Abstract
In this work, we designed novel-based catalysts composed of metals and tungstophosphoric acid supported mesoporous clay through wetness impregnation and reduction methods for getting good activity and selectivity of menthols from catalytic hydrogenations of citral and citronellal. Clay was treated with 4 M HCl and then doped with 12-tungstophosphoric acid and Pd/Ni metals through wetness impregnation method. These catalysts were activated through hydrogen reduction method and applied in high-pressure hydrogenation reactions at optimum reaction conditions. Nickel-doped catalysts showed a good catalytic activity in liquid-phase citral hydrogenation and produced 56% menthols (e.g., selectivity). Similarly, Ni-doped and Pd-doped catalysts could produce 98% and 82% menthols yield/selectivity from citronellal hydrogenation with excellent catalytic activity (e.g., fast conversion), respectively. The catalytic activity and selectivity were connected with balanced metal–acid sites ratio (strong Lewis and medium Bronsted acid-sites) and the presence of mesopores. This multistage reaction was possible in the presence of strong Lewis and medium Brønsted acid sites along with metal active sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Owing to wide range of industrial applications of menthol such as pharmaceuticals, cooling agent, cosmetics, and tobacco, the synthesis of organic menthol has gained great attention in scientific world [1]. The total annual menthol demand is around 12,000 tons, 80% of which comes from natural resources and remaining 20% from synthetic routes which accounts 2500 tons [2]. Different synthetic methods for menthol production have been used which include (1) myrcene transformation to diethyl geranyl amine, then citronellal enamine to menthol, (2) piperitenone catalytic hydrogenation to pulegone and then to menthols, and (3) methyl esters hydrogenolysis with enzyme [2]. These synthetic methods are very complex. Besides these methods, citronellal hydrogenation to menthol is easy route, which is possible though metal–acid catalyst. Citronellal hydrogenation to menthol is mainly comprised of consecutive reactions: (1) isopulegol produced by citronellal cyclization, (2) menthol production by isopulegol hydrogenation as described in Scheme 1 [3].
Reaction network of liquid phase hydrogenation of citral and citronellal to menthols [2]
Menthol synthesis though this method is possible through active and selective metal–acid catalyst with optimized process parameters. Some side effect reactions (e.g., hydrogenolysis, dehydration, or defunctionalization) occurrence is expected due to unsuitable design of catalysts, which may result in less menthol production. The acidic catalyst such as SiO2-Al2O3 [4], H-BEA zeolite [5], SiO2 [6], H-beta zeolite[1], zeolites, SiO2-Al2O3 [7], and sulfated zirconia [8] is previously being widely applied but, owing to certain issues such as recyclability, activity loss, imbalance metal–acid sites, less selective, and lifetime stability, their utilization is limited these days. In citronellal hydrogenation, low menthol selectivity problems have been reported by many researchers. About 70% of menthol selectivity was reported in the study performed by Mertens et al. [1] through Pt/H-zeolite. Milone et al. [9] performed a study for the optimization of selectivity of menthol using silica and carbon catalyst supported by ruthenium (Ru); the results show 37 and 80% menthol selectivity. Similarly, Silva et al. [10] tested silica-oxide catalyst supported by Pd-heteropoly acid and found 36–85% menthol selectivity. Other researchers have also developed different kinds of functional catalyst such Negoi et al. [3] synthesized a catalyst by attaching two metals, gold, and fluorides in order to develop a bi-functional catalyst and tested for menthol selectivity. The results revealed about 43–60.8% of menthol selectivity. Thus, researchers are in great intention to develop an efficient catalyst for in order to overcome currently reported issues.
Heteropoly acids are the class of strong acids, which have strong Bronsted acidity and catalytic activity [11]. In heteropoly acid family, phosphotungstic acid is the most stable (300–500 °C) and possesses highest Brønsted acidity [12]. However, the low surface properties of 12-tunsgtophosphoric acid and other its similar group acids have limited its wide range application [13]. This problem can be overcome by dispersing heteropoly acids on mesoporous materials in order to improve mass transfer and reaction rate.
In comparison with porous supports, montmorillonite clay can be considered as catalyst support in acid catalyzed reactions, because of its good catalytic, thermal, and acidic properties along with high surface area characteristics. Besides its advantages, it possesses a few drawbacks which can be resolved through acid-treatment (dealumination). The dealumination technique is known to improve the internal structure, surface area, acidic sites, and catalytic properties of the montmorillonite clay. The cation exchange capacity and physical properties could be changed by leaching alumina, magnesium, and iron cations from interlayer tetrahedron structure of montmorillonite clay [14].
The aim of this study is to overcome the drawbacks previously reported by dispersing HPA (12-tunsgtophosphoric acid) over mesoporous media. The clay was treated with acid in order to enhance the mesoporosity material for getting good mass transfer rate in reaction [15]. The active and selective metal–acid catalysts were prepared using economical methods and were applied in catalytic hydrogenations of liquid-phase citral and citronellal reactions for menthol synthesis. The effects of metal loading, metal type, and pressure variations have been studied on catalytic hydrogenations of liquid-phase citral and citronellal reactions and their co-relation has been compared with menthol production.
2 Material and methods
2.1 Materials
For performing experiments, some specific laboratory-based chemicals such as NiNO3.6H2O, citral (95% pure), citronellal (95% pure), montmorillonite clay (K10), nickel nitrate hexahydrate, PdCl2 (99.98%), 12-tunsgtophosphoric acid, ethyl alcohol, and HCl have been directly purchased from Sigma Aldrich, whereas cyclohexane solvent was obtained from Dae-Jung Chemicals. For the product identification (3,7-dimethyl1-octanol, citronellol, menthol, and isopulegol) of citral hydrogenation reaction, laboratory analysis-grade chemicals were purchased from Sigma Aldrich.
2.2 Preparation of mesoporous montmorillonite clay
Ten grams of montmorillonite clay was poured into 100 ml HCl (4 M) solution and stirred at 80℃ for 4 h. After 4 h, clay was washed with distilled water and dried in an oven at 105℃ for 24 h. The dried clay was calcined at 200℃ for 4 h in muffle furnace at rate of 5℃ temperature rise. This calcined material is being used as support for metal–acid catalyst preparation. It is assumed that HCl treatment helps in dealumination of montmorillonite clay and modify framework structure that might bring surface properties. The prepared support was coded as mesoporous clay “MC.”
2.3 Preparation of metal doped 12-tungstophosphoric acid supported clay catalysts
12-tungstophosphoric acid (TPA)-supported mesoporous clay (MC) acid catalyst was prepared using wetness impregnation method. Metal-supported catalysts such as 5%Pd-MC, 2.5%Pd-TPA-MC, 5%Pd-TPA-MC, 5%Ni-TPA-MC, and 10%Ni-TPA-MC were designed using standard wetness impregnation method by doping metal precursors solution drop-wise over the surface of TPA-supported clay (acidic support). The metal precursors were dissolved into methanol solvent. The prepared catalysts were dried at 60 °C for 24 h and then calcined at 250–400 °C for 4 h at 5 °C/min temperature rise. Pd-supported catalysts were reduced through hydrogen gas at 300 °C at flow rate of 20 ml/min, whereas Ni-supported catalysts were reduced through hydrogen gas at 500 °C at 20 ml/min flow rate. This reduction step was performed into glass type tubular furnace in the presence of hydrogen gas. Before and after catalyst reduction, system was purged with helium gas. Freshly reduced catalysts were applied in catalytic liquid phase citral and citronellal hydrogenation reactions.
2.4 Catalyst characterization
The characterization of prepared catalysts was carried out using XRD, pyridine adsorption, and BET techniques. Nitrogen sorption measurements were carried out at − 196 °C using Micrometrics TriStar II 3020. Prior to analysis, the prepared catalyst samples were degassed at 150 ~ 300 °C for 2 h. The Brunauer–Emmett–Teller (BET) method was applied to estimate specific surface areas [16]. Mesopore size distribution was calculated from the adsorption branch of the isotherm (BJH pore size model). The t-plot, according to Lippens and de Boer, was used to estimate micro and mesoporosity contribution [15]. Fourier transform infrared spectroscopy of prepared materials were performed on a Nicolet, iS10 FTIR spectrometer (400–4000 cm−1, 8 cm−1 optical resolution, co-addition of 32 scans) using dehydrated KBr-supported pellets. For the measurement of pyridine-adsorbed Lewis and Bronsted acid sites of prepared materials, self-supporting wafers (11 tons cm−2, 30 mg, 1 cm2) were dehydrated into especial designed stainless steel IR cell under vacuum (10−3 mbar) at 300 °C for 2 h. The hot pyridine vapors were further introduced into IR cell until pressure inside IR cell reached 5 bar pressure. The pyridine vapors were adsorbed on the pellet surface at 100 °C for 2 h in order to avoid pyridine physisorption. After pyridine adsorption saturation, remaining pyridine was removed from IR cell at 150 °C for 15 min using vacuum condition. After physisorbed pyridine removal, IR cell was connected to infrared spectroscopy and recorded IR spectrum of sample under conditions of 600–4000 cm−1, 8 cm−1 optical resolution and co-addition of 32 scans. The accurate IR spectrum of material was collected after subtracting the spectrum of dehydrated sample from pyridine adsorbed spectrum [17]. The total concentrations of Bronsted acid sites (CB) and Lewis acid sites (CL) were calculated from the band areas of adsorbed pyridine at 1440 cm−1 and 1540 cm−1 using calculated molar extinction coefficients [18]. The acidity of prepared catalysts was determined by amine titration technique as described in procedure [19] using 0.1 N n-butyl amine (in dry benzene) solution as weak base and dimethyl yellow as indicator. A total of 0.1 g of each acid type catalysts were poured along with addition of 9 mL dry benzene and dimethyl yellow indictor into 20-mL glass vials. These samples were further titrated drop-wise with butyl amine weak base until pink color changed to yellow color. Again, sample was stirred for 4 h and titrated again for getting complete saturation point and final acidity of catalyst was calculated.
2.5 Catalytic reaction study
2.5.1 Catalytic hydrogenation of liquid phase citral
Fifty-milliliter autoclave reactor was used for carrying out consecutive reactions. Optimized amounts of citral (4.5 mmol), cyclohexane (25 ml), and activated catalyst (0.2 g) were poured in the 50-ml autoclave reactor. The autoclave reactor was connected with nitrogen and hydrogen gas cylinders. The reactor was purged three times with nitrogen gas and 0.5–1.0 MPA pressure was maintained inside reactor and temperature approximately 80 °C was fixed through temperature controller. When required process parameters were achieved, then stirring (speed ~ 200 rpm/min) was started in the presence of hydrogen gas. The reaction was further preceded for long time (0–24 h). The reaction samples were taken at different time intervals and analyzed.
2.6 Catalytic hydrogenation of liquid phase citronellal
Similar citronellal hydrogenation reaction procedure has been applied as mentioned in Sect. 2.5.1. Optimized amounts of citronellal (4.5 mmol), cyclohexane (25 ml), and activated catalyst (0.2 g) were poured in the 50-ml autoclave reactor. The autoclave reactor was connected with nitrogen and hydrogen gas cylinders. The reactor was purged three times with nitrogen gas and 0.5–1.0 MPA pressure was maintained inside reactor and temperature approximately 80 °C was fixed through temperature controller. When required process parameters were achieved, then stirring (speed ~ 200 rpm/min) was started in the presence of hydrogen gas. The reaction was further preceded for long time (0–12 h). The reaction samples were taken at different time intervals and analyzed. The product samples of both reaction were analyzed using chiral column (Cyclodex-B (Agilent), with temperature conditions as described [20]. The unknown-formed products were identified by GC–MS (Agilent, Model# 7890A).
3 Results and discussion
3.1 Catalyst’s characterization
In our previous published paper [21], we have already discussed characterization of HPA-MM catalysts in detail. Therefore, we have provided here brief details of catalyst characterization. Table 1 shows BET and pyridine adsorption data. BET surface area and pore volume of montmorillonite increased with acid-treatment, but it further decreased with impregnations of HPA and Pd/Ni metals. The prepared catalysts are more mesoporous in nature and possess high surface areas and pore volumes. The montmorillonite contains weak Lewis acidity, which enhanced with HPA impregnation. Ni- and Pd-supported catalysts contain strong Lewis acid sites and weak Brønsted acid sites as shown in Table 1. Furthermore, in this research work, we authors have mainly focused on reaction performance and determined parametric effects on menthol synthesis.
3.2 Reactions study
Bifunctional catalysts (e.g., Pd and Ni doped over tungstophosphoric acid-supported clay) were prepared using wetness impregnation method and then were activated or reduced in tubular furnace through hydrogen at specific temperature and gas flow. These prepared catalysts were applied in both hydrogenation reactions (e.g., citral and citronellal hydrogenation. The influence of metal loading, types of metals, and pressure loading on menthol synthesis were studied in both reactions; the details are shown in Scheme 1 [5].
In the 1st step, citral hydrogenation to menthol was studied. According to literature study, Pd [5] and Ni [22] metal precursors are found more selective for menthol synthesis. The main controlling reaction parameter is design of catalysts and presence of required acid sites. In this perspective, we have tried to design catalysts according to reaction requirements. In our previous work [23], acidic catalysts were found more active and selective in citronellal cyclization for isopulegol formation; this performance was correlated with balanced acid sites and presence of mesopores. For this perspective, TPA-supported clay catalyst was used as acidic support for the preparation of metal–acid-supported catalysts for both hydrogenation reactions. Mesoporous clay is porous and acidic nature. Further acidity strength (Lewis and Bronsted acid sites) of mesoporous clay is improved and imbalanced acid sites with impregnation of tungstophosphoric acid. This type of modification helps in catalytic activity and selectivity enhancement. TPA possesses low surface area, whereas mesoporous clay is porous in nature that helps reactant molecules fast mass transfer and diffusion.
3.3 Catalytic reaction of liquid phase citral hydrogenation
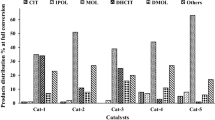
Table 2 shows reaction performance of catalysts in citral hydrogenation to menthols and other products. In first stage, various supports such as alumina, silica, activated carbon, clay, and carbon nanotubes were combined with Pd metal precursors and then applied in citral hydrogenation. All catalysts except silica and montmorillonite resulted formation of citronellal and dihydrocitronellal products. No more menthol yield was found. The silica and montmorillonite-supported metal could yield 5 to 35% menthol. From our experiment observation, it has been observed that catalyst does not show any conversion (e.g., citronellal to isopuelgol) in the absence of acid sites on catalyst surface. Only metal sites can promote hydrogenation reactions.
When Pd and Ni metals were attached with TPA-MC catalysts, the path of reaction shows higher selectivity for menthol synthesis. In initial reaction study, Pd metal showed a good catalytic activity and conversion of substrate as compared to Ni metal. With increase of Pd or Ni loading, the catalytic activity further enhanced. When Pd loading is increased (~ 5% Pd), the acid sites were covered by metal sites and promoted hydrogenation instead of cyclization. That factor reduced menthol selectivity. The cyclization and hydrogenation were promoted by less dispersion of Pd over acid sites (surface catalyst). Experimental/reaction observations supported this mechanism.
A total of 2.5% Pd-MM catalyst could produce 35% menthol within 12 h with formation of other hydrogenated products at full conversion of citral substrate. This less selectivity is to be assumed because of weak acid site presence, which favored hydrogenation as compared to cyclization. When heteropoly acids were impregnated on montmorillonite surface, the acid sites were increased and high Lewis acidity was produced on the surface of catalyst, which restrict hydrogenation of the citronellal to dihydrocitronellal and favor to citronellal cyclization to isopulegol. A total of 2.5% Pd-TPA-MC could yield 51% menthol within 12 h at low pressure (0.5 MPa). Furthermore, we determined effect of high pressure (1.0 MPa) using same catalyst. This high-pressure effect reduced menthol yield and resulted approximately ~ 39% menthol at full substrate conversion. The high-pressure hydrogen gas activates metal sites and promotes them for hydrogenation of citronellal to dihydrocitronellal then 3, 7 dimethyl octanols instead of cyclization. High hydrogenation pressure promotes hydrogenolysis product formation that caused low formation of menthols, because higher pressure increased hydrogenation reaction rate and was too difficult to control hydrogenation of citronellal intermediate to dihydrocitronellal and to favor towards cyclization of citronellal to isopulegols [20]. Low hydrogenation pressure (0.5 MPa) showed low reaction rate and it was possible to cyclize citronellal intermediate to isopulegols, then menthols effectively. Pd also showed optimum reaction selectivity towards menthols synthesis, due to low hydrogenation reaction rate of citronellal intermediate towards dihydrocitronellal, and it caused 94% citronellal conversion to menthols (59%) and other cyclic products (26%). During hydrogenation, citronellal intermediate molecules have contact with sites of Lewis and Bronsted acid of catalyst and promoted it into cyclic isopulegol (28%), and hydrogenated it to desired menthol product.
Further menthol synthesis study was extended, and nickel-based catalysts were prepared, and above-described parameters were evaluated as shown in Table 2.
The 5% Ni-TPA-MC at low pressure (0.5 MPa) results 37% menthol within 24 h. When hydrogen pressure was increased up to 1.0 MPa, that resulted more production of menthol which is approximately 44% at full substrate conversion. It is observed that nickel metal-based catalysts are found less active and consuming more time in multistage reactions. Therefore, Ni loading was enhanced up to (~ 10%) and applied in citral hydrogenation at low and high pressures. More Ni (~ 10%) loading at low pressure showed more activity and high selectivity (~ 44%) as compared to 5% Ni-based catalyst. Further increasing hydrogen pressure, catalytic activity, and menthol selectivity enhanced. This catalyst (10% HPA-MM) could yield highest menthol yield (~ 56%) with formation of 30% other cyclic products. In multistage reaction, many side reaction products were produced due to dehydration, defunctionalization, and isomerization of isopulegols and menthols. The presence of strong Brønsted acid sites can result dehydration or isomerization reactions, which can result more reduction of menthol yield.
In general discussion, low Pd content (~ 2.5 wt%) favored full conversion of citral to citronellal in 1st step of reaction. Afterwards, citronellal can be transformed into various products like isopulegol, dihydrocitronellal, 3,7-dimethyl-1-octanol, and other cyclic compounds. Pd also dehydrated and defunctionalized menthols and isopulegols and generated 25% side products like citronellal ethers, methyl-4-propyl cyclohexane, cyclohexane-1-methyl-4-propylidene, and menthatriene [5]. Pd metal has strong capability for hydrogenation reaction. High Pd content strongly favored to hydrogenate citral to citronellal, then to dihydrocitronellal [3]. The catalytic activity rate was improved on increasing Pd percent. Higher Pd content was not suitable for menthols yielding and all citronellal intermediate product was converted to dihydrocitronellal effectively [3]. Low Pd content reduced catalytic activity for hydrogenation of citronellal intermediate product to dihydrocitronellal and favored direct synthesis chemical route to menthols. The rate of dehydration or defunctionalization of menthols/isopulegols was dependent on Pd content and acid site strength and their nature [7]. The role of metal type, acid sites, chemical compound nature, and process parameters is very important for getting high yield of menthols. Nickel-based catalysts produced high percentage of menthols but menthols/isopulegol products were further dehydrated or cracked into other side cyclic products. Our optimum menthol production (~ 56%) is best as compared to others. Literature review justified that silica and alumina contain only Lewis acidic characteristics which are not suitable for citronellal cyclization. Our prepared catalysts contain good catalytic activity and selectivity because of strong Lewis acidity and weak Brønsted acidity characteristics.
Above-described same parameters were considered for one pot synthesis of citral hydrogenation to menthols (Scheme 1) [2]. Pd- and Ni-impregnated PTA-supported mesoporous K-10 montmorillonite catalysts were tested for higher menthols yielding.
3.4 Effects of metal type on menthol yielding
Palladium and nickel metals were impregnated on PTA-supported mesoporous K-10 montmorillonite and were tested in citral hydrogenation for menthol yielding. Pd and Ni metals favored to one pot synthesis route towards menthols. Palladium’s catalytic activity was higher than Ni. Low content (2.5 wt. %) of Pd slightly favored to hydrogenation of citronellal to dihydrocitronellal (6%). Pd also dehydrated and defunctionalized menthols and isopulegols and generated 25% side products like citronellal ethers, methyl-4-propyl cyclohexane, cyclohexane-1-methyl-4-propylidene, and menthatriene [7]. Ni catalyst also favored direct one pot synthesis route to menthols and also slightly favored hydrogenation of citronellal to dihydrocitronellal, then 3, 7 dimethyloctanol. Ni catalytic activity was slow that caused in slow conversion of citral to citronellal, then isopulegols and menthols. Ni-type catalyst also generated many cyclic side products (29%). According menthol selectivity, Pd could yield 59% menthols as compared with Ni, it optimized menthol yield about 56% (Fig. 1). Pd-PTA-MMT catalyst was found best than Ni-PTA-MMT. Our optimum menthol yielding rate was higher than compared to here [2, 3].
Shows experimental set up and catalyst preparation and characterization equipments (a) Muffle furnace used for calcination and (b) tubular furnace used hydrogen reduction of catalyst (c) Pyridine adsorption system (d) Pyridine adsorption system connected with FTIR and (e) Autoclav hydrogenation apparatus
3.5 Effects of metal loadings on menthols yielding
Pd metals have strong capability for hydrogenation reaction. High Pd content strongly favored to hydrogenate citral to citronellal, then to dihydrocitronellal 5 [3]. The catalytic activity rate was improved on increasing Pd percent. Higher Pd content was not suitable for menthols yielding and all citronellal intermediate product citronella was converted to dihydrocitronellal 5 effectively [3]. Low Pd content reduced catalytic activity for hydrogenation of citronellal intermediate product citronellal to dihydrocitronellal and favored direct synthesis chemical route to menthols. The rate of dehydration or defunctionalization of menthols/isopulegols was dependent on Pd content on acid support [2], whereas 2.5 wt. % Pd-PTA-MMT could yield 59% menthols at 6% hydrogenolysis product formation rate. Catalytic activity and reaction rate were improved on increasing Ni content. At 5 wt. %Ni content, conversion rate was slow as compared to 10 wt. % Ni content. Five percent Ni-PTA-MMT could convert all citronellal within 24 h as compared 10 wt. % Ni-PTA-MMT (11 h) (Fig. 2).
3.6 Effects of pressure variations on menthol yielding
Same reaction behavior of citronellal hydrogenation was observed in citral hydrogenation on hydrogen pressure variations (Fig. 3). High hydrogenation pressure promotes hydrogenolysis product formation that caused low formation of menthols, because higher pressure increased hydrogenation reaction rate and was too difficult to control hydrogenation of citronellal intermediate to dihydrocitronellal and to favor towards cyclization of citronellal to isopulegols. Low hydrogenation pressure (0.5 MPa) showed low reaction rate and it was possible to cyclize citronellal intermediate citronellal to isopulegols, then menthols effectively. Pd also showed optimum reaction selectivity towards menthol synthesis, due to low hydrogenation reaction rate of citronellal intermediate citronellal towards dihydrocitronellal and it caused 94% citronellal conversion to menthols (59%) and other cyclic products (26%). During hydrogenation, citronellal intermediate citronellal molecules have contact with sites of Lewis and Bronsted acid of catalyst and promoted it into cyclic isopulegol, and hydrogenated it to desired menthol product as shown in Fig. 3(a) and (b). Due to effect of hydrogen pressure, molecules of reactant and hydrogen molecules are colliding each other and reacting on bifunctional catalyst structure. High hydrogenation pressure (1.0 MPa) increased chemical reaction rate of citral. During continuous citral hydrogenation reaction, all citral reactant was converted within 12 h and yielded 24% menthols as compared low pressure (0.5 MPa) hydrogenation that resulted zero menthol yield in same reaction period. High hydrogenation pressure and high Ni content were considered best for menthols yielding and high chemical reaction rate. At 1.0 MPa, 5 wt. % and 10 wt. % Ni-PTA-MMT yielded 37% and 56% menthols and 17% and 30% cyclic side products respectively within 50 h. Ni-type catalyst in citral hydrogenation also favored hydrogenation of citronellal intermediate to dihydrocitronellal, then 3,7- dimethyloctanol. This catalytic performance rate of Ni bifunctional catalysts in accordance with menthol selectivity was different in citral and citronellal hydrogenation. Finally concluded that optimum reactivity and menthols yielding were obtained at high pressure (1.0 MPa) using low Pd (2.5 wt. %) and high Ni (10 wt. %) content during catalytic citral hydrogenation [11].
(a) Citral hydrogenation to menthols. Product yields and citral conversion as function of different metal loading and hydrogenation reaction pressures Autoclave hydrogenation apparatus for one pot menthol synthesis from citral/citronellal. (b) Products distributions in one pot synthesis from citral hydrogenation by using catalyst-1 at 0.5 MPa pressure condition. (c) Citronellal hydrogenation to menthols. Product yields and citronellal conversion as function of different loadings of metal and pressures
3.7 Effects of reaction time on menthol yielding
The catalytic performance of Pd- and Ni-type catalysts was determined for menthols yielding on the basis of metal loading and pressure variations. The complete conversion rate of citral was possible optimally within 1-h reaction period. High Pd (> 2.5 wt. %) and pressure (> 0.5 MPa) increased hydrogenation reaction rate and catalytic activity. On continuous hydrogenation reaction, 3–4% more menthol-dehydrated products were reported within 19-h reaction. In the case of Ni, catalytic performance and overall chemical reaction rate performance was slow [2]. The menthols yielding was possible when all citral reactants were converted to citronellal and isopulegols intermediates, then hydrogenation of isopulegols promoted to menthols. Mostly menthol yielding was possible after 20 and 10 h of citral hydrogenation reaction by use of 5 wt. % and 10 wt. % Ni-TPA-MC at 1.0 MPa. In considering low hydrogenation pressure, menthols yielding was possible after 20 h citral hydrogenation reaction using 10 wt. % Ni at 0.5 MPa at 81% conversion rate. The menthol selectivity was improved with reaction time; besides it, side products were also formed. It was an interesting point that menthol product not appeared in reaction until all citral was totally converted to citronellal and isopulegols. After full conversion of citral, menthol formation rate was increased, because molecules of isopulegols were activated and hydrogenated to menthols. The catalytic reaction behavior might be changed due to interaction of reactants with bifunctional catalyst surface, adsorption and desorption of reactants and products, process parameters, and metal type [2]. Menthol yield was improved with reaction time in the use of Ni catalyst, but menthols yielding was decreased in case of Pd due to decomposition of menthols by dehydration reaction as highlighted in Fig. 4.
3.7.1 Catalytic reaction study of citronellal hydrogenation
In one pot menthol synthesis from citral hydrogenation, very important observations were measured. Furthermore, we extended our experimental study on one pot menthol synthesis from citronellal hydrogenation and determined effects of metal type, metal loading, and pressure variations on catalytic activity and menthol selectivity. In this experiment, we started reaction in the presence of nitrogen gas in order to promote citronellal cyclization to isopulegol and avoid hydrogenation of citronellal to dihydrocitronellal and 3, 7-dimethyl 1-octanol. Within 1 h, all citronellal substrate molecules were completely converted to isopulegol isomers (~ 98% isopulegol selectivity at full conversion). This result was possible because metal acid sites were inactive because in absence of hydrogenation and acid sites became more active and promoted cyclization reaction. This cyclization reaction was possible in the presence of strong Lewis acid sites and weak Brønsted acid sites, which is already explained its reaction phenomena in our published paper [23].
Table 3 shows reaction performance of catalysts in citronellal hydrogenation. A total of 2.5% Pd-TPA-MC catalyst could produce 82% menthol within 3 h at low pressure and remaining isopulegol and menthol products were defunctionalized or dehydrated in other cyclic compounds, whereas 6% citronellal molecules were hydrogenated into dihydrocitronellal and 3,7-dimethyl-1-octanol products. Less formation of hydrogenated products were due to low pressure of hydrogen (~ 0.5 MPa). When hydrogen gas pressure was increased up to 1.0 MPa, the reaction scenario became changed and menthol yield obtained 64%, because Pd metal sites were more active in presence of hydrogen gas molecules and promoted citronellal hydrogenation to dihydrocitronellal and 3,7 dimethyl octanol partially (~ 20%). The catalytic activity or conversion rates were enhanced under high pressure of hydrogen gas; this change might be due to fast collision of molecules inside reactor.
Further loading of Pd (~ 5%) on acidic support was increased and pressure effects were determined as earlier discussed above. With doping of high amount of Pd precursor on support, menthol yield decreased (approximately obtained 60%) under low hydrogen pressure (~ 0.5 MPa). Further hydrogen gas pressure increased up to 1.0 MPa; the menthol yield further decreased (approximately obtained 46%). This change more favored to citronellal hydrogenation to dihydrocitronellal and 3,7-dimethyl octanol. This drastically reduction in menthol production has close connection with number of metal acid sites and their distribution over acidic support. It can be assumed that more Pd loading can block acid sites of support and citronellal reactants had more contact with metal acid sites that promotes citronellal hydrogenation reaction. In other words, we can say that metal sites suppress acidic sites that restrict cyclization reaction.
Similarly following reaction study, Ni-based catalyst was applied in citronellal hydrogenation as same as described above. Five percent Ni-TPA-MC catalyst yielded 96% menthols under low hydrogen pressure within 12 h. Nickel-based catalyst did not promote citronellal hydrogenation to dihydrocitronellal and 3,7-dimethyl-1-octanol. Experimental results declare that approximately 100% citronellal substrate was converted to isopulegol and menthol. When pressure was increased up to 1.0 MPa, the reaction rate became low and yielded 77% menthols along with other side product formation. It was thought that catalytic activity and selectivity were suppressed with the effect of high pressure of hydrogen gas.
With increase in nickel loading (~ 10% Ni), the catalytic activity (conversion ~ 100%) and selectivity (~ 98%) improved at 0.5 MPa pressure as compared to 5% Ni-HPA-MM catalyst. Further selectivity rate decreased (98 to 95%) on increasing hydrogen pressure. Some menthol isomers did not remain stable and decomposed into other cyclic compounds under high pressure. On the basis of catalytic activity, Pd-supported catalysts were found more active, whereas Ni-based catalysts were considered more selective for menthols production. Nickel-based catalysts had low catalytic activity as compared to Pd-supported catalysts, but nickel-supported catalysts did not favor to hydrogenolysis and dehydration reactions. In comparison, citronellal hydrogenation to menthol was carried out by using two steps: (1) citronellal cyclization to isopulegol using nitrogen gas instead hydrogen gas, and (2) isopulegol hydrogenation to menthol using hydrogen gas. Pd-supported catalyst could yield 85% menthols along with 15% side reaction product formation, whereas Ni catalyst yielded 99% menthols but catalytic activity remained lower than Pd catalyst. On the basis of GC–MS analysis, many side products such as cyclohexane-1-methyl-4-propylidene, methyl-4-propyl cyclohexane, citronellal ethers, and menthatriene [6, 10] were formed. High Pd metal content favored hydrogenolysis and dehydration reactions. Experimental data and our previous work suggest that citronellal cyclization to isopulegol occurs through protonation–deprotonation mechanism with the help of strong Lewis and weak Brønsted acid sites. When citronellal molecule binds with Lewis acid site via the aldehyde oxygen, this brings the citronellal into an orientation favorable for ring closure through an intramolecular carbonyl-ene (C–C) reaction. In the transition state, protonation of the oxygen occurs via a neighboring Brønsted hydroxyl group, together with an abstraction of hydrogen from the isopropyl group followed by ring closure to form isopulegol.
3.8 Recyclability test
The recyclability test of good catalyst Ni-doped mesoporous clay has been evaluated in liquid-phase citral hydrogenation reaction. In 1st cycle, 100% citral reactant was consumed and catalyst was washed with cyclohexane solvent and dried then used in reaction as 2nd cycle, whereas the citral conversion remained 98% and 96% in 2nd and 3rd reaction cycle, respectively (Fig. 5).
4 Conclusion
Dual properties (metal–acid)-type catalysts were designed by doping metals and 12-tungstophosphoric acid over mesoporous type clay. These catalysts were calcined and activated using high-temperature calcination and reduction methods, respectively, and characterized. The novel catalysts were applied in catalytic hydrogenations of citral and citronellal reactions. The reaction kinetics study and various process parameters were studied and correlated with catalytic activity and menthol production. In chemical reaction study, Ni/TPA-MC (10% Ni-doped) catalyst yielded 56% menthols at low pressure (0.5 MPa), whereas 44% side products were formed. When hydrogen gas pressure was enhanced up to 1.0 MPa, the menthol yield was improved (in case of Ni-doped catalyst, viva versa for Pd-doped catalyst) in catalytic citral hydrogenation reaction, whereas Pd-doped catalysts showed highest catalytic activity but menthol formation was low as compared to Ni-doped catalyst. With increase in Pd concentration, catalytic activity enhanced but menthol formation was low. In case of Ni, catalytic activity and menthol production were improved with increase in Ni content (maximum 10%).
In citronellal hydrogenation, 10% Ni-TPA-MC catalyst at low hydrogen pressure (0.5 MPa) showed highest menthol selectivity (~ 98%) as compared to Pd-supported catalyst (~ 82%). With increase in Pd metal sites and hydrogen pressure, the catalytic activity improved but menthol selectivity decreased. The direct conversion of citral to menthol is complex reaction; it majorly depends on designing of catalyst, optimized process parameters, and balanced ratio of metal–acid sites. The reaction rate and mass transfer are also enhanced due to presence of mesopores and high surface area of support. The strong Lewis and medium Bronsted acid sites are main responsible for complete cyclization of citronellal to isopulegol under desired metal acid sites and process parameters.
Abbreviations
- MC:
-
Mesoporous clay
- TPA-MC:
-
12-Tungstophosphoric acid supported mesoporous clay
- Pd-TPA-MC:
-
Pd-doped over tungstophosphoric acid supported mesoporous clay
- Ni-TPA-MC:
-
Ni-doped over tungstophosphoric acid supported mesoporous clay
References
Mertens P, Verpoort F, Parvulescu A-N, De Vos D (2006) Pt/H-beta zeolites as productive bifunctional catalysts for the one-step citronellal-to-menthol conversion. J Catal 243(1):7–13
Mäki-Arvela P, Kumar N, Kubička D, Nasir A, Heikkilä T, Lehto V-P et al (2005) One-pot citral transformation to menthol over bifunctional micro- and mesoporous metal modified catalysts: effect of catalyst support and metal. J Mol Catal A: Chem 240(1):72–81
Negoi A, Teinz K, Kemnitz E, Wuttke S, Parvulescu V, Coman S (2012) Bifunctional nanoscopic catalysts for the one-pot synthesis of (±)-menthol from citral. Top Catal 55(7–10):680–687
Trasarti AF, Marchi AJ, Apesteguía CR (2013) Synthesis of menthols from citral on Ni/SiO2–Al2O3 catalysts. Catal Commun 32:62–66
Plößer J, Lucas M, Claus P (2014) Highly selective menthol synthesis by one-pot transformation of citronellal using Ru/H-BEA catalysts. J Catal 320:189–197
Ravasio N, Poli N, Psaro R, Saba M, Zaccheria F (2000) Bifunctional copper catalysts. Part II. Stereoselective synthesis of (-)-menthol starting from (+)-citronellal. Top Catal 13(3):195–9
Trasarti AF, Marchi AJ, Apesteguía CR (2007) Design of catalyst systems for the one-pot synthesis of menthols from citral. J Catal 247(2):155–165
Cortés CB, Galván VT, Pedro SS, García TV (2011) One pot synthesis of menthol from (±)-citronellal on nickel sulfated zirconia catalysts. Catal Today 172(1):21–26
Milone C, Gangemi C, Neri G, Pistone A, Galvagno S (2000) Selective one step synthesis of (−)menthol from (+)citronellal on Ru supported on modified SiO2. Appl Catal A 199(2):239–244
da Silva Rocha KA, Robles-Dutenhefner PA, Sousa EMB, Kozhevnikova EF, Kozhevnikov IV, Gusevskaya EV (2007) Pd–heteropoly acid as a bifunctional heterogeneous catalyst for one-pot conversion of citronellal to menthol. Appl Catal A 317(2):171–174
Yadav GD (2005) Synergism of clay and heteropoly acids as nano-catalysts for the development of green processes with potential industrial applications. Catal Surv Asia 9(2):117–137
Bhorodwaj SK, Dutta DK (2010) Heteropoly acid supported modified Montmorillonite clay: an effective catalyst for the esterification of acetic acid with sec-butanol. Appl Catal A 378(2):221–226
Adams JM (1987) Synthetic organic chemistry using pillared, cation-exchanged and acid-treated montmorillonite catalysts — A review. Appl Clay Sci 2(4):309–342
Mishra T, Parida KM, Rao SB (1996) Transition metal oxide pillared clay: 1. a comparative study of textural and acidic properties of Fe(III) Pillared Montmorillonite and Pillared acid activated Montmorillonite. J Colloid Interface Sci 183(1):176–183
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73(1):373–80
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60(2):309–319
Mäki-Arvela P et al. (2004) Cyclization of citronellal over zeolites and mesoporous materials for production of isopulegol. Journal of Catalysis 225(1):155–169
Emeis CA (1993) Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J Catal 141(2):347–354
Benesi HA (1957) Acidity of catalyst surfaces. II. Amine titration using Hammett indicators. J Phys Chem 61(7):970–3
Virtanen P, Mikkola J-P, Salmi T (2007) Kinetics of citral hydrogenation by supported ionic liquid catalysts (SILCA) for fine chemicals. Ind Eng Chem Res 46(26):9022–9031
Zhu B, Jang BWL (2014) Insights into surface properties of non-thermal RF plasmas treated Pd/TiO2 in acetylene hydrogenation. J Mol Catal A Chem 395:137–144
Nie Y, Chuah G-K, Jaenicke S (2006) Domino-cyclisation and hydrogenation of citronellal to menthol over bifunctional Ni/Zr-Beta and Zr-beta/Ni-MCM-41 catalysts. Chem Commun 7:790–792
Shah AK, Park S, Khan HA, Bhatti UH, Kumar P, Bhutto AW et al (2018) Citronellal cyclisation over heteropoly acid supported on modified montmorillonite catalyst: effects of acidity and pore structure on catalytic activity. Res Chem Intermed 44(4):2405–2423
Acknowledgements
We are thankful to Dawood University of Engineering and Technology for the providing laboratory facilities and chemicals for research work.
Author information
Authors and Affiliations
Contributions
All authors did write the manuscript and proof reading.
Corresponding authors
Ethics declarations
Ethics approval
N/A.
Consent to participate
N/A.
Consent for publication
N/A.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, A.K., Abro, M.A., Ahmed, S. et al. Catalytic conversion of liquid phase citral and citronellal hydrogenations to menthols over metal-12- tungstophosphoric acid supported mesoporous clay catalysts. Biomass Conv. Bioref. 13, 14023–14034 (2023). https://doi.org/10.1007/s13399-022-02847-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02847-w