.
2 Samples preparation
The liquid insulation utilized in this work is mineral oil and pongamia ester oil. Initially, the oils should be pretreated to remove foreign particles and moisture content which affects the performance of liquid insulation. In the pretreatment process, the oils are heated up to 100 °C to remove moisture content and filtered with Whatman filter paper to remove foreign particles. After the pretreatment process, moisture content in mineral oil and pongamia ester oil is 5.2 ppm and 18.6 ppm, respectively. The base properties of mineral oil and pongamia ester oil are given in Table 1.
In this work, three nanoparticles namely Fe3O4, ZnO and TiO2 are used for analysis purpose. The shape of the nanoparticles is in spherical as shown in Fig. 1, SEM images of nanoparticles. Similarly, the size distribution of the nanoparticles is less than 70 nm. With these nanoparticles, two different sets of mineral oil and pongamia ester oil based nanofluids samples are prepared for laboratory oriented experiments. During the nanofluids preparation process, the samples are placed in magnetic stirring process for 1 h. Further, the samples undergo ultra-sonication process for 1 h to avoid agglomeration of nanoparticles and to have uniform and stable blending of nanoparticles as shown in Fig. 2. In the first set, Fe3O4, ZnO and TiO2 nanoparticles individually blended with mineral oil and pongamia ester oil at 0.1%, 0.2%, 0.3% and 0.4% volume concentration. Subsequently, in the second set, mixtures of Fe3O4, and TiO2 nanoparticles and mixtures of Fe3O4, and ZnO nanoparticles are blended with mineral oil and pongamia ester oil. The detailed samples list is given in Table 2, and some of the samples are shown in Fig. 3.
3 Experimental results
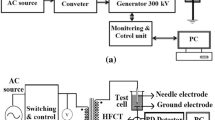
The BDV test is an important aspect of the liquid insulation acceptance testing. With the spacing of 2.5 mm, two copper plate electrodes are used for BDV test as shown in Fig. 4. In order to generate high AC voltage, the samples are electrically strained using step up transformer. The BDV tests are conducted with the same electrodes as the ac breakdown experiments. All the BDV tests are carried out in the same temperature range as a thermal test in an incubator to study the effect of temperature on breakdown behavior [14]. For the purpose of decreasing the influence of production and growth of vapor bubbles, the highest temperature is limited to 70 °C, which is less than the boiling point of liquid samples.
The average BDV of pure MO, pure EO and nanofluid samples is shown in Table 3. It is inferred that nanofluid samples have higher average BDV than average BDV of pure MO and EO samples. In particularly, Fe3O4-blended nanofluid samples (both MO- and EO-based nanofluids) have higher average BDV than TiO2- and ZnO-blended nanofluid samples. The Fe3O4 nanofluid sample with 0.4% concentration in both MO and EO (i.e., sample number S6 and S18) registered best average BDV as 62.8 kV for MO and 91.5 kV for EO compared to other nanofluid samples as shown in Figs. 5 and 6. Moreover, when comparing the enhancement percentage of nanofluid samples BDV, Fe3O4-blended nanofluid samples (both MO and EO) performed better and have higher enhancement percentage than TiO2- and ZnO-blended nanofluid samples. In particular, nanofluid sample with 0.4% Fe3O4 concentration registered highest enhancement percentage as 52% for MO (sample S6) and 53% for EO (sample S18) than other nanofluid samples.
The effect of blending mixtures of nanoparticles in MO and EO on average BDV is shown in Table 4. The nanoparticles mixtures are prepared by addition of two nanoparticles such as Fe3O4 + ZnO and Fe3O4 + TiO2 nanoparticles at various ratios as given in Table 2. The experimental results of mixtures of nanoparticles on MO and EO are given in Table 4 and for better understating it are shown in Fig. 7. It is noted that in all concentrations, nanoparticles mixtures enhance the BDV of MO and EO than nanoparticle samples as individually (i.e., S3 to S14 for MO and S15 to S26 for EO). Moreover, the mixture samples S27 (MO + 75% Fe3O4 + 25% TiO2) and S33 (EO + 75% Fe3O4 + 25% TiO2) performed better and produce some appreciable improvement in average BDV 68.1 kV and 99.2 kV, respectively. Moreover, samples S29 and S35 recorded highest enhancement percentage as 65% and 69%, respectively.
4 Statistical analysis
The widely used statistical techniques to analyze experimental results of liquid dielectrics breakdown voltage are Weibull and normal distribution. In order to verify the distribution level of experimental results, hypothesis test will be carrying out. As a result, we determine the p value, which is a probability that evaluates the proof in favor of the null hypothesis. Stronger evidence is presented in contrast to the null hypothesis by lower p values [15].
When the p value and significance level are compared, it is possible to assess whether or not the data have a normal distribution. A significance threshold of 0.05 usually produces good results. If the significance threshold is set at 0.05, there is a 5% chance that the results will be interpreted as not following a normal distribution. The choice is to reject null hypothesis and come to the conclusion that the data do not reflect a normal distribution if the p value is below or equal to the probability value. Furthermore, if the p value exceeds the import level, the null hypothesis is not rejected since there is insufficient evidence to support the conclusion that the data do not obey a normal distribution. The data do not, however, necessarily follow the normal distribution; therefore, this cannot be said. The outcomes of the hypothesis test are additionally supported by additional standards, including those imposed by the Jarque–Bera and Pearson, Lillie tests [16].
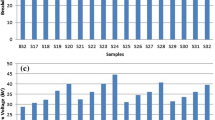
The histogram distribution of BDV of examined nanofluids samples is shown in Fig. 8. This permits us to observe the abnormalities in the distribution of BDV in various ranges of voltages. It is inferred that with the irregularities in BDV of nanofluid samples S5 (MO + 0.3% Fe3O4), S10 (MO + 0.4% TiO2), S13 (MO + 0.3% ZnO), S17 (EO + 0.3% Fe3O4), S21 (EO + 0.3% TiO2), S26 (EO + 0.4% ZnO), S30 (MO + 75% Fe3O4 + 25% ZnO) and S36 (EO + 75% Fe3O4 + 25% ZnO) are having lower p value. In addition, the experimental data of examined nanofluid samples follow the normal distribution as shown in Table 5.
The measures of skewness and kurtosis are also used to determine whether there is any issue with non-normality. Skewness is a metric for symmetry or, more specifically, for the absence of symmetry. An experimental data set is said to be symmetric if it has the same appearance to the right and left of the center point. Kurtosis measures a distribution’s peakedness and the degree to which the data are heavily or lightly tailed in relation to a normal distribution. In other words, data sets with a higher kurtosis have heavier tails. Low kurtosis data typically have lighter tails. Some researchers reported that the asymmetry and kurtosis values between -2 and + 2 are considered to prove the distribution is normal [16].
Tables 6, 7 and 8 show the Skewness and kurtosis values of examined nanofluids. Except the nanofluid sample S30 (MO + 75% Fe3O4 + 25% ZnO), skewness and kurtosis are higher than 3, and the kurtosis value of samples S5 (MO + 0.3% Fe3O4), S10 (MO + 0.4% TiO2), S13 (MO + 0.3% ZnO), S17 (EO + 0.3% Fe3O4), S21 (EO + 0.3% TiO2), S26 (EO + 0.4% ZnO) and S36 (EO + 75% Fe3O4 + 25% ZnO) is between 2 and 3. Remaining all other nanofluid samples, the skewness and kurtosis values are lesser than 2. Nevertheless, the higher kurtosis value 3 does not eliminate the data of normality. In fact, most statistical software programmes, including SPSS [16], offer “extra” kurtosis that is determined via subtracting 3 from the kurtosis for various practical purposes.
For a fully normal distribution, the extra kurtosis ought to be zero. The samples S30 (MO + 25% Fe3O4 + 25% ZnO) sample out of all the examined nanofluid samples has a kurtosis value of 3.19176, giving it an extra of 0.19176. This an extra is not that significant when taking into account data that deviate from normality. Leptokurtic distributions, which have a high peak, are defined as having a positive extra kurtosis, while Platykurtic distributions, which have a negative extra kurtosis, are defined as having a flat-topped curve. In light of the foregoing, it can be said that all samples follow the normal distribution. Based on the p value, the samples S5 (MO + 0.3% Fe3O4), S10 (MO + 0.4% TiO2), S13 (MO + 0.3% ZnO), S17 (EO + 0.3% Fe3O4), S21 (EO + 0.3% TiO2), S26 (EO + 0.4% ZnO), S30 (MO + 75% Fe3O4 + 25% ZnO) and S36 (EO + 75% Fe3O4 + 25% ZnO) do not follow normal distribution.
Weibull probability plots for dielectric strength of mineral and pongamia ester oil-based Fe3O4, TiO2 and ZnO nanofluids for different volume concentrations of nanoparticles are shown in Fig. 9. The scale parameter is connected to data dispersion and reflects the degree of failure. The shape parameter is equal to the line’s slope. The probabilities for the different examined nanofluid samples are listed in Table 5 along with their conformity to the normal and Weibull distribution. It is observed from the calculated p value, for experimental data of dielectric strength for most of the nanofluids samples are higher when compared to the significance value. Moreover, the BDV of samples S5 (MO + 0.3% Fe3O4), S10 (MO + 0.4% TiO2), S13 (MO + 0.3% ZnO), S17 (EO + 0.3% Fe3O4), S21 (EO + 0.3% TiO2), S26 (EO + 0.4% ZnO), S30 (MO + 75% Fe3O4 + 25% ZnO) and S36 (EO + 75% Fe3O4 + 25% ZnO) are lesser than the significance value. It confirms that the experimental data comply with normal and Weibull distributions. As mentioned in the results, experimental data for sphere gap electrode perfectly fit the normal and Weibull distribution.
Table 9 depicts the possible breakdown voltages at 10%, 25% and 50% of examined nanofluids, and this supports to determine voltage limitation for continuous reliable operation of transformers. The BDV at 10% is an approximate possible lowest breakdown voltage. Moreover, it evidences the reliable performance of liquid insulation and it enables the determination of a safety coefficient of electrical equipments. Commonly, the BDV of nanofluid samples is higher than that of pure MO and EO for all three types of nanoparticles and its blending concentrations. For MO and EO, the BDV at 0.4% concentration (samples S6, S10, S14, S17, S22 and S26) of Fe3O4, ZnO and TiO2 nanoparticles is higher than other concentrations. Similarly, for mixed nanofluids, the concentration at 75% Fe3O4 + 25% TiO2 (sample S27) and 75% Fe3O4 + 25% ZnO (sample S33) has higher BDV than other concentrations. This clearly shows that the blending of nanoparticles enhances the BDV of both MO and EO.
5 Discussion
For the majority of the examined nanofluid samples, the statistical analysis of BDV of experimental data follows both the normal and Weibull distributions. The experimented BDV values of MO- and EO-based nanofluids as well as mixed nanofluids combinations often follow normal rather than Weibull distribution according to the experimental data presented in above section.
The experimental results depict that among MO-based nanofluids Fe3O4 nanoparticles (sample S6) at a concentration of 0.4% registered a higher average BDV with an enhancement of 45% than other nanoparticles. For EO-based nanofluids, Fe3O4 nanoparticles (sample S18) at a concentration of 0.4% registered 52% enhancement in BDV. Similarly, in mixed nanofluids the samples S29 (MO + 75% Fe3O4 + 25% TiO2) and S33 (EO + 75% Fe3O4 + 25% TiO2) registered higher enhancement in BDV as 65% and 69%, respectively. It is inferred from this enhancement in average BDV of MO, EO and mixed nanofluids, the conductive nanoparticles register higher enhancement percentage than other nanoparticles.
The enhancement in BDV of nanofluids is influenced by type, size, shape, and volume concentration of the nanoparticle. Whereas the similar nanoparticle type and shape and volume concentration, BDV is influenced by size of the nanoparticle, i.e., for smaller size nanoparticle the BDV is higher and vice-versa for increase in size of the nanoparticle [5]. This is caused by collisions between the polarized nanoparticle surface, liquid molecule and volume concentration of interfaces in the liquid insulation. As a result, size and surface area are crucial for breakdown in liquid insulation. The surface area becomes increasingly crucial as nanoparticles get smaller, which increases the likelihood of charges building up at liquid insulation interfaces.
Many scientists agree that the growth of streamers is responsible for the change or enhancement of the BDV of insulation liquid by the addition of nanoparticles. By acting as electron scavengers, nanoparticles lower the charge density and prevent the growth of streamers in nanofluid, raising the breakdown voltage. The enhancement in BDV of nanofluids by the electron scavenging effect is clearly explained by Peppas [17] and Makmud [18]. This postponed the generation of streamers and slowing down the streamer development process. The BDV of the nanofluids is often influenced by the conductive nanoparticles. According to a paper, conductive nanoparticles swiftly catch fast-moving electrons and change them into slower negatively charged nanoparticles, which slows streamer development and raises breakdown voltage [19]. Our experimental findings confirm that conductive nanoparticle (Fe3O4) performs superior than semi-conductive nanoparticles (TiO2 and ZnO), whereas insulating particles increase BDV quite similarly.
The higher BDV of Fe3O4 nanoparticles compared to semi-conductive nanoparticles (TiO2 and ZnO) is the result of Fe3O4’s easier ability to accumulate charges at its surfaces and to become polarized than the other two types of nanoparticles. Conductive nanoparticles entrap moving electrons when an electric field is present; these surface charges are referred to as induced charges.TiO2 and ZnO nanoparticles, which are nonconductive, trap electrons due to polarization effects. Devins et al. [20] provided a model that they considered the field ionization happens in the liquid to describe how electronic scavenger agents affect streamer propagation. To estimate the amount of positive and negative carriers present in a cylinder-shaped conducting channel, they applied Zener’s tunneling theory for solids. This model assumes that the positive streamer speed is constant over the entire electrode gap. While electron injection and trapping are followed by ionization within the liquid in order to propagate negative streamers. It produces plasma that is comparable to that created if the point is in positive. The time taken in either the injection stage or the trapping step determines the negative streamer velocity. If this model needs to be justified for some of its assumptions [20], it is backed by two fascinating facts: (i) Electronic scavengers are added, which reduces the time spent in the trapping distance, first step and increasing the negative velocity. (ii) With the addition of low ionization potential compounds, increase in rate of ionization shortens the time spent in the second step and enhances the velocity.
When the polarization-relaxation time τ of nanoparticles is subjected to an electric field that can have an impact on the streamer production or not is discussed in [21]. The streamer is suppressed, and the BDV will rise if the τ is lesser than the streamer’s growth time. If τ is longer than the streamer’s growth time, on the other hand, neither the streamer nor the breakdown voltage will be impacted. This explanation is in conflict with findings related to the impact of electronic scavenger substances including carbon tetrachloride, iodobenzene, and Weibull hexafluoride [22]. It is true that these substances speed up the spread of streamers in liquids.
When nanofluids conduct, nanoparticles may behave as charge carriers’ scavengers. If so, they will minimize the amount of charges by trapping, which will in turn diminish the overall space charge. The liquid insulations BDV raises as a result of this decrease in conductivity [18]. The dielectric constant and conductivity of the liquid insulation and nanoparticles influence the improvement in BDV. When these two parameters for nanoparticles and nanofluids are close together, the BDV suffers; however, if these two parameters are dissimilar, the BDV improves.
By acting as electron scavenger at the electrodes and nanofluid interface, nanoparticles play two key roles in enhancing breakdown voltage: (1) increasing the starting voltage of streamers; and (2) forming a double layer at the insulation liquid and nanoparticle interfaces that trap electrons moving within the majority of nanofluid. The effect of electron scavenging stops when the double layers are saturated, which explains why the ideal concentration of nanoparticles produces the highest value of BDV for nanofluids. Due to the enormous volume percentage of interfaces in nanofluid and subsequent linkages between liquid insulations molecular structure and nanoparticles surface, it is noticed that at a given concentration of nanoparticles, the BDV are greater for smaller nanoparticles. As a result, charge carrier motion is delayed, which in turn slows the growth of streamers.
6 Conclusion
In this work, the BDV of MO- & EO-based Fe3O4, ZnO and TiO2 nanofluids at various concentrations is examined. Moreover, the BDV of mixed nanofluids (i.e., blending of more than one nanoparticle at various concentrations) is also examined. From the results, the conductive nanoparticles blended MO- and EO-based nanofluids reported with highest enhancement percentage in BDV as 45% (sample S6 (MO + 0.4% Fe3O4)) and 52% (sample S18 (EO + 0.4% Fe3O4)), respectively, compared to other nanoparticles. Among the mixed nanofluids, the samples S27 (MO + 75% Fe3O4 + 75% TiO2) and S33 (EO + 75% Fe3O4 + 25% TiO2) reported with highest enhancement percentage in BDV as 65% and 69%, respectively. While comparing the BDV of individual nanofluid samples and mixed nanofluid samples, the mixed nanofluid samples have higher enhancement percentage.
Furthermore, Weibull and normal distribution statistical analysis are carried out for examined nanofluids. This depicts that most of the nanofluids follows both Weibull and normal distribution except the samples S5 (MO + 0.3% Fe3O4), S10 (MO + 0.4% TiO2), S13 (MO + 0.3% ZnO), S17 (EO + 0.3% Fe3O4), S21 (EO + 0.3% TiO2), S26 (EO + 0.4% ZnO), S30 (MO + 75% Fe3O4 + 25% ZnO) and S36 (EO + 75% Fe3O4 + 25% ZnO). One of the major requirements of transformers is higher BDV, and this is fulfilled by these nanofluids. Hence, this may be considered to replace traditional MO in transformers.
Data availability
Not applicable.
References
Bartnikas R (1994) “Electrical Insulating Liquids,” Engineering Dielectrics. ISBN: 9780803120556, Ame Soc Testing Mater 3
Fofana I (2013) 50 Years in the Development of Insulating Liquids. IEEE Electr Insul Mag 29(5):13–25
Oommen TV (2002) “Vegetable oils for liquid-filled transformers. IEEE Electr Insul Mag 18(1):6–11
Madavan R, Balaraman S (2017) Comparison of Antioxidant Influence on Mineral Oil and Natural Ester Properties under Accelerated Aging Conditions. IEEE Trans Dielectr Electr Insul 24(5):2800–2808
Rengaraj Madavan, Mariappan Ravindran and Subburaj Senthil Kumar (2020) “Evaluating critical characteristics of vegetable oil as a biodegradable insulating oil for transformer”. Int J Emerg Electr Power Syst 21(5)
Madavan R, Balaraman S (2017) Investigation on effects of different types of nanoparticles on criticalparameters of nano-liquid insulation systems. J Mol Liq 230:437–444
Choi S (1995) Enhancing thermal conductivity of fluids with nanoparticles. Dev Appl Non-Newtonian Flows ASME FED 231:99–105
Segal V, Hjortsberg A, Rabinovich A, Nattrass D and Raj K (1998) “AC (60 Hz) and impulse breakdown strength of a colloidal fluid based on transformer oil and magnetite nanoparticles,” in Proceedings of the Conference Record of the IEEE International Symposium in Electrical Insulation, 619–622
Takada T, Hayas e Y, Tanaka Y, Okamoto T (2008) Space charge trapping in electrical potential well caused by permanent and induced dipoles for LDPE/MgO nanocomposite. IEEE Trans Dielectr Electr Insul 15(1):152–160
Yuefan Du, Lv Y, Li C, Chen M, Zhong Y, Zhou J, Li X, Zhou Y (2012) Effect of semiconductive nanoparticles on insulating performances of transformer oil. IEEE Trans Dielectr Electr Insul 19(3):770–776
Yuefan Du, Yuzhen Lv, Chengrong Li, Mutian Chen, Jianquan Zhou, Xiaoxin Li, You Zhou and Youping Tu (2011) “Effect of electron shallow trap on breakdown performance of transformer oil-based nanofluids”. J Appl Phys 110(10)
R Madavan, S Senthil Kumar, M Willuice Iruthyarajan (2018) "A comparative investigation on effects of nanoparticles on characteristics of natural esters-based nanofluids" Colloids Surf. A Physicochem. Eng 556: 30-36
Yuan-xiang Z, Yun-shan W, Ji-huan T, Yan-chao S (2010) JIANG Xin-xin, GAO Sheng-you, SUN Qing-hua, and NIE Qiong, “Breakdown characteristics in transformer oil modified by nanoparticles.” High Volt Eng 36(5):1155–1159
Yang B, Han ZH (2006)“Temperature-dependentthermalconductivityof nanorod-based nanofluids”. J Appl Phys 89(8)
IEC (1995) 60156 International Standard, Insulating liquids - Determination of the breakdown voltage at power frequency - Test method, Second edition
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika. 52(3 and 4):591–611
George D, Mallery M (2010) SPSS for Windows Step by Step: A Simple Guide and Reference. Pearson, Boston
Peppas G, Charalampakos P, Pyrgioti E (2016) Statistical investigation of AC breakdown voltage of nanofluids compared with mineral and natural ester oils. IET Sci Meas Technol 10(6):1–9
Ibrahim M, Abd-Elhady A, Izzularab M (2016) Effect of nanoparticles on transformer oil breakdown strength: Experiment and theory. IET Sci Meas Technol 10:839–845
Sima W, Shi J, Yang Q et al (2015) Effects of conductivity and permittivity of nanoparticle on transformer oil insulation performance: Experiment and theory. IEEE Trans Dielectr Electr Insul 22(1):380–390
Devins C, Rzad S, Schwabe J (1981) Breakdown and pre-breakdown phenomena in liquids. J Appl Phys 52(7):4531–4545
Hwang J, Zahn M, O’Sullivan E et al (2010) Effects of nanoparticle charging on streamer development in transformer oil-based nanofluids. J Appl Phys 107:014310
Beroual A (1993) Electronic and gaseous processes in the pre-breakdown phenomena of dielectric liquids. J Appl Phys 73(9):4528–4533
Funding
There is no funding source available for this research.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, experiments and writing were contributed by M.Yamuna.
Conceptualization, methodology, review and editing were contributed by R.Madavan.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamuna, M., Madavan, R. Impact of nanoparticles mixtures on mineral and pongamia ester oils dielectric strength. Biomass Conv. Bioref. 14, 7167–7179 (2024). https://doi.org/10.1007/s13399-022-03235-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03235-0