Abstract
The decrystallization or hydrolysis of lignocellulosic biomass is usually carried out either with concentrated solutions at moderate temperature or with dilute solutions at high temperatures. In contrast to this, agricultural waste biomasses (sunflower stalk, rapeseed stalk, and rice hull) were treated with dilute acidic or alkaline aqueous solutions (5 mol %) in this study to test the variations in cellulose crystallinity under ambient temperature. Solutions of HCl, H3PO4, CH3COOH, HNO3, H2SO4, HF, NaOH, Ca(OH)2, C2H5OH, and CS(NH2)2 were used. Effects of the treatment on cellulose crystallinity were evaluated based on the crystallinity index (CrI) calculations through the reflection intensities in X-ray diffraction (XRD) and the absorbance ratios in Fourier transform-infrared (FTIR) spectroscopy at A1429/A897 (lateral order index) and A1374/A2900 (total crystallinity index). It was found that the CrI values based on the total crystallinity index suited more than lateral order index to the CrI values found by XRD method. HF solutions led to most striking decreases in CrI, while the solutions of neither strong acids nor NaOH resulted in reductions in CrI. Derivative thermogravimetry (DTG) and differential scanning calorimetry (DSC) profiles revealed that the applied treatment influenced the pyrolytic degradation characteristics and the reactivity of biomass in range of 300–400 °C where cellulose decomposed.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lignocellulosic biomass, which is largely carbon dioxide-neutral, is a renewable and sustainable energy source and has enormous potential on a global scale. The use of biomass is of great importance in eliminating the negativities caused by the use of fossil fuels and reducing foreign dependence on energy. However, due to some properties of lignocellulosic biomass, such as low calorific value and density, and high moisture and volatile matter contents and inhomogeneous structure, there are difficulties in transportation, storage, and energy production [1, 2]. The macromolecular structure of lignocellulosic biomass is largely composed of cellulose, hemicellulose, and lignin, with cellulose (40–60%) being the predominant component [1]. Cellulose is a very vital biopolymer and an almost inexhaustible and renewable raw material. Cellulose is formed by combination of semi-crystalline fibers with ordered crystalline regions and disordered amorphous regions [3]. The free hydroxyl groups in cellulose are able to involve hydrogen bonds that originate different ordered crystalline arrangements [4]. Thus, cellulose crystallinity, which is the proportion of crystalline cellulose in cellulosics, is one of the most important properties of cellulose because it affects their physical, chemical, and mechanical features [5,6,7]. Among the macromolecular components of lignocellulosics, only cellulose is crystalline, while lignin and hemicellulose are non-crystalline [4]. Woody biomass shows higher crystallinity (ca. 44%) than stalk-type (ca. 34%) or shell-type ones (ca. 29%) [7]. The rigidity of cellulose fibers enhances, and their flexibility reduces with increasing ratio of crystalline to amorphous regions [4]. The amorphous form of cellulose is more easily hydrolyzed than the crystalline form since the hydrogen bonds per unit repeat is bigger in crystalline cellulose (8 versus 5.3) [8]. Therefore, industrial manufacturing of cellulose from lignocellulosics usually includes alkaline treatment and bleaching so that amorphous substances are removed and the crystallinity of cellulose is improved [9]. Cellulose crystallinity affects almost all of the downstream applications where cellulose is processed. Increase in crystallinity is associated with rising tensile strength, dimensional stability, and density, while weakening chemical reactivity and swelling property [10]. Therefore, various characteristics of materials, including Young’s modulus, density, hardness, dimensional stability, and chemical reactivity, are related to crystallinity [11, 12]. Since the hydrolysis of cellulose proceeds across the amorphous region, one of the most significant factors that estimate the difficulty of the hydrolysis process and how much energy will be spent is the degree of crystallinity [11]. Therefore, cellulose crystallinity is one of the key factors that complicate the anaerobic digestion of lignocellulosic biomass into fermentable sugars. Also, amorphous cellulose contributes to the formation of char and gaseous products, whereas crystalline cellulose leads to the formation of levoglucosan during pyrolysis [13].
Physical, chemical, physicochemical, and biological pretreatment processes are applied to lignocellulosic biomass. These pretreatment methods include extrusion, sonication, milling, steam explosion, organosolv, ozonolysis, wet oxidation, liquid hot water, ammonia fiber explosion, acid treatment, alkali pretreatment, supercritical CO2 explosion, ionic liquid, and microwave- and ultrasound-assisted methods, and biological reactions of bacteria, microbes, and fungi [14, 15]. The crystallinity of cellulose in lignocellulosic biomass can be altered by pretreatments. Zhang et al. [16] applied anaerobic digestion to yard waste/food waste mixture and reported 23% reduction in cellulose crystallinity of yard waste after hydrolysis that improved biodegradability of cellulose. In another study, effects of mechanical fragmentation on cellulose crystallinity was investigated using wheat straw during alkali treatment and found out that mechanical fragmentation contributes to cellulose crystalline transformation under low NaOH concentration [3]. Pulp beating of tobacco stems was also reported to change the cellulose crystallinity [10]. The reduction in cellulose crystallinity is attributed to the destroyed hydrogen bonding that transformed the crystalline region into the amorphous region [3]. Yin et al. [17] examined the effects of compression combined with steam treatment on the crystallinity of wood (spruce) cell walls and determined that the crystallinity of cellulose largely increased (from 33.6 to 58.6%) due to crystallization in semi-crystalline region upon treatment. Song et al. [9] employed superfine pulverization pretreatment to Lycium barbarum leaves to enhance the cellulose crystallinity (up to 18.3%). Moreover, Okon et al. [12] applied silicon oil heat treatment to Chinese parasol wood and obtained thermally modified wood with increased cellulose crystallinity (from 38.8 to 63.8%) due to degradation of hemicellulose. Poletto et al. [4], who investigated the thermal decomposition of several wood samples by thermogravimetry, concluded that lower crystallinity associated with higher extractives content accelerates the degradation process and reduces the thermal stability of wood. Likewise, Wang et al. [13] found out that the samples with lower crystallinity start to degrade at lower temperatures, showing sharper DTG curves and lower activation energies during pyrolysis.
Crystallinity index (CrI) is defined as the fraction of crystalline matter within a sample, and it is a parameter frequently considered to quantify the extent of crystallinity. CrI also helps to monitor the changes in structure caused by physicochemical, thermochemical, and biological methods [10, 11]. Several classical methods including X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, solid-state 13C nuclear magnetic resonance (NMR), Raman spectroscopy, and their modified versions have been used to measure the CrI [5, 6, 10]. Of which, XRD is by-far the most prevailing method [10]. XRD-based procedures usually consider peak height, peak area, deconvolution of crystalline and amorphous peaks, and amorphous subtraction techniques [6, 10, 17]. On the other hand, CrI calculated by FTIR method is usually compared with those by XRD or NMR methods, and thus, it is regarded as a complementary approach [10].
However, the physical pretreatment methods generally base on energy-intensive techniques and biological pretreatment methods require long time and use of expensive bacteria and enzymes. Besides, treatment with chemicals is the fastest and the most efficient method to affect the cellulose crystallinity [16]. Chemical decrystallization methods include the use of ionic liquids, NaOH/urea, or phosphoric acid leaching [18]. Swelling or dissolving biomass in chemicals such as concentrated acids or ionic liquids increases the accessibility of enzymes in enzymatic conversion of cellulosic biomass into fermentable sugars [19]. Acids such as sulfuric acid (H2SO4), nitric acid (HNO3), hydrochloric acid (HCl), and phosphoric acid (H3PO4) have widely been used as catalyst for hydrolysis and degradation of cellulose [20]. Acid pretreatment provides efficient breaking down the biomass structure and promoting crystalline-amorphous cellulose conversion [15]. Jin et al. [21] reported that sulfomethylation-aided H3PO4 treatment of bamboo residues at room temperature for 1–4 h resulted in lower lignin content and lower CrI (from 64.7 to 16.0%) that made the enzyme accessibility to cellulose easier. Zhang et al. [22] investigated the changes in cellulose crystallinity of lignocellulosic biomass (switchgrass, corn stover, and rice husk) through treatment by ionic liquids and found out that the CrI dropped because of swelling of crystalline cellulose. Goto and Yokoe [23] compared the CrI of untreated and NH3-treated barley straws and reported the reduction (14–24%) of CrI for treated sample owing to the effect of NH3 as a weak base on ester bonding within the cell wall. However, sodium hydroxide (NaOH) that is a stronger alkali was reported not to have influence on cellulose crystallinity in barley and wheat straw [23].
Acid pretreatment of biomass targets solubility of hemicelluloses and making the cellulose susceptible to hydrolysis as well as penetration of enzymes. Pretreatment by dilute acids is applied at high temperatures ranging between 120 and 215 °C, while concentrated acids (> 30%) are employed at moderate temperatures (< 100 °C) [15]. Concentrated solutions including 72% H2SO4, 44% HCl, and 85% H3PO4 are reported to destruct cellulose completely [24, 25]. Oil palm empty fruit bunch fiber was extracted and hydrolyzed using H2SO4 (95–98%), acetic acid (CH3COOH) (99.5%), and NaOH (99%) [26]. However, these potent solutions also lead a certain depolymerization of cellulose due to cleavage of the glycosidic bonds [24]. As regards the use of dilute solutions, temperature is raised to enhance the effect of treatment. Zhao et al. [24] used 0.05 M H2SO4 at 175 °C, while Kanchanalai et al. [27] treated cellulose by H2SO4 (10–50 wt %) at temperatures between 80 and 100 °C. Consequently, it is difficult to comparatively examine the effectiveness of dilute solutions at ambient temperature on cellulose crystallinity of different biomass species.
Aqueous solutions have advantages over non-polar organic solvents in terms of dissolving polar sugars and furanics [28]. In fact, the benefits of using aqueous solutions lie on the ability to break polar bonds, low environmental impacts, and being simple and economical. Dilute solutions include less chemical substance that makes the pretreatment process to operate in a more economical way. If diluted solutions could be applied at low temperatures, the contribution to the process economy would be even higher due to the reduction in heating requirement. For this purpose, the changes in cellulose crystallinity of agricultural waste biomass such as sunflower stalk, rapeseed stalk, and rice hull treated with solutions of 5 mol% concentration at ambient temperature were investigated comparatively. This study differs from the literature in terms of the types of biomass used and the treatment conditions applied. There are no detailed studies under the aforementioned conditions on the cellulose crystallinity of these lignocellulosic biomass, which has a large agricultural waste potential. Sunflower, rapeseed, and rice are among the agricultural products that are cultivated in large quantities globally, and so the waste biomass potential of these products is great as well. The sunflower stalk (SS) is the stem part of the sunflower plant. This stalk has a hairy structure and its bark is rich in lignin. SS is used as fuel or also used in paper making. Sunflower is the oil plant with the largest cultivation area and nearly 2.5 million tons yearly production in Turkey. SS left in the field pose a problem for farmers and are habitually destroyed by burning. That is, 600 thousand tons of SS are burned annually that can be eliminated with the use of SS in an alternative manner. Besides, the rapeseed plant is among the oilseed plants. Rapeseed can enter crop rotation with wheat under suitable climatic conditions and draws attention as an alternative that will help fill a significant gap in both cooking oil and fuel (biodiesel) production. Rapeseed production in Turkey is around 125 thousand tons per year and tends to increase quickly. Rapeseed stalk (RS) was used as an alternative biomass in this study. Also, rice hull (RH) is the shell that covers the outer part of the rice grain. This shell can be used in various fields including as fuel and insulation material. Turkey’s annual rice production is around 1 million tons, and accordingly, a large amount of RH is obtained. For these reasons, SS, RS, and RH were chosen as the biomass species to be used in treatment by dilute solutions at ambient temperature.
2 Materials and methods
2.1 Characterization of samples
The particle size of SS, RS, and RH samples was reduced below 250 µm by grinding in a ring mill and sieving through 60 US mesh sieve by Retsch AS200 sieve shaker. The ground samples were kept in closed containers in order to minimize interaction by surrounding conditions. The proximate analysis of samples was carried out in accordance with ASTM standards. The higher heating value (HHV) of samples was determined by IKA C2000 model calorimeter, and the lower heating value (LHV) was calculated. An elemental analyzer (Leco TruSpec CHN with S module) was used for ultimate analysis. Compositional analyses of the samples were done by applying wet chemical methods. That is, the biomass was first extracted with benzene and ethyl alcohol solutions to get the extractives-free bulk and to determine the extractive matter content according to ASTM D1105 method. Then, the extractives-free bulk was used in further analyses to find the holocellulose (sum of cellulose and hemicellulose) content according to Wise’s chlorite procedure [29] and the lignin content according to van Soest method [30]. The experiments were repeated several times, and the average values were taken if they were not deviated more than ± 5%. The results are given in Table 1.
2.2 Treatment of biomass by solutions
In the second step of the experimental study, aqueous solutions with concentration of 5 mol% were prepared. The reason why the solution concentration was chosen as 5 mol% is because even the dilute acid solutions with concentration < 5% is able to hydrolyze hemicellulose if operating conditions are suitable [15]. The theoretical basis of these experiments lies on the fact that solutions interact the cellulose structure through hydrogen bond breaking due to complex formations and partly depolymerization of macromolecular chains [20]. Meanwhile, hemicellulose is also degraded. Even at low temperatures, solutions are able to diffuse into the interstices between fibrillary structural units of cellulose and penetrate both ends into elementary crystallites, leading changes in the crystallinity of cellulose [31]. The dilute solutions were prepared using acids such as HCl (hydrochloric acid), H3PO4 (phosphoric acid), CH3COOH (acetic acid), HNO3 (nitric acid), H2SO4 (sulfuric acid), and HF (hydrofluoric acid) as well as bases such as NaOH (sodium hydroxide) and Ca(OH)2 (lime). These acids and NaOH are among the chemicals most commonly used to biomass for pretreatment. In addition, it is known that lime is such an alkaline reagent that is cheap, low toxic, and not having a significant impact on the environment. Treatment of lignocellulosics with lime removes amorphous substances such as lignin and hemicellulose since it cleaves ether bonds in phenolic units [32]. Moreover, effects of C2H5OH (ethanol) and CS(NH2)2 (thiourea) solutions at 5 mol% were also tested. Schematic flow diagram of the experimental procedure is given in Fig. 1.
Ten grams of ground biomass was mixed in a polypropylene beaker with the solutions as to get a total volume of 100 mL. The samples were kept in interaction with the solutions by stirring with a magnetic stirrer at 100 rpm for 2 h at ambient temperature. In fact, the time for efficient interaction of biomass with dilute solutions is reported to last up to 2 h [15]. Given the mild conditions of treatment, we decided to apply this procedure for 2 h. Then, the treated biomass was separated from solution by vacuum filtration using the Gooch crucible porosity grade 2 and washed with distilled water. Some of the treated samples are shown in Fig. 2. These images show that more or less swelling has occurred in samples. The treated samples were then dried in oven at 60 °C for 16 h. The dried samples are packaged as shown in Fig. 3. The treatment by solutions was carried out in two parallel sets of experiments. The treated biomass obtained from both sets was subjected to FTIR, XRD, and pyrolysis experiments to examine the reproducibility of the results. Data with deviations of less than 5% were averaged. A third set of experiments was performed for deviations exceeding 5%.
2.3 Determination of crystallinity index
XRD and FTIR tests were performed to determine how cellulose crystallinity in biomass was affected by wet treatment. Powder X-ray diffraction (XRD) analysis was implemented using Bruker™ AXS D8 Advance XRD system operated at 40 kV and 40 mA. The diffracted intensity of Cu-Kα radiation was measured in the range 2θ between 10° and 90°, and data were recorded at every 0.0101°. CrI was calculated from the peak height method that calculated from the height ratio between intensity of the crystalline peak (I002-Iam) and the total intensity (I002) after subtraction of the background signal measured without cellulose [10]. Accordingly, the following empirical formula was used to calculate the crystallinity index based on diffracted intensity:
where I002 is the maximum intensity of the 002 lattice diffraction. I002 is the diffraction intensity of peak at 2θ = 22.5°, while Iam is the intensity of the amorphous peak at 2θ = 18.7° [7, 9, 10, 12, 23, 33]. Xylans and other-non-cellulosic polysaccharides contribute to the intensity of the amorphous peak Iam [23].
FTIR spectra of the samples were obtained using Perkin Elmer Spectrum 100 FTIR Spectrometer between 650 and 4000 cm−1. For this, pellets were prepared by mixing biomass with spectroscopic grade KBr. The cellulose crystallinity index was evaluated by absorbance ratios such as A1429/A897 (lateral order index) and A1374/A2900 (total crystallinity index) [10, 13]. Lateral order index (LOI) bases on the ratio of the absorption peaks between crystalline structure of cellulose (A1429 cm−1) and glycosidic bond β-(1,4) in cellulose (897 cm−1). Besides, the total crystallinity index (TCI) bases on the ratio of the absorption peaks –CH bending (1374 cm−1) and the vibration of hydroxymethyl group from crystalline form of cellulose (2900 cm−1).
2.4 Pyrolytic degradation of biomass
Pyrolytic degradation tests were performed by TA Instruments SDTQ600 thermal analyzer that has alumina reference, Pt/Pt–Rh thermocouples, and a temperature sensitivity of 1E-3 °C. Approximately 10 mg sample was heated up to 700 °C with a heating rate of 10 °C/min under N2 flow of 100 mL/min. DTG (derivative thermogravimetry) and DSC (differential scanning calorimetry) profiles were derived from the thermal data. In this way, effects of the applied treatment on the pyrolytic degradation behavior of the samples were studied.
3 Results and discussion
3.1 Sample characteristics
Analysis results of the biomasses are given in Table 1.
It is clear that the proximate analysis results for SS and RS are close to each other, while RH seriously differs. RH has higher contents of ash and fixed carbon than the others, while its volatile matter content is lower. Waste biomass species are typically rich in ash content, and the ash contents given in Table 1 are comparable with the ash of other agricultural waste biomasses [34]. Besides, the LHV values of all three samples are comparable. In literature, LHV of trees from oak to pine are reported to change in the range of 19–21 MJ/kg [35]. Given the fact that the biomass species used in this study are waste biomasses, accordingly, their LHV values (14–15 MJ/kg) are lower than those of the trees. On dry-ash-free basis, RH is richer in elemental carbon and poorer in oxygen than other samples. Although the cellulose contents are close to each other, the richest sample in terms of holocellulose (sum of cellulose and hemicellulose) is SS, while the sample with the highest lignin content and the lowest extractive substance content is RS. It can be said that the selected biomass samples are structurally dissimilar from each other. These results are comparable with literature [36, 37].
FTIR spectra of raw samples are shown in Fig. 4. A sharp absorbance peak originating from ether bonds (C–O–C) around 1030 cm−1 was observed in all of the raw samples. Since this peak is characteristic for both cellulose and lignin, it is the most striking peak. Apart from this, the absorbances of O–H stretching at approximately 3335 cm−1, C-H stretching at 2920 cm−1, C = O stretching at 1733 cm−1, and C = C vibration at 1594 cm−1 are among the obvious peaks. In terms of absorbance intensity, SS is the sample that creates the strongest absorptions. According to the ultimate analysis results, SS is the sample containing the most oxygen, and accordingly, it is also rich in oxygen-containing functional groups. Being rich in oxygen-containing functional groups increases the hydrophilic property of a sample and is thus considered to be active in terms of wettability and swelling properties. Also, the wavenumbers 668, 897, 1374, 1429, and 2920 cm−1 that are considered when estimating cellulose crystallinity based on the ratios of absorbance levels in the FTIR spectra are also marked on Fig. 4.
3.2 CrI determination by XRD
The XRD spectra before and after the samples were treated with solutions are shown in Fig. 5. However, it is unsuitable to include all of spectra on the same graph due to the overlapping of curves. Therefore, the spectra of raw biomasses and some selected examples of treated samples are shown in Fig. 5. However, Table 2 includes the CrI calculations for all of the treatments as well as the percentage changes in CrI based on variation in the value of raw biomass upon treatment.
CrI values of raw samples were calculated as 46.6%, 37.4%, and 43.1% for SS, RS, and RH, respectively. These results are in accordance with literature [3, 4, 7, 12, 38]. The results in Table 2 show that all of tested solutions have more or less effects on cellulose crystallinity. It is known that, the interactions of cellulosic materials with water, enzymes, and other reactive or adsorptive substances first occur in non-crystalline areas or on the surface of cellulose crystallites. Therefore, an increase or decrease in the degree of crystallinity of cellulose is observed depending on the intensity of interaction and swelling taking place in structure. However, studies in literature carried out using RH and corn stover showed that CrI value was not affected at low temperatures from treatment even by ionic liquid due to limited swelling [22]. Under such mild pretreatment conditions, plant cell wall and the structure of lignin maintain its integrity with only partial swelling of cellulose crystals [22]. We assumed that if the change in CrI value after treatment is below 5%, its effect may be ignorable. From this point of view, it is clear that HCl solution caused negligible changes in the CrI values of two out of three samples and led to some increase only in crystallinity of RS. In contrast to this, Tao et al. [39] reported that dilute HCl solutions applied at higher temperatures seriously improved the enzymatic hydrolysis of biomass. Also, the solution of Ca(OH)2 only produced a significant decrease in the CrI value of the RH, while it created almost neglectable effect on the other samples. Besides, the role of H2SO4, C2H5OH, and H3PO4 solutions on the CrI value varied from sample to sample, and it is difficult to generalize. Likewise, HNO3 solution did not reduce cellulose crystallinity in any samples. In contrast, an increase of 25.1% in CrI of RS and 7.2% in RH occurred. Emam et al. [40] reported that this increase in CrI arises from removal of hemicellulose and lignin contents. During the interaction of cellulose with acids, the acids primarily act on the amorphous regions they can easily reach. Therefore, an increase in the degree of crystallinity of the cellulose that remains intact is expected. Interaction of cellulose with strong acids has been a classical way to obtain highly crystalline cellulose by removal of the amorphous cellulose and improvement of the crystallinity [9]. However, in the hydrolysis of biomass with dilute acids, swollen cellulose is hardly observed as an intermediate product. Since the concentration is low and the temperature is not high enough in the present study, the dilute acidic solutions may be expected not have any potential to extract the cellulose [20]. Therefore, hemicellulose removal by dilute acid treatment is usually followed by alkali treatment for lignin removal to produce high-purity cellulose [15]. Nevertheless, even 5 mol% of concentration of HNO3 led to remarkable increases in CrI. Removal of some acid-soluble ingredients such as minerals and organic extractives might enrich the polysaccharides relatively in the treated biomass.
Other solutions caused acceptable changes for all—or at least—two samples. Even treating the ground biomasses with pure water reduced the CrI values. In case of RS, the reduction was in the order of 10.3%. In literature, however, the effects of water on cellulose crystallinity have not been clarified, and instead, the steam treatment was reported to increase the CrI value in wood [17]. In fact, among the pretreatment methods using water, the use of liquid hot water (LHW) is more common, in which the temperature is kept between 150 and 260 °C [28].
When HF solution was used, the maximum drop in CrI was achieved. This decrease reached 53.2% for SS. This shows that if this biomass (with a high CrI of 46.6%) is treated with a 5 mol% HF solution at ambient temperature for 2 h, the CrI decreases to a very low value of 21.79. Significant decreases were also observed in CrI of other biomass species such as 32.3% for RH and 24.1% for RS.
Although thiourea solution caused little change in the CrI of RH, it caused a decrease of up to 19.9% in CrI of other samples. Although we applied the thiourea solution directly, thiourea solutions are commonly used in combination with NaOH to dissolve cellulose to prepare a transparent solution for wet spinning [41] or to improve the softness of the cellulose fibers from hardwood in paper industry [42]. Our findings revealed that the use of dilute solution of thiourea without the support of NaOH also has a non-negligible effect on CrI of lignocellulosic biomass.
CH3COOH solution led to an increase in CrI value for RS, but created reductions as much as 12.5% and 28.3% for other samples. Acid-chlorite delignification with CH3COOH and NaClO2 (sodium chlorite) is known as an efficient way to remove lignin from biomass [43]. In literature, delignified and bleached pine flower was hydrolyzed with CH3COOH (10–60%) at 45 °C for 1 h [44], and effects of this treatment on cellulose crystallinity revealed that if the concentration is low, the effect is less. However, despite CH3COOH is a weak acid, its effectiveness in our study even at ambient temperature may be regarded higher than expected.
Treatment with NaOH solution resulted in higher CrI values in two of the samples. In comparison to acidic hydrolysis methods, treatment with alkaline methods is known to cause less degradation of cellulosic fraction [32]. Likewise, Gao et al. [3] treated coarse milled wheat straw by NaOH solutions (1–10%) at 80 °C and found out that treatment with solutions up to 8% concentrations increased the CrI value, while reduction in CrI could only be seen in case of 10% concentration. Also, Goto and Yokoe [23] reported that NaOH did not have any effect on cellulose crystallinity.
H3PO4 treatment can be concluded to have remarkable decreasing or increasing effects on CrI depending on the type of sample. The CrI values of H3PO4-treated biomass changed in range of 38.7–43.0%. Similarly, Wei et al. [45] also reported cellulose crystallinity that changed in range of 32–45% when cellulose was treated with H3PO4 at temperatures between 25 and 45 °C. The effectiveness of H3PO4 is reported to increase beyond a critical concentration (around 80 wt.%) [27, 46], and anhydrous H3PO4 is known as an excellent solvent for cellulose even under mild conditions [46, 47]. Besides, H3PO4 at low concentration is known to be able to solubilize the hemicellulose fraction [48]. Conversely, H3PO4 treatment is also known to remove some of amorphous components and lignin, slightly increasing the CrI [21]. The lack of a regular trend of change in the CrI value can be explained by the emergence of these different effects; accordingly, there were decreases in SS and RH and an increase in RS. However, it is also stated that hydrolysis of cellulose using H3PO4 at low temperatures (below 70 °C) is highly limited [25].
The most effective solutions in reducing the crystallinity of cellulose can be shown in order of decreasing effectiveness as follows:
The solutions of strong acids (with negative pKa values) such as HCl, H2SO4, and HNO3 and the solution of NaOH (with a high positive pKa value) do not have a reducing effect on CrI. The effective solutions mentioned above are the solutions of chemicals that show neither too strong acidic nor too strong alkaline character, namely, HF whose solution decreased crystallinity at most for all biomass species is a relatively weak acid with a pKa value of + 3.2. Likewise, the pKa values of CH3COOH and H3PO4 are + 4.76, and + 2.12, respectively.
3.3 CrI determination by FTIR
FTIR spectra were examined in order to check whether the FTIR method gave results consistent with those determined by XRD method. Because of overlapping spectra, all spectra could not been included on the same graph. Therefore, raw biomass, HNO3-treated, CH3COOH-treated, and HCl-treated samples are arbitrarily selected, and their FTIR spectra are presented in Fig. 6. From these spectra, it is clear that each biomass structure was affected dissimilarly. Treatment with HNO3 solution had different effects on the absorbance intensity of the 1030 cm−1 peak, where the highest absorbance values were observed for raw biomasses. While this peak intensified in SS, there were remarkable decreases in RS and RH. Besides, CH3COOH or HCl solutions led to reductions in the absorbance intensities.
The absorbance ratios of A1429/A897 (lateral order index (LOI)) and A1374/A2900 (total crystallinity index (TCI)) are given in Table 3. LOI denotes the ratio between intensity of the bands of crystalline structure of cellulose (1429 cm−1) to glycosidic bond β-(1,4) in cellulose (897 cm−1). The increase in TOI is a measure of the rupture of hydrogen bonds in the cellulose components [15]. Except for a few cases, TCI values were generally found to be greater than LOI values. The results found for these two different absorption ratios were evaluated both for their compatibility with each other and with the XRD results. There was an increase or decrease in the CrI value of the treated biomass compared to the CrI value of the raw biomass. The compatibility of this change trend determined by FTIR method with the change trend determined by XRD was compared. The numbers shown in bold and underline in Table 3 are the values that are similar to the trend determined by XRD. Thus, there is compatibility with XRD for 18 of 33 treatments for the absorbance ratio of A1374/A2900. Besides, this compatibility is limited for the absorbance ratio of A1429/A897, and only 9 out of 33 treatments showed compatibility. Zhao et al. [10] also compared the CrI values of tobacco stems calculated from A1429/A897 and A1374/A2900 absorbance ratios and found out serious deviations for most of data. Nonetheless, the TCI is relatively more consistent with the XRD results than the LOI. When both absorbance ratios are evaluated together, at least one of the absorbance ratios for 20 out of 33 treatments provided the similar trend of change in cellulose crystallinity with those found by XRD.
It is known that crystallinity index can vary considerably depending on the measurement technique, and XRD peak height method produces significantly higher values than other methods [49]. The biomass that showed the highest dissimilarity in XRD and FTIR findings was RS. In contrast, it was determined that at least one absorbance ratio was compatible with the XRD findings for 9 out of 11 different solutions applied to SS and only the solutions of water and CS(NH2)2 did not give consistent results. Figure 7 shows the comparison of LOI and TCI values. In cases where LOI was greater than TCI or the results were negative, the relevant bar was left blank. While all bars could be shown for RS, few bars could be included for RH. The mean ratio of LOI/TCI values calculated using the available data was 39%, 38%, and 49% for SS, RS, and RH, respectively.
3.4 Pyrolytic degradation
It is reported that the intramolecular dehydration of cellulose chains initiates at temperatures below 300 °C and then decarbonylation, ring-opening polymerization, and aromatization take place beyond 300 °C [1]. On the other hand, the presence of acid changes the cellulose pyrolysis mechanism [50]. The pyrolysis of untreated cellulose generally proceeds with depolymerization reactions, while dehydration reactions are dominant during the pyrolysis of acid-impregnated cellulose [50]. Figure 8 shows the possible pathways for pyrolytic degradation of acid-treated cellulose [50].
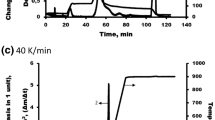
The effects of changes in CrI value on the pyrolytic degradation behavior of biomass were investigated by thermal analysis. For this purpose, the DTG and DSC curves of the two treated samples (SS–HF and RS-HNO3), for which the greatest decrease (− 53.2%) and increase (+ 25.1%) in CrI values were determined, were compared with their raw samples. Decomposition temperature ranges of hemicellulose, cellulose, and lignin in an inert environment are 220–315 °C, 300–400 °C, and 150–900 °C, respectively [1, 51]. Therefore, the rate of mass losses between 300 and 400 °C is included to Fig. 9 in order to focus on degradation characteristics of cellulose.
Hemicellulose-rich raw SS has high low-temperature reactivity, and consequently, it showed a rapid decomposition behavior especially up to 325 °C. The weight losses occurring after the completion of hemicellulose degradation were due in part to lignin degradation and largely due to degradation of cellulose. The treatment with HF resulted in a very significant increase in the decomposition rates within 330–380 °C. The decrease in CrI caused an increase in thermal reactivity. The strong endothermic peak in range of 338–360 °C in the DSC curve confirms this reactivity, since cellulose absorbed significant amount of heat for decomposition.
Besides, the situation is somewhat different for HNO3-treated RS, where an increase in CrI value occurred. The large difference in mass loss rates in the 330–380 °C range stated above has largely disappeared, and the degradation rates of the raw HS-treated sample have converged. Although there was an increase in the decomposition rates in the range of 335–350 °C, this increase was limited. In contrast to this, it is seen that there is a slight decrease in reactivity between 350 and 375 °C. Accordingly, no endothermic peak occurred in the DSC curve.
In similar studies in literature, the effects of the applied pretreatment methods were investigated usually on the pyrolytic liquid yield and product distribution produced from whole lignocellulosic biomass [15, 52]. The positive contribution of pretreatment to the yield and product distribution as well as reduction in char formation has been attributed to the removal of inorganics. Mohammed et al. [53] subjected Napier grass to H2SO4 solutions (0.5–2.5 wt%) at 70 °C for 1 h and determined serious reductions in potassium and sodium contents. David et al. [54] treated sugarcane bagasse by 0.1% HNO3 and 0.2% H2SO4, and then, pyrolysis of treated sample at 350 °C yielded seven times as bigger amount of anhydro sugar. However, conclusions based on the effect of the applied treatment only on the inorganic structure should be combined with the effects on the crystal structure of cellulose.
4 Conclusion
It is concluded that various dilute solutions (5 mol %) influence the cellulose crystallinity at ambient temperature in a way that is more than expected. Depending on the type of chemical in solution, either decrease or increase occurred in the CrI of cellulose. For a certain type of biomass, some dilute solutions may be recommended for applications aiming to increase the crystallinity of cellulose by affecting the amorphous structure of biomass, while some other dilute solutions may be proposed for applications aiming to reduce the crystallinity of cellulose, namely, treatment with dilute solution of HF enabled the most significant decrease in CrI of all of the biomass species. On the other hand, effect of a particular solution on cellulose crystallinity differs depending on biomass. Because the lignocellulosic biomass has a complex structure and consists of many components, each biomass was affected differently under the same conditions of treatment. It is also concluded that instead of comparing the CrI values determined by different methods such as XRD and FTIR, comparison should be made between the values determined by the same method. Nevertheless, the TCI value specified by FTIR method is relatively more compatible with the XRD results compared to the LOI. Thermal analysis tests revealed that the reactivity of pyrolytic degradation in the temperature range of 300–400 °C is significantly affected by the treatment due to change in cellulose crystallinity. In the literature, the thermal reactivity changes observed for treated biomass were generally attributed to changes in the inorganic structure. However, in the interpretation of changes in reactivity, not only the effects of inorganic matter but also variation in crystal structure of cellulose should be taken into account. The performance of this chemical treatment process on cellulose crystallinity can be tested by using biomasses with different properties such as hardwood or softwood as a future recommendation. In addition, the influences of different solution concentrations and slightly increased temperature on the process may be investigated. Moreover, the accessibility of cellulase enzyme to the treated biomass and its effect on fermentable sugar yield may be examined.
Data availabilty
Data will be made available on reasonable request.
Code availability
Not applicable.
Abbreviations
- ASTM:
-
American Society for Testing and Materials
- CrI:
-
Crystallinity index
- DSC:
-
Differential scanning calorimetry
- DTG:
-
Derivative thermogravimetry
- FTIR:
-
Fourier transform infrared
- HHV:
-
Higher heating value (MJ/kg)
- Ka:
-
Acid dissociation constant
- LOI:
-
Lateral order index
- LHV:
-
Lower heating value (MJ/kg)
- NMR:
-
Nuclear magnetic resonance
- RS:
-
Rapeseed stalk
- RH:
-
Rice hull
- SS:
-
Sunflower stalk
- TCI:
-
Total crystallinity index
- XRD:
-
X-ray diffraction
References
Hoang AT, Ong HC, Fattah IMR, Chong CT, Cheng CK, Sakthivel R, Ok YS (2021) Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process Technol 223:106997. https://doi.org/10.1016/j.fuproc.2021.106997
Haykiri-Acma H, Yaman S (2022) Effects of torrefaction after pelleting (TAP) process on strength and fuel characteristics of binderless bio‑pellets. Biomass Convers Biorefine 1007/s13399-022-02599-7
Gao C, Yang J, Zhang H, Xiao W, Han L (2020) Quantitative and qualitative characterization of dual scale mechanical enhancement on cellulosic and crystalline-structural variation of Na OH treated wheat straw. Bioresour Technol 312:123535. https://doi.org/10.1016/j.biortech.2020.123535
Poletto M, Zattera AJ, Forte MMC, Santana RMC (2012) Thermal decomposition of wood: influence of wood components and cellulose crystallite size. Bioresour Technol 109:148–153. https://doi.org/10.1016/j.biortech.2011.11.122
Agarwal UP, Ralph SA, Reiner RS, Baez C (2018) New cellulose crystallinity estimation method that differentiates between organized and crystalline phases. Carbohydr Polym 190:262–270. https://doi.org/10.1016/j.carbpol.2018.03.003
Ju X, Bowden M, Brown E, Zhang X (2015) An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr Polym 123:476–481. https://doi.org/10.1016/j.carbpol.2014.12.071
Chen H, Liu Z, Chen X, Chen Y, Dong Z, Wang X, Yang H (2020) Comparative pyrolysis behaviors of stalk, wood and shell biomass: correlation of cellulose crystallinity and reaction kinetics. Bioresour Technol 310:123498. https://doi.org/10.1016/j.biortech.2020.123498
Crooker M (2010) Thermochemical conversion of biomass to liquid fuels and chemicals. RSC Publishing, London. ISBN-13: 978-1849730358
Song P, Zhou F, Li F, Han Z, Wang L, Xu J, Zhang B, Wang M, Fan J, Zhang B (2021) Superfine pulverisation pretreatment to enhance crystallinity of cellulose from Lycium barbarum L. leaves. Carbohydr Polym 253:117207. https://doi.org/10.1016/j.carbpol.2020.117207
Zhao D, Yang F, Dai Y, Tao F, Shen Y, Duan W, Zhou X, Ma H, Tang L, Li J (2017) Exploring crystalline structural variations of cellulose during pulpbeating of tobacco stems. Carbohydr Polym 174:146–153. https://doi.org/10.1016/j.carbpol.2017.06.060
Caliari IP, Barbosa MHP, Ferreira SO, Teofilo RF (2017) Estimation of cellulose crystallinity of sugarcane biomass using nearinfrared spectroscopy and multivariate analysis methods. Carbohydr Polym 158:20–28. https://doi.org/10.1016/j.carbpol.2016.12.005
Okon KE, Lin F, Chen Y, Huang B (2017) Effect of silicone oil heat treatment on the chemical composition, cellulose crystalline structure and contact angle of Chinese parasolwood. Carbohydr Polym 164:179–185. https://doi.org/10.1016/j.carbpol.2017.01.076
Wang Z, McDonald AG, Westerhof RJM, Kersten SRA, Cuba-Torres CM, Ha S, Pecha B, Garcia-Perez M (2013) Effect of cellulose crystallinity on the formation of a liquid intermediate and on product distribution during pyrolysis. J Anal Appl Pyrolysis 100:56–66. https://doi.org/10.1016/j.jaap.2012.11.017
Hoang AT, Nizetic S, Ong HC, Mofijur M, Ahmed SF, Ashok B, Bui VTV, Chau MQ (2021) Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere 281:130878. https://doi.org/10.1016/j.chemosphere.2021.130878
Hoang AT, Nizetic S, Ong HC, Chong CT, Atabani AE, Pham VV (2021) Acid-based lignocellulosic biomass biorefinery for bioenergy production: advantages, application constraints, and perspectives. J Env Manage 296:113194. https://doi.org/10.1016/j.jenvman.2021.113194
Zhang L, Loh KC, Zhang J (2018) Food waste enhanced anaerobic digestion of biologically pretreated yard waste: analysis of cellulose crystallinity and microbial communities. Waste Manage 79:109–119. https://doi.org/10.1016/j.wasman.2018.07.036
Yin J, Yuan T, Lu Y, Song K, Li H, Zhao G, Yin Y (2017) Effect of compression combined with steam treatment on the porosity, chemical compositon and cellulose crystalline structure of wood cell walls. Carbohydr Polym 155:163–172. https://doi.org/10.1016/j.carbpol.2016.08.013
Zhang J, Zhang J, Lin L, Chen T, Zhang J, Liu S, Li Z, Ouyang P (2009) Dissolution of microcrystalline cellulose in phosphoric acid— molecular changes and kinetics. Molecules 14:5027–5041. https://doi.org/10.3390/molecules14125027
Chundawat SPS, Agarwal U (2019) Swelling by hydrochloric acid partially retains cellulose-I type allomorphic ultrastructure but enhances susceptibility toward cellulase hydrolysis such as highly amorphous cellulose. In Book: Smith MD (Ed.) Understanding lignocellulosic synergistic computational and analytic methods. ACS Symposium Series. ACS Publications, Washington, DC: pp 69–88
Huntley CJ, Crews KD, Abdala MA, Russell AE, Curry ML (2015) Influence of strong acid hydrolysis processing on the thermal stability and crystallinity of cellulose isolated from wheat straw. Int J Chem Eng 658163. https://doi.org/10.1155/2015/658163
Jin Y, Liu J, Yang H, Shi Z, Zhao P, Yang J (2021) Improving enzymatic saccharification and ethanol production of bamboo residues with sulfomethylation-aided phosphoric acid pretreatment. Ind Crops Prod 170:113733. https://doi.org/10.1016/j.indcrop.2021.113733
Zhang J, Wang Y, Zhang L, Zhang R, Liu G, Cheng G (2014) Understanding changes in cellulose crystalline structure of lignocellulosic biomass during ionic liquid pretreatment by XRD. Bioresour Technol 151:402–405. https://doi.org/10.1016/j.biortech.2013.10.009
Goto M, Yokoe Y (1996) Ammoniation of barley straw. Effect on cellulose crystallinity and water-holding capacity. Anim Feed Sci Technol 58:239–247. https://doi.org/10.1016/0377-8401(95)00903-5
Zhao H, Kwak JH, Wang Y, Franz JA, White JM, Holladay JE (2006) Effects of crystallinity on dilute acid hydrolysis of cellulose by cellulose ball-milling study. Energy Fuels 20:807–811. https://doi.org/10.1021/ef050319a
Satari B, Karimi K, Kumar R (2019) Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: a review. Sustaianble Energy Fuels 3:11–62. https://doi.org/10.1039/C8SE00287H
Kuthi FABA, Badri KH (2014) Effect of cooking temperature on the crystallinity of acid hydrolysed-oil palm cellulose. AIP Conf Proc 1614:456–462. https://doi.org/10.1063/1.4895240
Kanchanalai P, Temani G, Kawajiri Y, Realff MJ (2016) Reaction kinetics of concentrated-acid hydrolysis for cellulose and hemicellulose and effect of crystallinity. BioResources 11:1672–1689. https://doi.org/10.15376/biores.11.1.1672-1689
Chen WH, Nizetic S, Sirohi R, Huang Z, Luque R, Agis M et al (2022) Liquid hot water as sustainable biomass pretreatment technique for bioenergy production: a review. Bioresour Technol 344:126207. https://doi.org/10.1016/j.biortech.2021.126207
Wise LE, Murphy M, D’Addiecs AA (1946) Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Paper Trade J 122:11–19
van Soest PJ (1963) Use of detergents in the analysis of fibrous feeds-II: a rapid method for the determination of fiber and lignin. J Assoc Off Anal Chem 46:829–835
Krässig HA (1993) Cellulose: structure, accessibility and reactivity. Gordon and Breach Science Publ, Philadelphia
Grimaldi MP, Marques MP, Laluce C, Cilli EM, Sponchiado SRP (2015) Evaluation of lime and hydrothermal pretreatments for efficient enzymatic hydrolysis of raw sugarcane bagasse. Biotechnol Biofuels 8:205. https://doi.org/10.1186/s13068-015-0384-y
Siripong P, Duangporn P, Takata E, Tsutsumi Y (2016) Phosphoric acid pretreatment of achyranthes aspera and sida acuta weed biomass to improve enzymatic hydrolysis. Bioresour Technol 203:303–308. https://doi.org/10.1016/j.biortech.2015.12.037
Haykiri-Acma H, Yaman S (2009) Thermogravimetric investigation on the thermal reactivity of biomass during slow pyrolysis. Int J Green Energy 6:333–342. https://doi.org/10.1080/15435070903106959
Klass DL (1998) Biomass for renewable energy, fuels, and chemicals. San Diego: Academic Press. ISBN: 9780124109506
Haykiri-Acma H, Yaman S, Kucukbayrak S (2015) Does carbonization avoid segregation of biomass and lignite during co-firing? Thermal analysis study. Fuel Process Technol 137:312–319. https://doi.org/10.1016/j.fuproc.2015.03.017
Haykiri-Acma H, Yaman S (2008) Thermal reactivity of rapeseed (Brassica napus L.) under different gas atmospheres. Bioresour Technol 99:237–242. https://doi.org/10.1016/j.biortech.2007.01.001
Filho GR, de Assunçao RMN, Vieira JG, Meireles CDS, Cerqueira DA, Barud HDS, Ribeiro SJL, Messaddeq Y (2007) Characterization of methylcellulose produced from sugar cane bagasse cellulose: crystallinity and thermal properties. Polym Degrad Stab 92:205–210. https://doi.org/10.1016/j.polymdegradstab.2006.11.008
Tao X, Li J, Zhang P, Nabi M, Jin S et al (2017) Reinforced acid-pretreatment of Triarrhena lutarioriparia to accelerate its enzymatic hydrolysis. Int J Hydrogen Energy 42:18301–18308. https://doi.org/10.1016/j.ijhydene.2017.04.149
Emam AA, Faraha SAA, Kamal FH, Gamal AM, Basseem M (2020) Modification and characterization of nano cellulose crystalline from Eichhornia crassipes using citric acid: an adsorption study. Carbohydr Polym 240:116202. https://doi.org/10.1016/j.carbpol.2020.116202
Ruan D, Zhang L, Zhou J, Jin H, Chen H (2004) Structure and properties of novel fibers spun from cellulose in NaOH/thiourea aqueous solution. Macromol Biosci 4:1105–1112. https://doi.org/10.1002/mabi.200400120
Zhai R, Chi F, Zhou X (2016) NaOH-Thiourea aqueous solution treatment of cellulose fiber and its effects on bulk and softness. BioResources 11:8703–8719. https://doi.org/10.15376/biores.11.4.8703-8719
Belgacem MN, Pizzi A (2016) Lignocellulosic fibers and wood handbook – renewable materials for today’s environment. Scrivener Publishing, Massachusetts, ISBN 978-1-118-77352-9
Aisiyah MM, Masruri M, Srihardyastutie A (2020) Crystallinity of nanocellulose isolated from the flower waste of pine tree (Pinus merkusii) The 2nd Int Conf on Chemistry and Material Science (IC2MS). IOP Conf. Series: Mate Sci Eng 833:12003. https://doi.org/10.1088/1757-899X/833/1/012003
Wei S, Kumar V, Banker GS (1996) Phosphoric acid mediated depolymerization and decrystallization of cellulose: preparation of low crystallinity cellulose - a new pharmaceutical excipient. Int J Pharm 142:175–181. https://doi.org/10.1016/0378-5173(96)04673-X
Zhang X, Qu T, Mosier NS, Han L, Xiao W (2018) Cellulose modifcation by recyclable swelling solvents. Biotechnol Biofuels 11:191. https://doi.org/10.1186/s13068-018-1191-z
Boerstoel H, Haatman H, Westerink JB, Koenders BM (2001) Liquid crystalline solutions of cellulose in phosphoric acid. Polymer 42:7371–7379. https://doi.org/10.1016/S0032-3861(01)00210-5
Haykiri-Acma H, Yaman S (2019) Effects of dilute phosphoric acid treatment on structure and burning characteristics of lignocellulosic biomass. J Energy Res Technol 141:082203. https://doi.org/10.1115/1.4042719
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3(1):10. https://doi.org/10.1186/1754-6834-3-10
Long Y, Yu Y, Chua YW, Wu H (2017) Acid-catalysed cellulose pyrolysis at low temperatures. Fuel 193:460–466. https://doi.org/10.1016/j.fuel.2016.12.067
Haykiri-Acma H, Yaman S, Kucukbayrak S (2010) Comparison of the thermal reactivities of isolated lignin and holocellulose during pyrolysis. Fuel Process Technol 91:759–764. https://doi.org/10.1016/j.fuproc.2010.02.009
Cao B, Wang S, Hu Y, Abomohra AEF, Qian L et al (2019) Effect of washing with diluted acids on Enteromorpha clathrata pyrolysis products: towards enhanced bio-oil from seaweeds. Renew Energy 138:29–38. https://doi.org/10.1016/j.renene.2019.01.084
Mohammed IY, Abakr YA, Kazi FK, Yusuf S (2017) Effects of pretreatments of napier grass with deionized water, sulfuric acid and sodium hydroxide on pyrolysis oil characteristics. Waste Biomass Valor 8:755–773. https://doi.org/10.1007/s12649-016-9594-1
David GF, Perez VH, Rodriguez Justo O, Garcia-Perez M (2017) Effect of acid additives on sugarcane bagasse pyrolysis: production of high yields of sugars. Bioresour Technol 223:74–83. https://doi.org/10.1016/j.biortech.2016.10.051
Author information
Authors and Affiliations
Contributions
Equal.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
> Effects of dilute solutions on cellulose crystallinity were studied comparatively.
> The solution of HF is the most efficient in terms of reduction in cellulose crystallinity.
> Compared to A1429/A897 (lateral order index), CrI values based on A1374/A2900 (total crystallinity index) are more consistent with those found by XRD.
> Applied treatment affected the pyrolytic degradation characteristics at temperatures between 300 and 400 °C where cellulose decomposed.
Statement of novelty
Insufficient attention has been paid to the treatment of lignocellulosic biomass using dilute solutions under ambient temperature, as it appears to be ineffective. In this study, it was aimed to determine how the cellulose crystallinity of lignocellulosic biomass treated by dilute solutions at ambient temperature is affected. In addition, a comparative evaluation was made to determine to what extent the cellulose crystallinity index (CrI) values determined according to XRD and FTIR techniques were compatible with each other. Also, effects of applied treatment on the pyrolytic degradation and the reactivity of cellulose were also studied. The results showed that the treatment with dilute solutions can have a non-negligible effect on the cellulose crystallinity of the lignocellulosic biomass, contrary to expectations.
Rights and permissions
About this article
Cite this article
H., HA., S., Y. Treating lignocellulosic biomass with dilute solutions at ambient temperature: effects on cellulose crystallinity. Biomass Conv. Bioref. 14, 9967–9981 (2024). https://doi.org/10.1007/s13399-022-03085-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03085-w