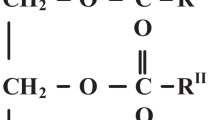Abstract
Transformers are most vibrant equipment of power system network and petroleum base mineral oil is used as dielectric insulating medium and cooling medium. At present more research works are focused towards natural esters based fluids as prospectivedisplace to traditional mineral oil, due to similar properties of transformer oil, high availability of resources, high biodegradability and environmental friendly nature. This work emphasizes on enhancement of properties of natural ester using antioxidants. Natural esters such as mustard oil, rice bran oil, punna oil and castor oil are chosen for investigation along with antioxidants such as gallic acid, citric acid, propyl gallate and tertiary butylated hydroxy quinone. Natural esters are mixed with 1 g, 2 g, 3 g and 5 g of antioxidants for the investigations. The critical properties like breakdown voltage, flash point, fire point, viscosity, interfacial tension, water content and acidity of natural esters are analyzed based on IEC/ASTM standards before and after addition of antioxidants. From this study, it is noted that antioxidants improve the characteristics of natural esters after addition of it and properties are superior to the traditional mineral oil.By the suitable proportion of fluids the 80–90% of characteristics betterment are achieved with the influence of fatty acid based additives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Power transformers are most imperative and strategic equipment in electrical networks. Without transformers, transmission and distribution of electricity for all sectors becomes highly impossible [1]. Important aspects in power transformers for uninterrupted power delivery are continuous monitoring and life assessment of insulating medium in it [2]. Dielectric fluids and solid insulation (press board and kraft paper) are basically used insulating medium in oil filled transformers. Dielectric fluids afford electrical insulation between windings (which are also provided with solid insulation for mechanical support) and cooling property by transferring heat generated inside the transformer [3].
Over 100 years, petroleum based mineral oil (transformer oil) is employed as dielectric fluids in the transformers owing to its admirable dielectric insulating properties, cooling properties and low cost [4]. Some drawbacks of transformer oil are poor biodegradability, poor recital at higher temperature and scarcity in future. Likewise transformer explosion and hazardous oil spill in the environment are also considered as downsides of mineral oil [5]. Later synthetic oils, silicone oil and esters are acquainted with the purpose for dielectric fluids. But their usages in transformers as direct replacement to mineral oil are impossible due to their higher cost and unpredicted in service performance [6].
In order to deserve optimized insulating medium with better dielectric properties, cooling properties, best grade of sustainability, least environmental influence, low cost and high resource, different alternate dielectric fluids are focused [7]. Natural esters based dielectric liquids are gaining more interest as a potential replacement to mineral oil.
Natural esters (vegetable oils) are natural products resulting from plants and seeds. Natural esters are classified based on the structure and constitutes of triglycerides. They are assorted as saturated, mono unsaturated and poly unsaturated. The performance and critical features are predominantlybe contingent on triglycerides [8]. Oxidation nature mainly depends on the higher concentration on mono unsaturated fatty acids. Poly unsaturated fatty acids are more vulnerable to oxidation of oil [9]. Viscosity is very much high for natural esters with higher saturated fatty acids. Oils with high poly unsaturated content have low viscosity. Natural esters have advantages such as low explosiveness, high flash point and fire point temperature, ecological friendly, renewable, non-hazardous and biodegradable. These advantages encourage the focus towards vegetable oil for alternate insulating medium [10, 11]. Already some biodegradable oil based insulating mediums are developed by few industries.
Natural esters hold some disadvantage namely elevated cost, improved viscosity and pitiable oxidation stability. Generally the natural esters have poor oxidation stability due to the removal of antioxidants [12]. Antioxidant is an additive compound which is capable of slow down the oxidation reaction and thereby terminates consequent chain process. It is clear that by adding some of the low toxic antioxidant with the fluids will definitely increase the oxidation stability to a better level. The addition of antioxidant not only increases the oxidation stability but also improves the operational properties like reduction of viscosity aside from pure natural esters. Subsequently the natural esters cost elevatedassociated to mineral oil, such shortcomings is overwhelmed by the features alike less formation of gas, low oxidation and slow ageing period [13, 14]. Antioxidants are broadly classified as natural and synthetic.
Natural antioxidants are extracted from natural products and synthetic antioxidants are obtained from chemical processes. Examples for natural antioxidants are tocopherol (alpha, beta, gamma and delta) and tocotrienol (alpha, beta, gamma and delta). Butylated hydroxy toluene, Butylated hydroxy anisole, 2, 6 tert butyl phenol, tertiary butylated hydroxy quinone and propyl gallate are some of the synthetic antioxidants [15]. Antioxidants are further classified as synergist which is one form of natural antioxidants. Gallic acid, citric acid, ascorbic acid and oryzanol are some of synergists. The mechanism of antioxidant as donors are represented in Eqs. 1 and 2.
where, AH is the Antioxidants, A* is the free radicals of Antioxidants.
Based on the major drawbacks identified with the employment of petroleum based mineral oil like vilest bio-degradation ability, poor behaviour at elevated temperature and availability of oil resource to support the future generation, the usage of natural esters are suggested. In order to achieve superior performance of natural esters for alternate liquid insulation, there is a requirement of chemical process to enhance the properties. In this paper, the addition of natural and synthetic antioxidants additives with some natural esters for the development of environmentally suitable dielectric fluids are recommended. It is also proposed that to overcome the existing fluid drawback, the natural esters are selected based on availability of resource, biodegradability and non-hazardous environmental friendlinessnature. By the addition of food grade additives in natural esters,the new dimension in dielectric fluids for high voltage applications are anticipated for making the superior power environment with green characteristics. The various contributions of this paper for the betterment of environment is emphasized as follows:
-
The highly advantageous natural esters along with the perfect addition of natural and synthetic additives for optimized transformer insulationsare identified.
-
The various qualifying properties like breakdown voltage, flash point, fire point, viscosity, interfacial tension, water content and acidity are estimated with the additive based natural ester fluids.
-
The mathematical based evaluation to identify the properties for intermediate additive with the functions are introduced and it is validated by analyzing the coefficient of determination.
The other portion of the paper is framed as follows. Section 2 of the paper reveals proposed methodology of this work including thepreparation of oil samples, importance and measurement techniques of critical properties. The experimental results of this works are given and discussed in Sect. 3. Mathematical evaluation is discussed in Sect. 4. Conclusion of this work is made in Sect. 5.
2 Proposed Methodology
In this effort, examinations are conceded to analyze the critical characteristics of natural esters with antioxidant additives in the aim of development of high performance environmental friendly dielectric liquids. Natural esters are selected from food grade vegetable based oils which have more obtainable resources in the near-by regions. For this work, mustard oil (MUO), rice bran oil (RBO), punna oil (PO) and castor oil (CO) is handpicked from local available manufacturer of virgin quality grade I (without the influence of chemical treatment). The different fatty acids structures of investigating natural esters based fluids are tabulated in Table 1. Antioxidants are chosen from natural and synthetic antioxidants groups which are given in Table 2 along with technical information. Additives are added besides natural esters based fluids in the ranges pertaining to 1 g, 2 g, 3 g and 5 g. The various samples and their combinational descriptions are given in Table 3. For scrutinizing the characteristics enactment of all oil samples, breakdown voltage, flash point temperature, fire point temperature, viscosity profile (at diverse temperatures), interfacial tension, moisture content and acidity are computed based on the standards. On behalf of investigating natural esters, standards related with mineral oil are used due to non-availability of specific standards for natural esters. By using nonlinear regression analysis, the mathematical modeling is derived to figure out best fitness for all the samples prepared with various proportions of antioxidant additives.
2.1 Sample Preparations
Each test samples consist of 500 ml of natural esters with the addition of 1 g, 2 g, 3 g and 5 g of antioxidant chemicals are placed in a separate beaker. With a constant temperature of 70 °C, the samples are heated in a heating chamber to dissolve the antioxidant with esters into liquid. These liquids are then blended with a magnetic stirrer at a speed of 450–500 rpm to have uniform scattering of antioxidant additives in liquids for 30 min. The blended samples are then stockpiled in a storage ampoule for the experimental evaluation.
2.2 Measurement of Properties
In view of the global guidelines, the properties of oil such as breakdown voltage, flash point, fire point, viscosity, interfacial tension, water content and acidity are analyzed.
2.2.1 Breakdown Voltage
Breakdown voltage of insulating medium defines the withstand capacity of insulating fluids [16]. Breakdown voltage is observed at a voltage which creates spark between the electrodes. The breakdown voltage is measured by using breakdown voltage test kit as endorsed by the approved IEC 60156 [17]. In view of the standard guidelines, measurement is made in test cell which consists of two spherical electrodes with a gap positioning of 2.5 mm. The oil is occupied above the externalsurface of the electrodes to a height of 40 mm. The voltage is actioned to the test cell at a rate of 2 kV/s. Breakdown voltage of oil sample is intended by taking the average of five consecutive values for the identical sample. In the middle of every estimation the time delay of 1 min is given in order to scatter the derivatives to drive out formerly the subsequent measurement. As per the standard, the breakdown voltagewould be superiorto 30 kV. Usually the pure oils have advanced breakdown voltage than the oil which has contaminations suchas moisture and acid content [18].
2.2.2 Flash Point and Fire Point
Flash point and fire point are used to identify the temperatures at which oil gets ignited [16]. The flash point and fire point measurement were carried out based on the standard ASTM D93. Theyare estimated by using Pensky martin flash point kit [19]. The Pensky martin flash point kit consists of a sealed test goblet in which 60 ml of oil sample is occupied and heated by using the energy regulator. A preliminary fire is coordinated to the opening on the outside locale to decide the temperature of the flash point and fire point. The flash point is characterized as where the fume inside the shut test cup blends in with air and delivers a transitory fire on the outside of the oil for not exactly a second, and if a constant fire happens on the outside of the oil, it is characterized as fire point where the fume inside the shut test cup blends in with air and creates a perpetual fire on the outside of the oil for over a second [20].
2.2.3 Viscosity
Viscosity of fluids indicates the resistance to flow on smooth surface that is associated with a hotness transfer capacity of fluids [16]. The oil with high viscosity takes lower hotness transfer characteristics whereas the low viscous oil has good cooling property [21]. The viscosity of oil is determined based on the recommendation in the standards ASTM D445 by using Redwood viscometer [22]. The redwood viscometer consists of oil beakerby an orifice. A 50 ml of oil is taken in oil cup and by opening the orifice, the time taken to gather the 50 ml of oil is noted to figure the viscosity [23]. The viscosity measurement is carried out at three different temperatures such as Room temperature, 60 OC and 90 OC.
2.2.4 Interfacial Tension
Interfacial tension is the molecular attractive force that occurs at the boundary of oil and water. It gives the potential of liquid to resist an external force [16]. The measurement of interfacial tension is used to detect the presence of decay and polar contaminants [24]. In view of the standard guidelines of ASTM D971, the interfacial tension is measured by using automatic interfacial tensiometer (Ring Method). The distilled water of 50 to 75 ml is filled in a beaker over which the oil is filled. The beaker is placed in an adjustable platform where the ring is progressively lifted through the water and oil interface. The reason for oil lying on the water surface is the density difference of two liquids. Since water has a higher interfacial tension than the oil, a force is desired to isolate the ring from the surface of water. The measured force is utilized towardscomputing the interfacial tension between oil and water. Pure oil has high interfacial tension and the oil with pollutants has low interfacial tension [25]. Based on the requirements of oil as liquid insulation, the interfacial tension should be superior to 30 mN/m.
2.2.5 Water Content
Existence of water molecule in oil is unacceptable which disturbs all properties of oil. The water content in oil reacts with solid insulation of core and windings of transformer which affects the properties by producing acidic contaminants [16]. These contaminants are main reasons for further reduction in properties while transformer in service [26, 27]. Water content in oil samples are measured by Karl Fischer Coulometer with moisture sensor and it is recommended by the standards ASTM D1533 [27]. A 50 ml of sample fluid is taken in a beaker and subjected to the coulometer with moisture sensor. The moisture sensor identifies the volume of water molecule content in fluid sample and demonstrated digitally. As per standard the water content should be less than 50 ppm [28].
2.2.6 Acidity
Acid content in insulating fluid is very harmful and if the oil gets acidified the oil becomes more soluble [16]. Acidity leads to deterioration of paper insulation of winding. According to the standard ASTM D974, acidity is measured by using Karl Fischer titration method [29]. In this method the potassium hydroxide is taken as a burette solution and oil sample is taken as a pipette solution. The phenolthelene is used as a color indicator to the pipette solution. The pipette solution is titrated against the burette solution until the color change occurs. The aggregate of potassium hydroxide requisite to neutralize the hydrogen ions in one gram of oil represents the acid level. As per standard, the good insulating oil wouldtake acidity lower than 0.4 mg KOH/g.
3 Results and Discussions
In this division, measured properties of all natural esters without and with antioxidants are provided and further discussions are carried out in the various aspects such as comparison on oil properties based upon fatty acid component and different concentrations of antioxidants.
3.1 Critical Properties of Base Samples
Acute properties likebreakdown voltage, flash point temperature, fire point temperature, viscosity profile (at diverse temperatures), interfacial tension, moisture content and acidity are measured for reference oil (RO) which is a petroleum based mineral oil and base oil samples (MUO, RBO, PO and CO) consistent with the specified standard guidelines. The obtained values are tabulated in Table 4.
Pure natural esters (base samples) are mixed with various concentrations (1 g, 2 g, 3 g and 5 g) of different antioxidants (GA, CA, PG and TBHQ). Properties are measured and tabulated in Tables 5, 6, 7 and 8 for oil samples prepared with base sample 1, base sample 2, base sample 3 and base sample 4 respectively.
3.2 Effect of Antioxidants on Properties of Oil Samples
In this section, properties of all oil samples are analyzed and discussed based on the fatty acid content of base oil samples and enhancement/decrement in properties after addition of antioxidants with base oil samples. Pure natural esters (base samples) are mixed with various concentrations (1 g, 2 g, 3 g and 5 g) of different antioxidants (GA, CA, PG and TBHQ). Properties are measured and graphically represented in Figs. 1, 2, 3, 4, 5 and 6.
3.2.1 Breakdown Voltage
Values of breakdown voltages for all oil samples are illustrated in Fig. 1. From the observations, it is noted that breakdown voltage of investigated vegetable based oil is higher to that of mineral oil. Among investigated natural esters, rice bran oil has high breakdown voltage.
Higher value of breakdown voltage for vegetable oils may be owing to the fatty acid composition of natural esters based fluids. From this investigation it is inferred that oil with higher content of mono unsaturated fatty acid has developed breakdown voltage. Increase in poly unsaturated fatty acid leads to reduction in breakdown voltage.
Breakdown can be further enhanced to a greater level by adding additives compound to the vegetable oil. Breakdown voltage has increased after addition of antioxidant additives compared to pure natural esters which is mainly due to less formation of carbon and it leads to less formation of gas.
Breakdown voltage of all base samples with antioxidant additives gives high breakdown voltage in that the base samples with 5 g of gallic acid provide higher breakdown voltage compared to other composition. This shows that the addition of gallic acid with base samples will enhance the breakdown voltage to a higher level. From the investigation, it is revealed that increase in concentration of antioxidant addition increase breakdown voltage of natural esters.
3.2.2 Flash Point and Fire Point
The changes in flash point and fire point of natural esters after addition of antioxidants are shown in Fig. 2. From this investigation, it is observed that the thermal stability nature esters are high. The formation of peroxide is less when the antioxidant additives combines with free radicals and there by controls the chain reactions. Based on the careful analyses, it is observed that the flash point and fire point temperature are advanced for natural esters subsequentlyby the addition of antioxidants compared to mineral oil.
From the investigation, it is found that the addition of antioxidant additives to natural ester shows good performance compared to additives free natural esters, in that the base fluids with 5 g of gallic acid provides higher flash and fire point temperatures. The influence of 5 g of gallic acid with base sample 3 shows high enhancement. Even though the samples prepared with 5 g of gallic acid with base sample 1, 2 and 4 gives good enhancement it is lower than base sample 3 with 5 g of gallic acid composition. Hence it is made clear that base samples have developed flash and fire point temperature when combined with antioxidant additives.
3.2.3 Viscosity
Viscosity profile of antioxidants added natural esters are showed in Fig. 3 for three diverse temperatures (at RTP, 60 °C and 90 °C). Generally when the temperature is increased, the considerable changes are observed with viscosity. Viscosity will reduce when temperature increases.
From the analysis of viscosity profile, it is evident that viscosity reduces for increase in temperature and viscosity reduces after the addition of antioxidants. Base sample with saturated fatty acid has greater viscosity and the base sample with unsaturated fatty acid has inferior viscosity. With reference to Table 4, at RTP, 60 °C and 90 °C the base sample 1, 2, 3 and 4 have high viscosities which are considerably higher than mineral oil. With the natural esters blended with antioxidant additives at RTP to 60 °C and 90 °C good decrement in viscosity is observed compared to pure natural esters based base samples. This may be due to antioxidants effects such as breaking a chain reaction by emitting a fresh electrons by offsetting the radicals. The base sample 1 with 5 g of gallic acid shows very good decrement compared to other composition at RTP. When the temperature is increased to 90 °C at 12 Cst, the base sample 2 with 5 g of gallic acid gives good decrement than base samples 1, 3 and 4 due to high unsaturated fatty acid content.
3.2.4 Interfacial Tension
Interfacial tension is helpful for deciding the occurrence of polar impurities and oil rot items. Great new oil for the most part displays high interfacial tension. Oil oxidation pollutants bring down the interfacial strain. The interfacial tension of all oil samples are presented in Fig. 4.
Since the exploration, it is clear that the interfacial tensions of natural esters based fluids are higher than the limits. After adding antioxidant compounds, the interfacial tension are enhanced to a high level and this enhancement indicates the purity of natural esters. It is also inferred that the base sample 2, 3 and 4 with 5 g of gallic acid and base sample 2 with 5 g of citric acid and TBHQ has high interfacial tension than other composition. The enhancement of interfacial tension under the influence of antioxidant additives indicates the reduction of water content that leads to acidity of a high level thereby achieve pure form of oil samples with less content of polar pollutants and other deterioration products.
3.2.5 Water Content
Moisture (water) content is designated as the amount of water molecules available in a material. Good natural ester based fluids mustconsumefewer water content molecule. When the water content molecule is reduced to low level, the acidity will be reduced which leads to high interfacial tension and breakdown voltage. The pictorial representations of water content of all samples are represented in Fig. 5.
After the examination, it is noted that the natural esters based fluids have higher water content than mineral oil other than base sample 2 which has lower water content. Reduction of water content of base samples is obtained by addition of antioxidant additives.Based on the analysis, it is inferred that the base sample 2 with 5 g of gallic acid shows good decrement than other compositions.Compared to the pure natural esters, the base sample 1, 2, 3 and 4 with 5 g of gallic acid gives lower water content with antioxidants which increases the purity of samples to a higher level.
3.2.6 Acidity
Acidity improves the oxidation practice in oil. Good natural ester based fluids would have fewer acidity. Acidity of oil samples are graphically represented in Fig. 6.
From the analysis, it is found that other than base sample 2, all other base samples are found to have high acidity compared to mineral oil. Since the investigation, it is observed that the addition of antioxidant additives to natural esters reduces acidity to a considerable level. The base sample 2 with 5 g of gallic acid and TBHQ gives good decrement of acidity compared to other composition. Similarly the base sample 1 with 5 g of gallic acid and base sample 3 and 4 with 5 g of TBHQ shows good decrement.
Enhancement/decrement in properties of oil samples after adding antioxidants with base oil samples are analyzed to know the influence of additives on the oil properties. Enhancement/decrement percentages in properties for all oil samples equipped with antioxidants are tabulated in Tables 9, 10, 11 and 12 for oil samples prepared with base sample 1, base sample 2, base sample 3 and base sample 4 respectively.
By comparing the obtained recorded experimental results with the various results of surveys, it is witnessed that the selected composition of fluids with the influence of diverse quantity of additives elevates the performance of fluids. The percentage of property enhancement and decrement of investigated additives based oil samples are analyzed. It is well recognized that addition of antioxidant additives with various base samples offer optimized property enrichment. The crucial properties like breakdown voltage, flash point, fire point and interfacial tension shows elevating behavior with the influence of 5 g of Gallic acid. Similarly, the challenging properties like viscosity, water content and acidity shows extreme declining behavior with the addition of 5 g of Gallic acid. Overall, it is well witnessed that 5 g of Gallic acid offers extreme property enrichment than other investigated antioxidant additives due to its phenolic and organic nature which are far superior to the results studied in surveys.
4 Mathematical Evaluation
For determining relations between different parameter variables, statistical based regression analysis is utilized as a unique tool [30]. Regression tool is also useful to develop mathematical relations (equations) among the variables. It is assorted as linear, exponential, logarithmic and nonlinear regressions based on the change of parameters with respect to other variables. The mathematical equations can be derived by doing regression analysis on linear, nonlinear, exponential and logarithmic scales for various parameters.Generally linear correlation coefficient (r) and coefficient of determination (r2) are measured to validate the relationship between variables while performing regression analysis. These values indicate the perfect fitting of data in the curves. For linear regression analysis, both linear correlation coefficient (r) and coefficient of determination (r2) exists. Only the coefficient of determination (r2) exists for nonlinear regression. The range of values for coefficient of determination (r2) is between 0 to 1. The value of ‘1’ specifies the apt fitting of data [30].
In this work, nonlinear regression tool is used to analyze the relation between properties of oil and concentration of antioxidant additives. The mathematical equations of properties are also developed for all antioxidants used in this investigation work. Various concentrations(x) of antioxidants are 1 g, 2 g, 3 g and 5 g. For all natural esters and antioxidants, the properties such as breakdown voltage (BV), flash point (FP1), fire point (FP2), viscosity (V), interfacial tension (IT), water content (WC) and acidity (A) are organized in Tables 13, 14, 15, 16, 17, 18 and 19 respectively. The validations of mathematical equations are measured by calculating the coefficient of determination (r2) for each equation. The mathematical functions are useful to calculate the properties for intermediates ranges of antioxidants.
For every sample with antioxidants, properties such as breakdown voltage, flash point, fire point, viscosity, interfacial tension, water content and acidity are derived as mathematical function of concentration of antioxidants using nonlinear regression analysis. These mathematical equations are very much useful to find out the properties for intermediate concentrations of antioxidants. These equations are also useful for finding optimized concentration of antioxidants at which oil samples have superior properties. Most of the derived equations have values nearer to one forcoefficient of determination (r2). These values show exact fitting of data in the equations.
5 Conclusion
Literally it is more significant that the insulating fluid mustofferimprovedstabilityamongstimprovedefficient performances againstdecreased environmental impact. In order to stumble on the alternate dielectric medium which has progressive properties, this effort is exploring the vegetable based oils like mustard oil, rice bran oil, punna oil and castor oil by blending with some of the natural and synthetic antioxidant compounds. With the help of individual focus, the assessmentelucidates that the behaviour of vegetable seed based oils with antioxidants become superior and in that the concert of 5 g of gallic acid with base sample 2 provides elevatedbetter properties than other samples due to the presence of high saturated and mono un-saturated fatty acids with low poly un-saturated fatty acids. This betterment is possible with high saturated, mono un-saturated fatty acids with low poly un-saturated fatty acids along with the influence of phenolic and organic nature based antioxidants. All oil samples have greater characteristics than that of traditional mineral oil is observed. During experiments, oil samples never get decelerate due to less formation of carbon in it. The cost is greaterrelated to mineral oil but such downside is overwhelmed by gentle ageing rate, improved oxidative stability and fewerdevelopment of gasses. By using the vegetable oil as a surrogate to mineral oil the environment becomes green. The use of vegetable oil offers the hands when insufficiencyarises with mineral oil which have a renewable resource so this methodology is extreme in technical and environmental aspects. Henceforth the completeexaminationaccomplishes that the vegetable oil blended with antioxidant is a suitable surrogate to mineral oil for transformer application. In future, the proposed work may be enhanced by analyzing the ageing behaviour for the identified vegetable oil with nanoparticles as additives.
References
Raj RA, Samikannu R, Yahya A, Mosalaosi M (2020) An overview of potential liquid insulation in power transformer. Int J Energy Convers 8(4):126–140
Associação Brasileira de Normas Técnicas—ABNT (2018) ABNT NBR 16361:2018—interpretation of dissolved gas analysis (DGA) in mineral insulating oil in electrical equipment factory tests. Rio de Janeiro-RJ, Brazil
Maharana M, Baruah N, Nayak SK, Sahoo N (2017) Comparative study of mechanical and electrical strength of kraft paper in nanofluid based transformer oil and mineral oil. In: 2017 international symposium on electrical insulating materials (ISEIM). IEEE, vol 2, pp 646–649
Fofana I (2013) 50 years in the development of insulating liquids. IEEE Electr Insul Mag 29(5):13–25
Aslam M, Arbab MN, Basit A, ul Haq I, Saher S, Khan AD, Khattak AN (2020) Improved insulation durability to improve transformer aging. Int J Emerg Electr Power Syst 21(1):586
Saha TK, Purkait P (2017) Transformer insulation materials and ageing, pp 1–33
Vanitha M, Narmadhai N, Karthik M (2016) Investigation of critical characteristics of mineral oil with activated carbon. Circuits Syst 7(09):2521. https://doi.org/10.4236/cs.2016.79218
Mahanta DK, Laskar S (2017) Electrical insulating liquid: a review. J Adv Dielectr 7(04):1730001
Zhang M, Liu J, Yin M, Jia H, Lv J (2019) Assessment on oil-paper insulation aging of transformer based on dielectric response model. Electr Power Compon Syst 47(13):1145–1155
Antunes HA, Wanderley Neto E, Muniz PR (2018) Monitoring method and fault identification in power transformers. In: de Tsuzuki MSG, Junqueira F (eds) 13th IEEE international conference on industry applications. Escola Politécnica da Universidade de São Paulo, Brazil (in Portuguese)
Associação Brasileira de Normas Técnicas—ABNT (2019) ABNT NBR 16788:2019—Insulating vegetable oil—Interpretation of the transformer dissolved gas analysis in operation, insulated with insulating vegetable oil. ABNT
Samikannu R, Raj RA, Karuppiah D, Dasari NR, Subburaj SK, Murugesan S, Akbar SS (2021) Reclamation of natural esters using nano-carriers as the biodegradable choice for the transformer insulation. Environ Technol Innov 23:101634
Yu N, Qi G, Qin J, Zhang R (2019) Study on the influence of antioxidants on transformer oil related indicators. In: IOP conference series: earth and environmental science. IOP Publishing, vol 223, no 1, p 012032
Polanský R, Hahn P, Kadlec P, Moravcová D, Prosr P (2020) Quantifying the effect of catalysts on the lifetime of transformer oil. Appl Sci 10(4):1309
Khaled U, Beroual A (2018) The effect of electronic scavenger additives on the ac dielectric strength of transformer mineral oil. Energies 11(10):2607
Karthik M, Narmadhai N (2020) A survey on natural esters based insulating fluid medium for transformer applications. Mater Today Proc. https://doi.org/10.1016/j.matpr.2020.09.482
Cao B, Dong J-W, Chi M-H (2021) Electrical breakdown mechanism of transformer oil with water impurity: molecular dynamics simulations and first-principles calculations. Curr Comput Aided Drug Des 11(2):123
Vanitha M, Narmadhai N, Karthik M (2016) Experimental analysis of liquid insulating medium for high voltage transformers. Asian J Res Soc Sci Hum 6(10):804–814. https://doi.org/10.5958/2249-7315.2016.01054.6
Standard test methods for flash point by pensky-martens closed cup tester, ASTM D 93 (2012)
Baruah N, Maharana M, Nayak SK (2019) Performance analysis of vegetable oil-based nano-fluids used in transformers. IET Sci Meas Technol 13(7):995–1002
Standard test method for kinematic viscosity of transparent and opaque liquids and calculation of dynamic viscosity, ASTM D 445 (2011)
Srinivasa DM, Surendra U (2019) Comparative study of breakdown phenomena and viscosity in liquid dielectrics. In: 2019 innovations in power and advanced computing technologies (i-PACT). IEEE, vol 1, pp 1–4
Dombek G, Nadolny Z, Marcinkowska A (2019) Thermal properties of natural ester and low viscosity natural ester in the aspect of the reliable operation of the transformer cooling system. Eksploatacja i Niezawodność 21(3):384–391
Standard test method for interfacial tension of oil against water by the ring method, ASTM D971 (2012)
Baka NA, Abu-Siada A, Islam S, El-Naggar MF (2015) A new technique to measure interfacial tension of transformer oil using UV-Vis spectroscopy. IEEE Trans Dielectr Electr Insul 22(2):1275–1282
Standard test method for water in insulating liquids by coulometric karl fischer titration, ASTM D1533 (2012)
Abdi S, Safiddine L, Boubakeur A, Haddad A (2019) The effect of water content on the electrical properties of transformer oil. In: The international symposium on high voltage engineering. Springer, Cham, pp 518–527
Yang Z, Zhou Q, Wu X, Zhao Z, Tang C, Chen W (2019) Detection of water content in transformer oil using multi frequency ultrasonic with PCA-GA-BPNN. Energies 12(7):1379
Standard test method for acid and base number by color-indicator titration, ASTM D974 (2012)
Abdi S, Harid N, Safiddine L, Boubakeur A, Haddad AM (2021) The correlation of transformer oil electrical properties with water content using a regression approach. Energies 14(8):2089
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karthik, M., Narmadhai, N. Experimental Evaluation on Suitability of Alternate Fluids with the Influence of Additives for Power System Transformer Applications. J. Electr. Eng. Technol. 17, 1883–1906 (2022). https://doi.org/10.1007/s42835-022-01035-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42835-022-01035-0