Abstract
This research work focuses on the potential usefulness of iron ion-exchanged Indian bentonite for one-pot synthesis of 3,4-dihydropyrimidine-2(1H)-thiones. Our goal was to produce mesoporous Fe3+-exchanged Indian clay (Fe3+-ExIC) and its supported forms through a wet impregnation technique. The physicochemical properties of the catalysts, such as XRD, FTIR, TGA, physisorption studies, SEM, and acidity, were investigated. It was found that Fe3+-ExIC catalysts are efficient and reusable acidic heterogeneous catalysts for the synthesis of 3,4-dihydropyrimidine-2(1H)-thiones by refluxing aldehyde, methyl acetoacetate and thiourea with acetonitrile in cyclocondensation. An optimized reaction condition was found for preparing 5-methoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione. A possible mechanism for the synthesis of 3,4-dihydropyrimidine-2(1H)-thiones has been proposed. Melting point, FTIR, and proton NMR were used to identify the products. Adequate catalyst activity was maintained over a period of six cycles, and a reduction in activity was attributed to a decrease in the acidity of the recycled catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the pharmaceutical field, 3, 4-dihydropyrimidin-2(1H)-thiones (DHPT) are an important class of compounds capable of blocking calcium channel activity, inhibiting inflammation, and preventing cancer. In addition, DHPT is used as a biologically potent scaffold in adrenoreceptor antagonist activity, antihypertensive agents [1, 2], anticancer [3], antibacterial [4], antioxidant [5, 6], and antiviral compounds [7].
The use of multicomponent reactions enhances sustainable development since they allow for the synthesis of structurally diverse and complex organic molecules in one pot, in a clean, step-efficient manner, with high value-added, and thus contribute to the realization of a perfect synthesis [8, 9]and the associated environmental benefits [10,11,12,13]. Clays are readily available in nature. The clays are modified by various methods such as pillaring, supporting, intercalation and cation exchange. There are many advantages to using clay-supported reagents for organic synthesis[14,15,16], including their ease of availability, safety, reusability[17], enhanced selectivity [18], and cost-effectiveness. Various strategies are used to synthesize substituted 3, 4-dihydropyrimidin-2(1H)-thione motifs. The synthesis of these Biginelli organic molecules under green conditions is most advantageous using the one-pot three-component condensation reaction. A lot of effort has been put into developing an easy and simple protocol for the synthesis of substituted 3, 4-dihydropyridine-2 (1H)-thiones by using catalysts including sulfated tungstate [19], Hydrotalcite [20], ZrOCl2/mont K10 [21], CSC-Star-SO3-AlCl2 [22], H5PW10V2O40/Pip-SBA-15 [23], Nano ZnO [24], Cellulose sulfuric acid [25], MnO2–MWCNT [26], SiO2–CuCl2 [27], Mo/γ-Al2O3 [28], Nanosized TiO2–SiO2 [29], Bi(III) complex of Fe3O4/SiO2-NH-ligand [30], Polyethylene-supported Fe/ionic liquid complex [31], Fe3O4@SiO2-APTMS-Fe(OH)2 [32], dendrimer-attached phosphotungstic acid nanoparticles immobilized on nanosilica [33], Polyionene/Br3 grafted on magnetic nanoparticles [34], strontium pyroarsenate nano-plates [35], N-Methylimidazolium acetate [36], and TiO2 nanoparticles [37], Acridine Yellow G [38, 39]. Fe+3-montmorillonite K-10 [40] catalyst had been reported for the synthesis of Biginelli compounds. Montmorillonite K-10 is an acid-treated catalyst that was further treated with FeCl3, which was not characterized by the researchers. In spite of these catalysts being effective for the synthesis of Biginelli derivatives, they are limited by molar ratios, catalyst amounts, reaction times, and homogeneity of the reaction medium.
Several substituted 3, 4-dihydropyrimidin-2(1H)-thiones have been synthesized using Fe3+-ExIC as a catalyst to overcome such drawbacks. Catalytic systems were developed using different strategies in the present study. The resulting catalysts were characterized by XRD, FTIR, TGA, BET surface area, BJH pore distribution, and SEM. Further, to find their acidity, the DRIFTS method was employed. In addition, the reduced activity of the catalysts after reuse has been studied by measuring the acidity of the reused clay catalyst for the first time.
Materials and methods
The Indian clay samples used in this study were purchased from Ashapura Group of Industries, India. Deionized water was used in the modification of the clay catalysts. The reactants were procured from Avra synthesis and Fischer-Scientific. p-Toluene sulphonic acid monohydrate (98%, p-TSA.H2O), 12-tungstophosphoric acid (98%, PWA), and zirconiumoxychloride octahydrate (98%, ZrOCl2.8H2O) were received from Loba Chemie Pvt. Ltd., India. Some solvents were utilized as such and others were distilled for purification.
Preparation of the catalysts
Iron ion exchanged Indian clay (Fe3+-ExIC)
5 g of Indian clay is added to 200 ml of 0.5 M aqueous FeCl3 solution. A continuous exchange procedure took place at room temperature for 16 h. The slurry was centrifuged repeatedly using fresh deionized water until it was free of chloride ions [by silver nitrate test]. After being dried overnight at 100 °C, the solid was ground into a fine powder.
p-TSA supported on Fe3+-ExIC
A homogeneous solution was formed by dissolving 750 mg (15 wt %) of p-toluene sulphonic acid monohydrate in 50 ml of methanol. Continuous stirring was used to mix methanolic p-TSA.H2O with 5 g of Fe3+-ExIC. A vacuum pump was used to remove the solvent from the methanolic p-TSA.H2O with Fe3+-ExIC and then the powder was dried in an oven for 3 h at 100 °C to obtain p-TSA/Fe3+-ExIC.
PWA supported on Fe3+-ExIC
In 50 ml of methanol, 750 mg (15 wt %) of 12-tungstophosphoric acid (PWA) was dissolved to form a homogeneous solution. With constant stirring, 5 g of Fe3+-ExIC was slowly added to the methanolic PWA solution. PWA/Fe3+-ExIC was obtained using a vacuum by removing the solvent and drying it at 100 °C for three hours.
ZrOCl2 supported on Fe3+-ExIC
The zirconium oxychloride octahydrate (ZrOCl2.8H2O) solution was prepared by dissolving 750 mg (15 wt %) in 50 ml of methanol. In a constant stirring process, 5 g of Fe3+-ExIC was slowly added to a solution of methanolic ZrOCl2. Under vacuum, the solvent was removed from the powder, which was then dried at 100 °C for three hours to yield ZrOCl2/Fe3+-ExIC.
Characterization of the catalysts
An X-ray diffractometer with a Rigaku XPert Pro was used to analyze clay catalysts. PXRD measurements were performed between 4° and 70°. With a JASCO 4600 FTIR spectrometer in transmittance mode, IR spectra were recorded between 400 and 4000 cm−1 using 32 scans for clay catalysts and organic products (KBr pellet method). On the TA SDT Q600 model instrument, thermo gravimetric analysis of clay catalysts (TGA) was done in nitrogen atmosphere (50 ml/min) with linear heating from room temperature to 800 °C. The specific surface areas of clay samples were determined by N2 adsorption–desorption at 77 K using a Micromeritics ASAP 2020 surface area analyzer, and the pore diameters were determined by Barrett-Joyner-Halenda (BJH) by studying the desorption branch of the isotherm. In a dynamic vacuum, samples were degassed at 150 °C for 12 h before sorbometric analysis was conducted. SEM morphology analysis was performed using a microscope LEO 1450. Several Biginelli products were tested using open capillaries. Bruker 300 MHz NMR spectrometer was used to record the 1H NMR spectra of organic compounds. Pyridine was used as a probe molecule for determining the acidity of fresh and recycled Fe3+-ExIC catalysts using diffuse reflection Infrared Fourier Transform spectroscopy (DRIFTS) [41].
Catalytic activity study
Fe3+-ExIC and its modified forms were evaluated for their catalytic activity for the synthesis of 3, 4-dihydropyrimidin-2(1H)-thiones (Scheme 1). For the production of 3, 4-dihydropyrimidin-2(1H)-thiones, acetonitrile medium, aldehyde or ketone (1 mmol), thiourea (3 mmol), and methyl acetoacetate (1 mmol) were refluxed together with catalyst. An analysis of the reaction progress was conducted using thin-layer chromatography. Catalytic activity was also assessed using a variety of catalysts, solvents, catalyst amounts, molar ratios of reactants, and time intervals. The catalyst was isolated after cooling and filtering the reaction mixture to room temperature. Following the evaporation of the solvent, the crude product was poured onto crushed ice and thoroughly mixed for five minutes. A 3, 4-dihydropyrimidin-2(1H)-thione product was obtained following filtration and additional purification with ethanol. The products were identified by melting points, IR specta, and NMR spectra.
Results and discussion
Characterization of Fe3+-ExIC& its modified forms
Figure 1 shows the PXRD patterns of Fe3+-ExIC together with its modified forms. As shown in Fig. 1, the peak at 6.82° corresponds to the periodicity in the direction of (0 01) for Fe3+-ExIC samples with a basal spacing of 12.96 Å. Observed peaks of the Fe3+-ExIC catalyst are 6.82°, 20.28°, 27.19°, 28.65°, and 35.92°. Supported catalysts are not altering their basal spacing values. Compared to the parent material, Fe3+-ExICs on support showed only a small increase in the XRD peak intensity. It may be due to p-TSA, PWA, and ZrOCl2 adsorption on Fe3+-ExIC.
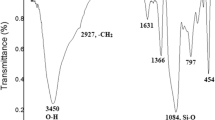
An FTIR spectrum of Fe3+-ExIC and its supported catalysts is shown in Fig. 2. A band at 3634 cm−1 corresponds to hydroxyls in the lattice. Stretching in the interlayer or bending in the surface water is responsible for the Infrared bands at 3426 and 1643 cm−1. A strong shoulder band has been observed at 1119 cm−1 associated with the Si–O bending vibrations, while another strong band has been observed at 1029 cm−1 associated with the Si–O–Si stretching vibrations. There was a shoulder band corresponding to the Al–O–H group at 916 cm−1 and a shoulder band at 878 cm−1 due to the skeletal vibrations of quartz. In p-TSA/Fe3+-ExIC, an extra peak at 690 cm−1 may be attributed to the influence of p-TSA [37], and in PWA/Fe3+-ExIC a band at 802 cm−1 may be assigned to the vibration of W–O–W. Despite this, in p-TSA/Fe3+-ExIC, PWA/Fe3+-ExIC and ZrOCl2/Fe3+-ExIC, all other bands corresponding to the parent clay, Fe3+-ExIC, were not visible and merged with those of p-TSA, PWA, and ZrOCl2.
Figure 3 shows the TGA profiles of air-dried Fe3+-ExIC, p-TSA/Fe3+-ExIC, PWA/Fe3+-ExIC, and ZrOCl2 /Fe3+-ExIC catalysts. The PWA/Fe3+-ExIC and Fe3+-ExIC catalysts show a sharp drop in weight at about 150 °C due to the loss of water physically adsorbed to them and a second drop beyond that due to the loss of hydroxyl groups [42, 43]. Gradual weight loss detectable at 400–700 °C indicates that clay’s structure is associated with hydroxyl water, which also indicates the thermal stability of PWA on Fe3+-ExIC. It is evident from the TGA profile of p-TSA/Fe3+-ExIC that the initial sharp decrease in weight is caused by the loss of physically adsorbed water and interlayer water, followed by a gradual reduction in weight after 120–350 °C due to hydroxyl group losses and dehydration. In the range of 350–800 °C, hydroxyl water is lost because of the structure of p-TSA and its gradual decomposition. However, when the TGA curve of Fe3+-ExIC/ZrOCl2 is investigated within the temperature range of 80–150 °C, Fe3+-ExIC and ZrOCl2.8H2O exhibit an initial sharp decrease in weight due to loss of adsorbed water and hydration water, and the removal of HCl. The gradual dehydration of ZrOCl2.8H2O and hydroxyl water associated with clay’s structure causes it to lose 8% weight as it is heated from 150 to 700 °C. Therefore, ZrOCl2 binds heterogeneously to Fe3+-ExIC. The possible explanations concluded that the formation of intermolecular bonds between Fe3+-ExIC and ZrOCl2.
The surface areas of the supported Fe3+-ExIC catalysts and pore sizes(adsorption branch) have been calculated. The isotherms of adsorption–desorption of nitrogen for Fe3+-ExIC, p-TSA/Fe3+-ExIC, PWA/Fe3+-ExIC, and ZrOCl2/Fe3+-ExIC are shown in Fig. 4. Hysteresis loops are observed for catalysts in the 0.45 to 0.99 relative pressure range. However, Fe3+-ExIC and p-TSA/Fe3+-ExIC exhibit slightly bigger hysteresis loops compared to PWA/Fe3+-ExIC, and ZrOCl2 /Fe3+-ExIC in the relative pressure (P/Pο) range of 0.45–0.99. Catalyst sorption isotherms (H3) indicate slit-shaped and panel-shaped pores [42]. In Fig. 5, there are curves for p-TSA/Fe3+-ExIC, PWA/Fe3+-ExIC, and ZrOCl2/Fe3+-ExIC distributions of pore size according to BJH. Modified forms possess mesopores and macropores, whereas PWA/Fe3+-ExIC has macropores. The average pore diameters of the modified forms have increased, and ZrOCl2/Fe3+-ExIC contains mesopores and macropores. Due to the adsorption process, the supported catalysts have a lower specific surface area (Table 1) than the patent clay.
Figure 6 illustrates the surface morphology of Fe3+-ExIC, p-TSA/Fe3+-ExIC, PWA/Fe3+-ExIC, and ZrOCl2/Fe3+-ExIC. The SEM images of samples containing the microsphere size ranging from 30 to 150 nm were observed. In the SEM image of Fe3+-ExIC, irregular stoned shapes with different sizes were observed, with an average particle size of 35 nm. Particles were free of agglomeration. In the SEM images of p-TSA/Fe3+-ExIC, PWA/Fe3+-ExIC and ZrOCl2/Fe3+-ExIC showed irregular agglomerated stoned-shaped particles with different sizes having an average size of 60 nm, 75 and 120 nm respectively. It was found that these results were in agreement with the BET surface area. Therefore, the modified catalysts have a higher level of particle aggregation than their parent clay.
After pyridine adsorption, the DRIFTS spectra of Fe3+-ExIC, p-TSA/Fe3+-ExIC, PWA/Fe3+-ExIC, and ZrOCl2/Fe3+-ExIC are shown in Fig. 7.
This technique can qualitatively determine an acidic site on the catalyst surface. After modification, the catalysts showed improved acidity [44]. It has been demonstrated that modified catalysts have a higher density of Brønsted acid sites at 1545 cm−1 and Lewis’s acid sites at 1490 cm−1, compared to Fe3+-ExIC. A small decrease in Lewis’s acidity can be observed in p-TSA/Fe3+-ExIC, PWA/Fe3 + -ExIC, and ZrOCl2/Fe3+-ExIC at 1442 cm−1.
Catalytic studies for the synthesis of 3, 4-dihydropyrimidin-2(1H)-thiones
Clay catalysts (150 mg, Table 1) are tested for their catalytic activity to determine which one is best suited for synthesizing 5-methoxycarbonyl-6-methyl-4-phenyl-3, 4-dihydropyrimidin-2(1H)-thione by using ethanol solvent to react equimolar quantities of aryl aldehydes, thiourea and methyl acetoacetate in one pot. The catalysts have shown improved activity after modification. However, the results didn’t give a conclusion on the selection of a catalyst for the reaction under study.
In methanol, ethanol, and acetonitrile solvents, a few iron-based clay catalysts were tested as catalysts for 5-methoxycarbonyl-6-methyl-4-phenyl-3, 4-dihydropyrimidin-2(1H)-thione synthesis (Table 2). Table 2 depicts the activity under specified conditions:
In acetonitrile solvent: Fe3+-ExIC > ZrOCl2/Fe3+-ExIC > FeCl3/mont K10 > p-TSA/ Fe3+-ExIC > PWA/Fe3+-ExIC.
In ethanol solvent: ZrOCl2/Fe3+-ExIC > FeCl3/mont K10 > Fe3+-ExIC > p-TSA/ Fe3+-ExIC > PWA/Fe3+-ExIC.
In methanol solvent: FeCl3/mont K10 > PWA/Fe3+-ExIC > ZrOCl2/Fe3+-ExIC > Fe3+-ExIC > p-TSA/ Fe3+-ExIC.
Table 2 shows that Fe3+-ExIC can be used as a catalyst for benzaldehyde, methyl acetoacetate, and excess thiourea reactions and that the yield of 5-methoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyridin-2 (1H)-thione was affected by a variety of parameters including solvent, molar ratio, catalyst amount, and time. Furthermore, various 3,4-dihydropyrimidin-2(1H)-thiones were synthesized under optimal conditions, and Fe3+-ExIC’s recyclability was examined.
Seven solvents were used to investigate the effect of the reaction medium (Fig. 8a). 5-methoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione was prepared in acetonitrile with a maximum yield of 97%. However, there was no reaction in the water. It was also observed that the yields were 59% and 63% in toluene and o-xylene (non-polar solvents), respectively. The yields were 67%, 81%, and 54% in methanol, ethanol, and n-butanol, respectively. Therefore, further reactions were studied in acetonitrile solvent. Thus, the reaction conditions, such as catalyst selection and solvent selection, were optimized [45].
The effect of the amount of catalyst has been investigated under optimized reaction conditions using 50–300 mg of Fe3+-ExIC catalyst for 4 h. With increasing catalyst amounts, 5-methoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione yields increased (Fig. 8b). The product yield gradually decreased after using 200–300 mg of catalyst. Consequently, the lower yield of the product with excess catalyst can be attributed to the restricted diffusion of the desired product within the active sites as a result of the increased adsorption of the desired product on the superfluous active sites, which was likely caused by an inappropriate solvation medium [36]. The preparation of 5-methyl-6-phenyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione was inhibited when catalyst quantities were increased. It was determined that 50 mg of catalyst should be used to synthesize 3,4-dihydropyrimidin-2(1H)-thiones.
The reaction of benzaldehyde, methyl acetoacetate, and thiourea with Fe3+-ExIC was carried out at various molar ratios (Fig. 9a). By increasing the molar ratio of benzaldehyde to methyl acetoacetate and thiourea from 1:0.5 to 1:3.5, a greater quantity of the product was formed. When the concentration of thiourea is increased, the equilibrium shifts toward the formation of 5-methoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione. There was no effect on the percentage yield of 5-methoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione (97) when the benzaldehyde/methyl acetoacetate/thiourea ratio was over 3:1. It was determined that a molar ratio of 1:1:3 (benzaldehyde: methyl acetoacetate: thiourea) would facilitate further investigation.
At seven intervals of time, Fe3+-ExIC catalyzed the reaction of benzaldehyde, methyl acetoacetate, and thiourea in acetonitrile. A decrease in yield occurs with increasing reaction time from 1 to 6 h, followed by an increase in yield with increasing reaction time (Fig. 9b). The decomposition of unreacted reactants and the formation of products due to prolonged heating can cause this phenomenon. The optimized reaction conditions were evaluated by using different aldehydes/ketones, thiourea, and the -keto ester in acetonitrile under reflux for 4 h.
The results of synthesized derivatives of 3,4-dihydropyrimidin-2(1H)-thione are given in Table 3. There were 75–80% yields of dihydropyrimidin-2(1H)-thiones produced by 3-nitro, 4-nitro, and 3,4-dimethoxy substituted benzaldehydes, and 62–72% yields of dihydropyrimidin-2(1H)-thiones produced by 2-nitro, 4-chloro, and 2-hydroxyl substituted benzaldehydes, compared to 66% and 58% yields for cinnamonaldehyde and 2-chlorobenzaldehyde, respectively. The yields of dihydropyrimidin-2(1H)-thione from acetophenone and halo-substituted acetophenone were 55–62%. A 51% yield was obtained with cyclopentanone, while a 54% yield was obtained with cyclohexanone (Table 3). The reactions produce lower yields due to a retarding effect.
In Scheme 2, the reaction proceeds via the acylimine intermediate (3) formed by the reaction of aldehyde (1) and thiourea (2), catalyzed by Fe3+-ExIC. An enolate keto ester (4) was added to the acylimine, followed by cyclization and the elimination of water to obtain 3, 4-dihydropyrimidin-2(1H)-thione (5).
Recyclability of Fe3+-ExIC
For the preparation of 5-methoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione, benzaldehyde (1 mmol) and methyl acetoacetate (1 mmol) were refluxed in acetonitrile medium for 4 h with the regenerated catalyst (150 mg). As shown in Fig. 10, the Fe3+-ExIC gradually lost its activity when regenerated. The reused catalyst had the same activity in both the first and second cycles. The yield declined by 3% in the third cycle, and another 3% in the fourth cycle. Despite the fact that the activity of the recycled catalyst was gradually decreasing, the percentage yield in all of these reactions was greater than 90%. However, the fifth, sixth, and seventh cycles had yield losses of 11%, 13%, and 16%, respectively. The reused catalyst’s gradual loss of activity is owing to its decreased acidity, as detailed in the following section.
DRIFTS of reused Fe3+-ExIC
Catalysts were separated after completion of the reaction by filtration, washed with dichloromethane, and activated at 110 °C. Using pyridine as a probe molecule, DRIFTS technique was used to determine the acidity of the reused catalyst. Generally, organic reactions can be catalyzed by a clay catalyst that functions as a Brønsted acid, Lewis’s acid, or both [16]. Figure 11 depicts a comparative study of fresh & reused Fe3+-ExIC catalyst with respect to the acidic sites on its surface which are responsible for organic transformations. The reused catalyst has shown reduced acidity after reuse. The densities of Brønsted acid sites at 1545 cm−1, Brønsted and Lewis acid sites at 1490 cm−1and Lewis acidic sites at 1442 cm−1 were reduced. This can be attributed to the gradual loss in the catalytic activity of Fe3+-ExIC after reuse.
Conclusions
A one-pot synthesis of 3,4-dihydropyrimidin-2 (1H)-thiones using a mesoporous Fe3+–ExIC heterogeneous clay catalyst is described in this study. It is simple, inexpensive, reusable, and eco-friendly. Three supported catalysts of Fe3+-ExIC have been prepared and characterized. In order to confirm the acidic nature of the catalysts, the DRIFTS method was applied after pyridine adsorption. 5-methoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione was synthesized by Fe3+-ExIC. Under optimized conditions, various substituted 3, 4-dihydropyrimidin-2(1H)-thiones have been synthesized in good to excellent yields. Until six cycles, the catalyst under green standards will retain its activity without a substantial loss. By investigating the reduced acidic nature (Brønsted & Lewis) of the reused catalyst, the catalyst’s gradual loss of catalytic activity was further explained. The iron ion exchanged Indian clay catalyst can be recommended to prepare several useful organic compounds such as coumarins, 2,3-dihydroquinazolin-4(1H)-ones, esters, imidazoles, etc. As a result of its unique properties, such as its large surface area and high catalytic activity, it is also useful as a catalyst in a wide variety of chemical reactions as well as pharmaceutical intermediates.
Data availability
Data will be made available upon reasonable request.
References
K.S. Atwal, B.N. Swanson, S.E. Unger, D.M. Floyd, S. Moreland, A. Hedberg, B.C. O’Reilly, J. Med. Chem. 34, 806 (1991)
C.O. Kappe, Eur. J. Med. Chem. 35, 1043 (2000)
W.M.B.I.A. Nagwa, M. Abdelazeem Farid, M. Sroor, M.A. Tantawy, Polycycl. Aromat. Compd. 43, 5840 (2023)
M. Brands, R. Endermann, R. Gahlmann, J. Krüger, S. Raddatz, Bioorg. Med. Chem. Lett. 13, 241 (2003)
H.A. Stefani, C.B. Oliveira, R.B. Almeida, C.M.P. Pereira, R.C. Braga, R. Cella, V.C. Borges, L. Savegnago, C.W. Nogueira, Eur. J. Med. Chem. 41, 513 (2006)
N. Hosseini Nasab, H. Raza, R.S. Shim, M. Hassan, A. Kloczkowski, S.J. Kim, J. Mol. Struct. 1286, 135638 (2023)
A. Kumar, R.A. Maurya, Tetrahedron Lett. 48, 4569 (2007)
N. Mohammadian, B. Akhlaghinia, Res. Chem. Intermed. 43, 3325 (2017)
G. Van Der Heijden, E. Ruijter, R.V.A. Orru, Synlett 24, 666 (2013)
M.M. Anastas, P.T. Kirchhoff, Acc. Chem. Res. 35, 686 (2002)
R.A. Sheldon, Green Chem. 9, 1273 (2007)
P. Beigiazaraghbelagh, A. Poursattar Marjani, Res. Chem. Intermed. 50, 485 (2024)
S. Bibak, A. Poursattar Marjani, Sci. Rep. 13, 17894 (2023)
A. Poursattar Marjani, F. Asadzadeh, A. Danandeh Asl, Appl. Organomet. Chem. 37, 1 (2023)
A. Cornelis, P. Laszlo, P. Pennetreau, Clay Miner. 18, 437 (1983)
G. Nagendrappa, Appl. Clay Sci. 53, 106 (2011)
S. Bikas, A. Poursattar Marjani, S. Bibak, H. Sarreshtehdar Aslaheh, Sci. Rep. 13, 2564 (2023)
J.M. Adams, K. Martin, R.W. McCabe, J. Incl. Phenom. 5, 663 (1987)
S.D. Salim, K.G. Akamanchi, Catal. Commun. 12, 1153 (2011)
J. Lal, M. Sharma, S. Gupta, P. Parashar, P. Sahu, D.D. Agarwal, J. Mol. Catal. A Chem. 352, 31 (2012)
B. Vijayakumar, G.R. Rao, J. Porous Mater. 19, 491 (2012)
P. Gupta, S. Paul, J. Mol. Catal. A Chem. 352, 75 (2012)
R. Tayebee, M.M. Amini, M. Ghadamgahi, M. Armaghan, J. Mol. Catal. A Chem. 366, 266 (2013)
F. Tamaddon, S. Moradi, J. Mol. Catal. A Chem. 370, 117 (2013)
A. Rajack, K. Yuvaraju, C. Praveen, Y.L.N. Murthy, J. Mol. Catal. A Chem. 370, 197 (2013)
J. Safari, S. Gandomi-Ravandi, J. Mol. Catal. A Chem. 373, 72 (2013)
G. Kour, M. Gupta, S. Paul, Rajnikant, V.K. Gupta, J. Mol. Catal. A Chem. 392, 260 (2014)
K. Kouachi, G. Lafaye, S. Pronier, L. Bennini, S. Menad, J. Mol. Catal. A Chem. 395, 210 (2014)
Y. Titova, O. Fedorova, G. Rusinov, A. Vigorov, V. Krasnov, A. Murashkevich, V. Charushin, Catal. Today 241, 270 (2015)
A. Mobinikhaledi, N. Foroughifar, A. Khajeh-Amiri, React. Kinet. Mech. Catal. 117, 59 (2016)
D. Elhamifar, D. Elhamifar, F. Shojaeipoor, J. Mol. Catal. A Chem. 426, 198 (2017)
M. Sheykhan, A. Yahyazadeh, L. Ramezani, Mol. Catal. 435, 166 (2017)
J. Safaei-Ghomi, M. Tavazo, G.H. Mahdavinia, Ultrason. Sonochem. 40, 230 (2018)
E. Dezfoolinezhad, K. Ghodrati, R. Badri, SILICON 11, 1593 (2019)
R. Esmaeili, L. Kafi-Ahmadi, S. Khademinia, J. Mol. Struct. 1216, 128124 (2020)
F. Ramezani Gomari, S. Farahi, H. Arvinnezhad, Iran. J. Chem. Chem. Eng. 40, 888 (2021)
B. Mohammadi, F.K. Behbahani, G.B. Marandi, B. Mirza, Russ. J. Org. Chem. 58, 1319 (2022)
F. Mohamadpour, Sci. Rep. 13, 13142 (2023)
F. Mohamadpour, Polycycl. Aromat. Compd. (2023)
M. Nikpassand, L.Z. Fekri, M. Gharib, O. Marvi, Lett. Org. Chem. 9, 745 (2013)
M. Kancherla, V. Badathala, J. Porous Mater. 24, 1187 (2017)
B. Vijayakumar, G.R. Rao, J. Porous Mater. 19, 233 (2012)
V. Singh, R. Ratti, S. Kaur, J. Mol. Catal. A Chem. 334, 13 (2011)
X.X. Zheng, Z.P. Fang, Z.J. Dai, J.M. Cai, L.J. Shen, Y.F. Zhang, C.T. Au, L.L. Jiang, Inorg. Chem. 59, 4483 (2020)
S. Sadjadi, M.M. Heravi, M. Malmir, Res. Chem. Intermed. 43, 6701 (2017)
Acknowledgements
BVK is thankful to the SERB, Ministry of DST, Government of India for sanctioning the research project (Project No. SB/FT/CS-089/2012). The authors thank Vel Tech High Tech Dr. RR Dr.SR Engineering College for providing research facilities.
Funding
The SERB, Ministry of DST, Government of India had provided financial support for this study (Project No. SB/FT/CS-089/2012).
Author information
Authors and Affiliations
Contributions
MK: Experimental and Characterization work analysis; KS: Data analysis, Visualization, and Characterization work analysis; VB: Conceptualization, Investigation, Writing-Original Draft, Funding Acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval and consent to participate
Does not require any ethical approval since we could not use any animals or Humans for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kancherla, M., Seku, K. & Badathala, V. An efficient three-component one-pot synthesis of 3, 4-dihydropyrimidin-2-(1H)-thione derivatives with a mesoporous iron ion exchanged Indian clay catalyst. Res Chem Intermed 50, 2307–2324 (2024). https://doi.org/10.1007/s11164-024-05247-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-024-05247-z