Abstract
Valorization of dairy-industry wastewater and stormwater energy is a new approach to establishment of sustainable agriculture, which is based on use of stormwater containing dairy wastewater for production of yeast-algae (Saccharomyces cerevisiae–Scenedesmus abundans) biomass and biofuel. Dairy wastewater (DW) has high COD (68,000 mg/L) and BOD (31,800 mg/L). To cultivate yeast-microalgae, dilution was performed using stormwater with the dilution rate of 10 to 100%. The objective of this study was to treat the dairy wastewater and stormwater (SW) with microalgae. In this study cultures of Scenedesmus abundans (microalgae), Saccharomyces cerevisiae + Scenedesmus abundans (yeast + microalgae) and Scenedesmus abundans + Chlorella minutissima (microalgae + microalgae) were cultivated on different dilution ratios (10–100%). The artificial consortium of yeast and microalgae has been able to remove 41.7% of total nitrogen (TN), 60.9% of total phosphorus (TP), 83% of COD, and 90% of BOD for 14 days. Reduction in bacterial load was also reported. Dry weight of yeast-algal biomass was found to be 1.9 g/L in DW and 1.2 g/L in control medium. Moreover, increased lipid content (27.5%) was also observed in DW cultivated biomass as compared to the control (21%) and further an increase in unsaturated fatty acids (USFA) and PUFA content was also observed. Increase in protein content while decrease in carbohydrate content was reported. Chlorophyll a and carotenoid content were high in yeast–algal pellets cultivated in DW and SW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The dairy business, which is one of the world’s most important food processing industries, produces a huge amount of wastewater after cleaning, cooking, floor washing, and sanitization [1]. Lactose, lipids, minerals, detergents, and sanitizers abound in dairy effluent [2]. Dairy wastewater is characterized by high organic (i.e., COD of 85–95,000 mg/L, BOD of 40–48,000 mg/L) and nutrient TN of 14–830 mg/L, TP of 9–280 mg/L) contents [3].
Stormwater is the water that comes from heavy rainfall or ice melting. Although harvesting and reuse of stormwater may reduce the demand of non-portable water, a wide range of pollutants and pathogens reported to be in stormwater may cause a serious health risk to human being [4, 5]. Stormwater can further wash out pollutants from industrial, domestic, or agricultural sites and is not suitable for human consumption and agricultural applications because of the presence of naturally occurring components. However, the profitable usage of stormwater can transform it into a more feasible and cheap material coming from the treatment methods of wastewater and water discharges [6]. Consequently, in recent years, increase in the demand of wastewater, stormwater harvesting, and reuse in different purposes have emerged as new field of sustainable water management [7]. From an environmental viewpoint, this is largely beneficial in lowering the amounts of liquid waste simultaneously decreasing the consumption of resources with respect to energy, material, and water utilization. Therefore, just like wastewater, stormwater should also be treated properly, because its improper management, mainly at the time of rainy seasons, can lead to serious influence on receiving waters [6].
To find the clean, economic, renewable energy source is the most challenging problem to replace conventional biofuel. Biofuel is a new opportunity to achieve global energy security and reduction in greenhouse gasses. The use of 1st generation biofuels has a lot of controversy and limitations, mainly as its competition with food security. The development of 2nd generation biofuels is considered to produce fuel from lignocellulosic biomass; however, high production cost and slow commercialization are major drawbacks. The use of 3rd generation biofuels could overcome these problems [8, 9]. In this context, the use of microalgae for biofuel production has several advantages like easy to cultivate on wastewater, small life cycle, rapid growth potential, store neutral lipids, did not required herbicides or pesticides, and convert sun energy and CO2 into chemical energy and O2. Microalgae species able to grow under different types of environmental conditions and able to produce different type of biofuel like biodiesel, bioethanol, hydrogen, and biogas [9,10,11,12].
Since plants developed from green algae, natural symbiosis between fungi and algae known as lichens has been widely documented [13]. Yeast is a single-celled organism that is mostly employed in the food processing industry [14]. It is utilized mostly in bakeries, distilleries, brewing, and winemaking and is good source of protein, vitamins, and minerals [15]. Yamada and Sgarbieri [16] found that yeast cells contain 62% protein, 8.2% total lipids, and 10.4% RNA.
Several researchers have been used microalgae to clean dairy effluent and integrated it into lipid and biofuel production [3, 17]. Nutrient (N, P) removal, cultivation period, biomass productivity, and lipid production vary depending on wastewater characteristics and microalgal species [2]. Chokshi et al. [3] reported the complete removal of N and P from dairy wastewater by Acutodesmus dimorphus microalgae. In another study, Kothari et al. [18] reported the 87% removal of P and 60% of N in dairy wastewater by Chlorella pyrenoidosa.
In recent years, microbial consortium has been produced by various researchers on laboratory scale for chemical production [19], feed [20], and wastewater treatment [21]. Zhang et al. [22] have postulated that, in yeast and microalgae consortium, the stresses caused by CO2 and O2 during growth can be eliminated by utilizing O2 by yeast and CO2 by algae. Liu et al. [23] conducted an experiment to demonstrate that artificial microalgae and yeast consortium enhances the lipid and biomass productivity. Similarly, Qin et al. [24] investigated on the advantage of mixed culture of yeast Yarrowia lipolytica and microalgae Chlorella vulgaris for treating liquid digestate of yeast industry and observed an increment in biomass productivity, lipid content, and higher heating value (HHV) of the mixed culture as compared to those of monoculture. Moreover, the nutrient removal efficiency was also higher in mixed culture and a feasible approach for cogeneration of biofuel feedstock was also indicated. The cultivation of microalgal and yeast consortia in 3:1 ratio is optimum inoculum to remove micropollutants from piggery wastewater [25].
Alam et al. [26] reported that consortium of microalgae with bacteria and yeast increase the algal biomass, bioremediation, and wastewater treatment. In another study, Jingrui et al. [27] reported that consortium of microorganism exchange and complete nutrients requirement of each other’s. Worldwide interest is rising in research on treating wastewater using the microalgae and yeast consortiums. In this study, we have examined the potential of removing nutrients from dairy and stormwater by artificial microalgae and yeast consortia and their effect on the yeast algae biomass and lipid content.
2 Material and methods
2.1 Yeast strain, microalgal strain, and dairy wastewater (DW)
Yeast Saccharomyces cerevisiae UUIND1 (KY385556) was isolated earlier. Yeast culture was maintained in YEPD (yeast extract peptone dextrose — glucose, 10; peptone, 5; yeast extract, 3 g/L) media. To avoid bacterial growth, YEPD media was supplemented with ampicillin 5–10 µL (100 mg/mL). Growth of yeast was monitored by measuring optical density (OD) at 660 nm using a spectrophotometer (UNICO model 2100) [28].
Scenedesmus abundans (NCIM 2897) was purchased from NCL (Pune, India). The strain was maintained in Bold’s Basal Medium (BBM) [29]. Chlorella minutissima (MCC-27) was purchased from IARI (New Delhi, India). Stock culture was cultivated at 25 °C, 300 µmol m−2 s−1 white light and an 18-h light and 6-h dark cycle. Light is provided by cold white Crompton Greaves LED lamps. Cultures were gently mixed twice a day.
Dairy wastewater was collected from the Graphic Era University dairy, Uttarakhand, India. Once collected, DW was filtered through a Whatman filter paper No. 1 to remove total suspended solids. The basic characteristics (COD, BOD, TN, and TP) of DW were determined following the standard methods [30].
2.2 Experimental design
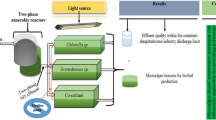
Cultures of Scenedesmus abundans (microalgae), Saccharomyces cerevisiae + Scenedesmus abundans (yeast + microalgae), and Scenedesmus abundans + Chlorella minutissima (microalgae + microalgae) were cultivated on different dilution ratios (10–100%) with microalgae: yeast 3:1 ratio with initial concentration 5 × 105 cells/mL [23] (Figs. 1 and 2). Dilution was done by stormwater, which was collected from the university footpaths, gardens, and lawns after heavy rainfall. Control culture for this experiment was Saccharomyces cerevisiae + Scenedesmus abundans (yeast + microalgae) in BBM.
The batch experiments were performed in a 250-mL Erlenmeyer flask with 150-mL control medium and raw DW. At a dilution ratio of 80%, the maximum growth was reported. The microalgae and yeast were inoculated in a flask containing both control medium and DW (w/w = 3/1) (Fig. 1). The flasks were inoculated with microalgae to set the cell density at 0.2 OD. Erlenmeyer flasks were incubated for 14 days at 28 °C, under 300 µmol m−2 s−1 and an 18-h light 6-h dark cycle. Light was provided by cold white Crompton Greaves LED lamps. Cultures were gently mixed on every 6 h to avoid formation of algal biofilm. Growth rate of microalgae was determined by spectrophotometer (Shimadzu-1900, UV–VIS Spectrophotometer) at OD at 680 nm [31]. The cultivation batch experiments were performed in triplicates. The maximum growth rate was reported by a yeast-microalgae consortium (Fig. 1), which was selected and then further evaluated for biodiesel production. Dry cell weight (DCW) was measured by drying the wet biomass at 80 °C overnight and then dry biomass was then measured gravimetrically.
2.3 Analysis
2.3.1 FTIR analysis
Fourier-transform infrared spectroscopy (FT-IR) was utilized for the identification of the chemical structural differences in microalgal-yeast pellets. The absorption spectrum of the sample was recorded between 4000 and ~ 450 cm−1.
2.3.2 Estimation of biomass productivity and lipid content
Biomass productivity (mg/L/d) was measured by following equation:
where, D1 and D2 are dry biomass (mg/L) at time t1 and t2 [32].
2.3.3 Total lipid extraction and analysis
Total lipids from dried biomass were extracted by a modified Bligh and Dyer’ method [33]. Briefly harvested algal biomass was sonicated with chloroform: methanol (2:1; v/v) and stirred at 200 rpm for 6 h and then centrifuged at 8000 rpm for 10 min and suspended solids were removed and upper phase was treated with 0.034% MgCl2 and centrifuge again at 6000 rpm for 10 min and discard the upper phase. Lower phase 2 N KCl was mixed and centrifuge at 6000 rpm for 10 min and then aspirating out lower phase. Lower phase was mixed with chloroform: methanol: water; 3:47:48 (v/v/v) followed by centrifugation at 6000 for 10 min and lower phase was dried. Lipid content in dry weight (% dw) was calculated by the following equation:
Accumulation of lipid in yeast-algal cells was analyzed by Nile red staining [34].
2.3.4 Estimation of biochemical composition and pigments
Total carbohydrate content was determined by Arora et al. [35] method. Briefly, 25-mg dried yeast-algal biomass was treated with 250 µl of 72% H2SO4 and incubated at 30 °C in water bath for 1 h. A 7 mL distilled water was added in the solution and autoclave at 121 °C for 1 h. Yeast-algal biomass hydrolysate was allowed to stand for some time, centrifuged at 8000 rpm for 15 min. Upper phase was used to total sugar estimation [36]. The total proteins were estimated nitrogen content was determined by using CHN and calculated by following formula:
For photosynthetic pigments isolation, 5-mL media containing yeast algal cell was treated with 10-mL acetone at 70 °C for 30 min, centrifuged at 6000 rpm for 10 min, and 3-mL upper phase was analyzed spectrophotometrically at 480, 649, and 665 nm. Chlorophyll a (chl a), chlorophyll b (chl b), and total carotenoid (carotenes and xanthophylls) concentrations were determined according to the equation proposed by [37].
2.3.5 Total bacterial count (CFU/mL)
Total CFU/mL count was estimated using Kumar et al. [38] method. Eighty percent diluted water and treated water were spread on nutrient plate. Petri dishes were incubated overnight at 37 °C for optimal growth of bacteria. After 18 h of incubation, bacterial colonies were counted using a CFU counter.
2.3.6 Transesterification and Biodiesel properties
Transesterification of the lipids extracted into FAMEs was done using 6% methanolic sulfuric acid [39]. Biodiesel profiling was done by GC–MS (Agilent Technologies, Athens, GA, USA) according to Arora et al. [34]. Biodiesel characterization such as specific gravity, saponification value, MUFA (%), PUFA (%), SV (mg/g), and density was analyzed online using Biodiesel Analyzer 2.2 (url: http://brteam.org/biodieselanalyzer).
2.4 Statistical analysis
Data analysis was carried out by repeating the experiments in triplicate (n = 3). The variability of the data was presented as mean ± standard deviation.
3 Results and discussions
3.1 Efficacy of algae-yeast consortium in dairy wastewater treatment
Wastewaters obtained from dairy industries are characterized by high COD and nutrients (N and P) [40]. The use of algae-based methods for dairy wastewater treatment has been argued by different researchers due to its high polluting load [41, 42]. However, the utilization of microbial consortia has been widely reported as an effective alternate in treating different kinds of wastewaters [43, 44]. Ghaly and Kamal [45] cultivated the Kluyveromyces fragilis yeast in cheese whey wastewater (COD: 59,640 mg/L) and observed about 91% reduction in COD after 28-h cultivation.
The microalgae-yeast consortium was grown in the raw DW and evaluated for its nutrient removal efficiency and biodiesel productivity. Initial inoculum size, pH, and the availability of nutrients, light, and temperature regulate algal development in the wastewater [46]. A high inoculum size of microalgae (i.e., 0.2 OD) was selected in this study. It is expected that the higher initial inoculum size leads to better microalgae growth and hence higher nutrient removal efficiency from wastewaters [46]. An algal inoculum size of 1 × 107 cells m/L has been reported optimum for microbal growth and nutrients removal from wastewater as compared with the inoculation sizes of 106 (medium) and 105 (low) cells mg/L [47]. The OD of microalgae and yeast cells were increased from 2nd day (Fig. 1). Iasimone et al. [48] reported that in combined yeast and microalgal cultivation, yeast cells grow from first day, and microalgal cells grows after 6th day. The difference in growth may be due to different metabolisms of two different microorganisms. Zhang et al. [22] also reported the microalgal slow growth as compared to yeast cells during co-cultivation of two microorganism.
The primary physico-chemical characteristics of DW are shown in Table 1. Due to high COD and BOD values, the microalgae-yeast consortium was able to tolerate 20% raw DW. Moreover, the symbiotic relation between yeast and microalgae resulted in the removal of 41% nitrogen and 90% BOD. Daneshvar et al. [2] reported 92.2% of total nitrogen and 100% of phosphate could be removed by microalgae when it was cultivated on DW. At the end of the cultivation period, 83% reduction was reported in COD. Cheirsilp et al. [49] measured the higher reduction in COD when wastewater treated with algae-yeast consortia as compared to single culture of microalgae or yeast. The increase in pH was obtained at the end of the stationary phase. This condition explained that there is decrease in bacterial growth. A similar change in pH was reported by Iasimone et al. [48] in microalgae-yeast culture.
A high bacterial load of 24 × 1022 cells/L was reported in raw DW (20%) and SW. After 14 days of treatment, reduction in bacterial load (9 × 10−11) was reported. Reduction of bacterial load in urban wastewater was also reported by Kumar et al. [38] after treatment with microalgae. A reduction in bacterial load might be due to adsorption of bacteria on algal cells surface [50]. Generally, bacteria and microalgae represent relationships fluctuating from parasitism to mutualism [51, 52]. The symbiotic relationship between cultured microalgae and bacterial populace in wastewater followed by the accumulation of nitrogen and phosphorus by microalgae might be the fundamental aspect related with the deduction of bacterial population in microalgae cultivated wastewaters [53]. In addition, microalgae also reduce the nutrient content in wastewater resulting in decreased bacterial load of wastewater [38, 50]. Some microalgae produce toxic polysaccharides which inhibit the growth of other microorganisms [54].
3.2 Biomass productivity and biochemical composition
An artificial consortium for the removal of nutrients from DW was utilized in this investigation. The initial microalgal biomass concentrations for DM-cultivated consortium and control were set at 3:1. Optical density measured at 680 nm and gravimetric data were examined for microalgal growth. Biomass concentrations of 1.9 ± 0.4 g/L for yeast-algal association and 1.2 ± 0.2 g/L for control were reported after 14-day cultivation. The maximum biomass productivity was 135 mg/L/day. It may be because yeast is a good source of protein that supplies enough nitrogen and CO2 to algae [22]. These results indicate that microalgae grow well when co-cultivated with yeast in DW. Acutodesmus dimorphus biomass in DW (0.84 g/L) was reported by Chokshi et al. [3].
Kim et al. [55] reported the 2.5 times higher growth rate of microalgae growing on yeast extract as a nitrogen source as compared to the other nitrogen sources. Gu et al. [56] reported that a growth rate of 10.4 g/L was achieved by supplying yeast extract to a freshwater microalgal strain, Scenedesmus acutus.
An increased lipid content (27.5%) was also observed in the DW cultivated biomass, compared to the control (21%) depicted in Fig. 3. The accumulation of lipid droplets within the microalgal and yeast cells was identified by Nile-Red-stained cells (Fig. S1). Similar findings were reported by Kim et al. [55]; lipid productivity can reach up to 36.0 mg/L/day when microalgae were cultivated with yeast extract. Existing studies have stated an obvious relationship between increase in algal biomass and lipid content of mixed culture. This might be because photoautotrophic culture of algae supplies additional substrates for lipid production [49]. Accordingly, in combination with increased biomass productivity, the lipid content of two species can also be higher as compared to those in monocultures. Hence, mixed culture of two species will possess symbiotic association and synergistic influence that can lead to higher biomass and lipid agglomeration in contrast with monocultures [22].
In the yeast-algal co-cultivation in DW medium, protein content was enhanced. A significant number of standard 98.1 amino acids could be found in yeast [57]. At a high nitrogen concentration, protein content increased [58]. No significant change was reported in the carbohydrate content. Kim et al. [55] showed a similar outcome during their investigation, where no change in the carbohydrate content was recorded when microalgae were grown on yeast extract.
FT-IR spectroscopy evaluated the chemical configuration of controlled-grown and DW-grown yeast and algae biomass. The functional group in the control and DW cultivated biomass was found to be almost similar. The band at 700–500 cm−1 represents the C–C stretching. The vibration band at 1200–1000 cm−1 is assigned to C = O/C–O–C stretching. The band at 1270–1230 cm−1 is assigned to C–O–C stretching. The band at 1400–1350 cm−1 represents C-H vibration. The C = O structural components are assigned the vibration band at 1630–1535 cm−1. The band at 1750–1640 cm−1 is responsible for C = O stretching. The band at 3000–2800 cm−1 is attributed to C–H stretching, aromatic. The band at 3700–3200 cm−1 in the spectra reveals the O–H stretching.
Chlorophyll a and carotenoid contents were high in yeast–algal pellets cultivated in DW and SW (Table 2). Arora et al. [34] reported that increase in chlorophyll content is associated with the nutrient-dependent growth of microalgae cells. Consequently, the study observed that deficiency in nitrogen content can lead to an increased prominent influence in enhancing Chl a, Chl b, and carotenoids ratios in comparison with phosphorus. The reason behind this fact might be the degradation of chlorophyll a due to its high nitrogen content. Similarly, Kamalanathan et al. [59] also reported that high nitrogen content would increase the pigments in microalgae.
3.3 Fatty acid profiles and biodiesel properties
The FTIR spectra of lipids obtained from yeast-algal biomass are illustrated in Table 3 and depicted in Fig. S2. The chemical structure of lipids in control and DW cultivated biomass showed a relatively analogous chemical composition.
Dairy wastewater affected the fatty acid composition of yeast and microalgae biomass. Figure 4 displays SFA, MUFA, and PUFA (> 1% of total fatty acids) corresponding to control biomass. Palmitic acid decrease (C16:0) and palmitoleic acid (C16:1) and oleic acid rise (C18:1) have been found in biomass grown in DW. FAME data displayed that co-cultivation of yeast-microalgae increased the unsaturated fatty acids (USFA) and PUFA content as compared to control. Besides this, the previous research has further reported that the fatty acid profile and lipid content of algae will enhance under the influence of multiple environmental stresses like nutrient starvation at the time of stationary growth phase after full maturation [60]. The kinematic viscosity of the C16:1 methyl ester is useful for making biodiesel suitable for use at low temperatures [61]. Dairy wastewater cultivated biomass biodiesel has a low cetane number with a high saponification value and iodine value as compared to control medium cultivated biomass (Table 4).
4 Conclusion
In this study, mixed cultivation of yeast and microalgae in DW and SW was assessed. The result showed that the nutrient removal efficiency of the yeast and microalgae consortium could be as high as 41.7%, 60.9%, 83%, and 90% for TN, TP, COD, and BOD, respectively, after 14-day treatment. Moreover, a higher biomass and lipid content was achieved at the end of the cultivation period. FAME data indicated that co-cultivation of yeast-microalgae consortium increased USFA and PUFA content as compared to control. Thus, the use of DW and SW can be a helpful and ecologically benign approach for yeast and microalgae cultivation to treat dairy effluent.
References
Tocchi C, Federici E, Fidati L, Manzi R, Vincigurerra V, Petruccioli M (2012) Aerobic treatment of dairy wastewater in an industrial three-reactor plant: Effect of aeration regime on performances and on protozoan and bacterial communities. Water Res 46:3334–3344
Daneshvar E, Zarrinmehr MJ, Koutra E, Kornaros M, Farhadian O, Bhatnagar A (2019) Sequential cultivation of microalgae in raw and recycled dairy wastewater: microalgal growth, wastewater treatment and biochemical composition. Bioresour Technol 273:556–564
Chokshi K, Pancha I, Ghosh A, Mishra S (2016) Microalgal biomass generation by phycoremediation of dairy industry wastewater: an integrated approach towards sustainable biofuel production. Bioresour Technol 221:455–460
Sidhu JP, Skelly E, Hodgers L, Ahmed W, Li Y, Toze S (2014) Prevalence of Enterococcus species and their virulence genes in fresh water prior to and after storm events. Environ Sci Technol 48:2979–2988
Müller A, Österlund H, Marsalek J, Viklander M (2020) The pollution conveyed by urban runoff: a review of sources. Sci Total Environ 709:136125
Tota-Maharaj, K (2020) Bioelectrochemical systems for stormwater treatment and energy valorization processes. Integ Microbial Fuel Cells Wastewater Treat. Butterworth-Heinemann 175–197
Begum, S, Rasul, MG, Brown, RJ (2008) Stormwater treatment and reuse techniques: a review. 2nd International Conference on Waste Management, Water Pollution, Air Pollution, Indoor Climate 144–50
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Fatima N, Kumar V (2020) Microalgae based hybrid approach for bioenergy generation and bioremediation: a review. Octa J Biosci 8:113–123
Jaiswal KK, Banerjee I, Singh D, Sajwan P, Chhetri V (2020) Ecological stress stimulus to improve microalgae biofuel generation: a review. Octa J Biosci 8:48–54
Bhatnagar P, Gururani P, Bisht B, Kumar V (2021) Algal Biochar: an advance and sustainable method for wastewater treatment. Octa J Biosci 9:79–85
Taylor TN, Hass H, Remy W, Kerp H (1995) The oldest fossil lichen. Nature 378:244–244
Øverland M, Skrede A (2017) Yeast derived from lignocellulosic biomass as a sustainable feed resource for use in aquaculture. J Sci Food Agric 97(3):733–742
Reed, G, Nagodawithana, TW (1991) Baker’s yeast production. Yeast Technol Springer, Dordrecht 261–314
Yamada EA, Sgarbieri VC (2005) Yeast (Saccharomyces cerevisiae) protein concentrate: preparation, chemical composition, and nutritional and functional properties. J Agric Food Chem 53(10):3931–3936
Hena S, Znad H, Heong KT, Judd S (2018) Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis. Water Res 128:267–277
Kothari R, Pathak VV, Kumar V, Singh DP (2012) Experimental study for growth potential of unicellular alga Chlorella pyrenoidosa on dairy waste water: an integrated approach for treatment and biofuel production. Bioresour Technol 116:466–470
Cai S, Hu C, Du S (2007) Comparisons of growth and biochemical composition between mixed culture of alga and yeast and monocultures. J Biosci Bioeng 104:391–397
Dong QL, Zhao XM (2004) In situ carbon dioxide fixation in the process of natural astaxanthin production by a mixed culture of Haematococcus pluvialis and Phaffia rhodozyma. Catal Today 98:537–544
Zhang K, Zheng J, Xue D, Ren D, Lu J (2017) Effect of photoautotrophic and heteroautotrophic conditions on growth and lipid production in Chlorella vulgaris cultured in industrial wastewater with the yeast Rhodotorula glutinis. J Appl Phycol 29:2783–2788
Zhang Z, Ji H, Gong G, Zhang X, Tan T (2014) Synergistic effects of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for enhancement of biomass and lipid yields. Bioresour Technol 164:93–99
Liu L, Chen J, Lim PE, Wei D (2018) Dual-species cultivation of microalgae and yeast for enhanced biomass and microbial lipid production. J Appl Phycol 30:2997–3007
Qin L, Wei D, Wang Z, Alam MA (2019) Advantage assessment of mixed culture of Chlorella vulgaris and Yarrowia lipolytica for treatment of liquid digestate of yeast industry and cogeneration of biofuel feedstock. Appl Biochem Biotechnol 187:856–869
Li H, Zhong Y, Lu Q, Zhang X, Wang Q, Liu H, Liu H (2019) Co-cultivation of Rhodotorula glutinis and Chlorella pyrenoidosa to improve nutrient removal and protein content by their synergistic relationship. RSC Adv 9:14331–14342
Alam, M, Wan, C, Tran, DT, Mofijur, M, Ahmed, SF, Mehmood, MA, Xu J (2022) Microalgae binary culture for higher biomass production, nutrients recycling, and efficient harvesting: a review. Environ Chem Letters 1–16
Jingrui, X, Alam, MA, Jing, W, Wenchao, W, Yusof, ZNB, Daroch, M, Russel M (2022) Enhanced removal of tetracycline from synthetic wastewater using an optimal ratio of co-culture of Desmodesmus sp. and Klebsiella pneumoniae. Bioresour Technol 351: 127056
Malairuang K, Krajang M, Sukna J, Rattanapradit K, Chamsart S (2020) High cell density cultivation of saccharomyces cerevisiae with intensive multiple sequential batches together with a novel technique of fed-batch at cell level (FBC). Processes 8:1321
Guarnieri MT, Nag A, Yang S, Pienkos PT (2013) Proteomic analysis of Chlorella vulgaris: potential targets for enhanced lipid accumulation. J Proteom 93:245–253
American Public Health Association, (2012) Standard methods for the examination of water & wastewater, twenty-second ed. American Public Health Association, Washington DC. (Accessed 10 Sept 2021)
Daneshvar E, Zarrinmehr MJ, Koutra E, Kornaros M, Farhadian O, Bhatnagar A (2019) Sequential cultivation of microalgae in raw and recycled dairy wastewater: microalgal growth, wastewater treatment and biochemical composition. Biores Technol 273:556–564
Bhola V, Desikan R, Santosh SK, Subburamu K, Sanniyasi E, Bux F (2011) Effects of parameters affecting biomass yield and thermal behaviour of Chlorella vulgaris. J Biosci Bioeng 111:377–382
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Arora N, Patel A, Pruthi PA, Pruthi V (2016) Synergistic dynamics of nitrogen and phosphorous influences lipid productivity in Chlorella minutissima for biodiesel production. Bioresour Technol 213:79–87
Arora N, Gulati K, Patel A, Pruthi PA, Poluri KM, Pruthi V (2017) A hybrid approach integrating arsenic detoxification with biodiesel production using oleaginous microalgae. Algal Res 24:29–39
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Lightenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Kumar V, Jaiswal KK, Verma M, Vlaskin MS, Nanda M, Chauhan PK, Kim H (2021) Algae-based sustainable approach for simultaneous removal of micropollutants, and bacteria from urban wastewater and its real-time reuse for aquaculture. Sci Total Environ 774:145556
Kumar V, Kumar R, Rawat D, Nanda M (2018) Synergistic dynamics of light, photoperiod and chemical stimulants influences biomass and lipid productivity in Chlorella singularis (UUIND5) for biodiesel production. Appl Biol Chem 61:7–13
Marazzi F, Bellucci M, Fantasia T, Ficara E, Mezzanotte V (2020) Interactions between microalgae and bacteria in the treatment of wastewater from milk whey processing. Water 12:297
Ji MK, Yun HS, Park S, Lee H, Park YT, Bae S, Choi J (2015) Effect of food wastewater on biomass production by a green microalga Scenedesmus obliquus for bioenergy generation. Bioresour Technol 179:624–628
Gupta S, Pawar SB, Pandey RA (2019) Current practices and challenges in using microalgae for treatment of nutrient rich wastewater from agro-based industries. Sci Total Environ 687:1107–1126
Marazzi F, Ficara E, Fornaroli R, Mezzanotte V (2017) Factors affecting the growth of microalgae on blackwater from biosolid dewatering. Wat Air And Soil Poll 228:1–14
Marazzi F, Bellucci M, Rossi S, Fornaroli R, Ficara E, Mezzanotte V (2019) Outdoor pilot trial integrating a sidestream microalgae process for the treatment of centrate under non optimal climate conditions. Algal Res 39:101430
Ghaly AE, Kamal MA (2004) Submerged yeast fermentation of acid cheese whey for protein production and pollution potential reduction. Water Res 38:631–644
Wang L, Li Y, Chen P, Min M, Chen Y, Zhu J, Ruan RR (2010) Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol 101:2623–2628
Lau PS, Tam NFY, Wong YS (1995) Effect of algal density on nutrient removal from primary settled wastewater. Environ Poll 89:59–66
Iasimone F, Zuccaro G, D’Oriano V, Franci G, Galdiero M, Pirozzi D, Pirozzi F (2018) Combined yeast and microalgal cultivation in a pilot-scale raceway pond for urban wastewater treatment and potential biodiesel production. Water Sci Technol 77:1062–1071
Cheirsilp B, Suwannarat W, Niyomdecha R (2011) Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. New Biotechnol 28:362–368
Wang Y, Guo W, Yen HW, Ho SH, Lo YC, Cheng CL, Chang JS (2015) Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour Technol 198:619–625
Kouzuma A, Watanabe K (2015) Exploring the potential of algae/bacteria interactions. Curr Opin Biotechnol 33:125–129
Ramanan R, Kim BH, Cho DH, Oh HM, Kim HS (2016) Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnol Adv 34:14–29
Arora N, Patel A, Pruthi PA, Pruthi V (2016) Boosting TAG accumulation with improved biodiesel production from novel oleaginous microalgae Scenedesmus sp. IITRIND2 utilizing waste sugarcane bagasse aqueous extract (SBAE). Appl Biochem Biotechnol 180:109–121
Mohamed ZA (2008) Polysaccharides as a protective response against microcystin-induced oxidative stress in Chlorella vulgaris and Scenedesmus quadricauda and their possible significance in the aquatic ecosystem. Ecotoxicol 17:504–516
Kim G, Mujtaba G, Lee K (2016) Effects of nitrogen sources on cell growth and biochemical composition of marine chlorophyte Tetraselmis sp. for lipid production. Algae 31:257–266
Gu H, Nagle N, Pienkos PT, Posewitz MC (2015) Nitrogen recycling from fuel-extracted algal biomass: residuals as the sole nitrogen source for culturing Scenedesmus acutus. Bioresour Technol 184:153–160
Caballero-Córdoba GM, Sgarbieri VC (2000) Nutritional and toxicological evaluation of yeast (Saccharomyces cerevisiae) biomass and a yeast protein concentrate. J Sci Food Agri 80:341–351
Prochazkova G, Kastanek P, Branyik T (2015) Harvesting freshwater Chlorella vulgaris with flocculant derived from spent brewer’s yeast. Bioresour Technol 177:28–33
Kamalanathan M, Pierangelini M, Shearman LA, Gleadow R, Beardall J (2016) Impacts of nitrogen and phosphorus starvation on the physiology of Chlamydomonas reinhardtii. J Appl Phycol 28:1509–1520
Chen M, Tang H, Ma H, Holland TC, Ng KS, Salley SO (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102:1649–1655
Knothe G (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Eng Fuels 22:1358–1364
Funding
This paper has been supported by the RUDN University Strategic Academic Leadership Program. MV would like to thanks to Brain Pool Program (2019H1D3A1A01102657). H. Kim was supported by the Korea Environment Industry & Technology Institute (KEITI) through the project for developing innovative drinking water and wastewater technologies funded by Korea Ministry of Environment (MOE) (NO. 2019002710006).
Author information
Authors and Affiliations
Contributions
VK: supervision, practical work, writing original draft. PG: experimental work. AP: experimental work. MV: analysis, writing review and editing. HK: editing and review. MV: editing and review. AVG: analysis, writing review and revision of manuscript. KGR: editing and revision of manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, V., Gururani, P., Parveen, A. et al. Dairy Industry wastewater and stormwater energy valorization: effect of wastewater nutrients on microalgae-yeast biomass. Biomass Conv. Bioref. 13, 13563–13572 (2023). https://doi.org/10.1007/s13399-022-02947-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02947-7