Abstract
The alcohol industry is material-intensive, where the cost of grain on the production of ethyl alcohol exceeds 60% of the total production costs. The most effective way to use agricultural raw materials is the introduction of new plants. Plants from the Asteraceae family are a promising alternative source due to their inulin content that can be used for ethyl alcohol production. However, the fermentation of alternative raw materials might bring undesirable sub-products that compromise the quality of the ethyl alcohol, such as fuse oil, ester, and aldehyde. This article aims to obtain ethyl alcohol from four alternative plants that belong to the Asteraceae family. To do so, we evaluated the ethyl alcohol production from raw materials from four different plant species from the Asteraceae family: Helianthus tuberosus L., Dahlia, Cichorium intybus L., and Arctium lappa L. Our findings revealed that, after the first distillation, the alcohol content ranged between 5 and 12%. The concentration of waste products (fuse oil, ester, and aldehyde) can decrease to 1519 mg dm−3 after adsorption with activated carbon and calcium oxide. Therefore, those four plants are viable alternatives to alcohol production due to their inulin content. These alternative plant materials can improve the state of the alcohol and alcoholic beverage industry, and the introduction of new technologies presented here can potentially increase the profitability of bioethanol production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The production of fermentation ethanol is a complex process. The process flow begins with a choice of raw materiał, substrate preparation, microbial conversion, ethanol recovery, and the utilization of by-products. A very wide variety of crops have been used for ethanol production (e.g., sugarcane, corn, grain crops). Generally, the selection of raw material depends on the climate and conditions of the different countries. For example, Zhukova et al. [1] explored the red algae of the Baltic sea Furcellaria lumbricalis and wheat straw as a potential polysaccharide-based raw material to produce bioethanol. In Kazakhstan, the main raw material for alcohol production is wheat, where 16 distilleries exist. However, wheat is used for feeding goats, bakery products, pasta, and several other products. Therefore, it is necessary to seek alternative plant species to maintain a sustainable production of ethyl alcohol.
Nowadays, the most promising alternative for ethyl alcohol from plants’ raw materials comes from plants from the Asteraceae family. Asteraceae plants are a rich source of inulin. Inulin is a polysaccharide whose molecules are built from elementary links — β-D-fructofuranose residues with end-group — α-D-glucopyranose residues [2]. Consequently, inulin is a water-soluble storage polysaccharide and belongs to the group of non-digestible carbohydrates called fructans. Inulin is commonly used as a prebiotic, fat substitute, sugar substitute, texture modifier, and functional foods to improve health due to its beneficial role in gastric health [3]. Inulin sources have recently attracted great interest, as they are a renewable raw material to produce bioethanol, fructose syrup, fructooligosaccharide, and other useful products for the alcohol industry [4].
Inulin is widely available from 36,000 plant species. Some of those plants are found in the territory of Kazakhstan: chicory (Cichorium intybus), Jerusalem artichoke tubers (Helianthus tuberosus L.), dahlia (Dahlia sp.), and large burdock (Arctium lappa L.). Those plants use inulin as a reserve polysaccharide in the tubers of plants. Inulin is found in the roots of chicory (10%), artichokes, and dahlias to 9%, a significant amount of inulin (14–18%) in the tubers of Jerusalem artichoke. Besides, inulin forms about 90% of the fructose when hydrolyzed.
Recently, Jerusalem artichoke (H. tuberosus) was recognized as a promising biomass for the development of bioeconomy with low-cost cultivation, high yield, extensive adaptation to climate and soil conditions, and plant diseases and insect pests. Jerusalem artichoke provides various biological products, including inulin, fructose, natural fungicides, antioxidants, and bioethanol [5,6,7,8,9].
Cichorium intybus is considered the main source of inulin for European countries. It is a perennial herb from the Asteraceae family. Best known as a coffee substitute, it is also used in alcohol production and has a wide range of medicinal effects. Previous studies showed that inulin content in chicory range between 12 and 30% per crude weight depending on the variety but can reach 75% in dry chicory roots [10].
The genus Dahlia Cav. belongs to the Asteraceae family and includes about 19 classes (cultivars). At present, dahlia tubers are being processed as a promising inulin-containing raw material in the USA and in European countries to produce inulin, which has a wide range of pharmacological effects [11].
Another interesting plant for inulin extraction is burdock (Arctium lappa L.). Burdock roots are used to a greater extent, less often leaves and fruits. The roots contain essential oil, inulin, fatty acids, sitosterol, and stigmasterol. For the A. lappa, Umarova et al. [12] studied the influence of ethyl alcohol in different concentrations. The authors established that the most acceptable method of obtaining tincture from the leaves of burdock is maceration. The method allows you to get a tincture with a high yield of tannins with the least time spent. In our study, we included the burdock given.
Large burdock has recently been introduced into cultivation and cultivated to obtain roots for consumer packaging. In terms of appearance, the raw material “burdock roots” are deeply cut, longitudinally-wrinkled, cone-shaped, sometimes spirally twisted roots. The color is brownish-brown on the outside, yellowish on the break. Burdock is a perennial herbaceous plant in the Asteraceae family with spindle-shaped roots up to 60 cm long. Decoction of the roots is used as a hypoglycemic, diuretic, choleretic, and diaphoretic [13].
The burdock roots are widely used in medicine as a diuretic, choleretic, antipyretic, anti-inflammatory, antibacterial, desensitizing, immune-stimulant, and antioxidant. Thus, for medical and pharmaceutical purposes, water extracts from medicinal plant raw materials are used, and pharmacological effect is due to water-soluble compounds, the basis of which is inulin (up to 45%) [14].
However, the fermentation process of raw materials generates waste products such as fuse oil [15], ester, and aldehydes. Therefore, the amount of waste products is key in determining the profitability of the plant raw material. The current study aims to obtain ethyl alcohol from plants belonging to the family Asteraceae growing in Kazakhstan and estimate the amount of ester mass, fuse oil, and aldehydes produced after the fermentation process and distillation.
2 Materials and methods
Four plant species from the Asteraceae family were used in the current study as raw materials growing in Kazakhstan. Helianthus tuberosus “Interest” variety, Dahlia tubers “Funny guys” variety, Cichorium intybus, and Arctium lappa roots were collected in 2018–2019 (Supplementary Figure S1). First, the plant raw materials were cleaned to remove dust and impurities. Then each material was cleaned according to the following requirements: avoid loss of solids; reduced damage that could result in excessive losses during subsequent operations, and discoloration during drying stage; and the water consumption must be economical.
The production of inulin and other fructose-containing products is based on the processing of tubers of Jerusalem artichoke, dahlia, chicory, and big burdock by the technology developed by us [16] at the stage of processing of raw materials, as well as a separation of the target product. For the Jerusalem artichoke, we also evaluated the inulin concentration in the stalk.
We studied the chemical composition of the four plants from the Asteraceae family. The humidity and ash content of plants were determined by the gravimetric method. Inulin by Bertrand method and pectin substances were determined by the volumetric method [16, 17]. The Bertrand method uses the aldehyde group of sugars to interact with Fehling’s reagent and restore the oxide of copper to cuprous oxide, which cipitate in the form of a red precipitate. Additionally, we investigated the content of anthocyanins, flavanoids, polyphenols, coumarins, and carotene in plants of four plants by photocalorimetric method on photocalorimeter brand KFK-2 and “KFK-3.” Mass fraction of protein was determined by the Kjeldahl method, cellulose — by Kürschner and Hafer method in modification of A. I. Ermakov. The content of crude oil was determined by gravimetric method using a Soxhlet apparatus [18]. The results are presented in Supplementary Table S1.
2.1 Preparation of raw materials and fermentation
Dzhanaev and Gobeev [10] evaluated different options for extracting the maximum amount of fermentable sugars from the green mass of Jerusalem artichoke, with subsequent use for the production of ethyl alcohol. Following their approach, we used wheat sprouts as an enzyme to obtain ethyl alcohol from the fruits and roots of inulin-containing plants. To do this, we evenly distribute 1 kg of wheat on the board and sprinkle water on top. After 3 days, when the wheat sprouts reached 1 cm, we pass them through a meat grinder, filter them, and extracted the wheat milk resulting in approximately 700 ml. The wheat milk was used as an enzyme to extract sugar from the plant raw materials.
Then, 200 g of the crushed plant raw material is filled with water in a ratio of 1:5 mixed and heat up to 100 °C in an electric stove. Cool to 50 °C temperature. After that, we poured the pre-prepared yeast, and the enzyme is mixed and put on for 2 weeks, where the temperature is about 20–24 °C. Dry alcoholic yeast was used as seeding yeast (Ty BY 100,104,781.010–2005 produced in Belarus dried alcoholic yeast wort Saccharomyces cerevisiae). The approach used consisted of the simultaneous saccharification and fermentation strategy [19]. With that we obtained a saccharification efficiency that ranged between 80 and 85%.
After 2 weeks, they are filtered out. Measure the amount of wort. The resulting wort is poured into a flask and heated in an electric stove and distilled. The first drops will start to drip at a temperature of 88–89 °C. After distillation, the composition of the raw alcohol is determined. The composition of the distilled raw alcohol contains impurities. Mature mash also contains secondary fermentation by-products: aldehydes, ketones, alcohols, fusel oil, glycerin, and carboxylic acids.
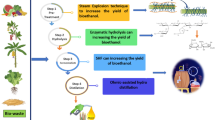
To clean the impurities, the raw alcohol is adsorbed with activated carbon (the process is repeated four times). To increase the concentration, the raw alcohol is adsorbed with calcium oxide (the process is repeated four times) [20]. Activated carbon (AG-3) GOST 20,464–75 was used to purify the raw alcohol and increase the concentration of calcium oxide GOST 8677–76. The complete scheme is described in Fig. 1.
2.2 Analysis of ester mass, fuse oil, aldehydes, and ethyl alcohol
The analysis of ester mass, fuse oil, aldehydes, and ethyl alcohol was performed for the raw alcohol and after each stage of distillation, followed by the adsorption by activated carbon and calcium oxide. Therefore, we determined the quality of ethyl alcohol produced during the five different stages of the producing process.
2.2.1 Determination of ester mass concentration
The method is based on photo electrocolorimetric measurement of the color intensity obtained after ferric chloride reaction with hydroxamic acid formed by the interaction of esters of the alcohol to be analyzed and hydroxylamine hydrochloride in an alkaline medium.
The analysis requires the preparation of test solutions A and B. In two 50-cm3 conical flasks, 6 cm3 each of the reaction mixture is added. Then 3 cm3 of the hydrochloric acid solution is added to one of the flasks and stirred for 1 min. The contents of this flask are called solution B; the contents of the second flask are called solution A. About 18 cm3 of test alcohol is added to each flask, and simultaneously, the mixture is stirred gently in a circular motion for 2 min. Add 3 cm3 of hydrochloric acid solution to the flask with solution A and stir for 1 min. Add 3 cm3 each of iron chloride solution to both flasks and simultaneously stir their contents as described above for 1 min. The intensity of the formed color of the analyzed solution A is measured compared to solution B on a photoelectrocolorimeter with a light filter at 540 nm in a cuvette with a light-absorbing layer thickness of 50 mm [21].
The final measurement result is taken as the arithmetic mean of the results of two parallel measurements. The discrepancy between each measurement and the arithmetic mean should not exceed 5% of the average value with a confidence probability of P = 0.95. The measurement range is 4 up to 30 mg dm−3 of anhydrous alcohol.
2.2.2 Determination of the mass concentration of fusel oil
The method to determine the concentration of fuse oil uses colorimetric measurement obtained after the reaction of higher alcohols present in alcohol with a salicylic aldehyde in the presence of concentrated sulfuric acid.
To calculate the fusel oil content, an adjustment should be made for the aldehydes present in the alcohol, which also react with salicylic aldehyde [22]. To do this, from the optical density value obtained after colorimetry, the calculated optical density value corresponding to the amount of aldehydes determined in the analyzed alcohol should be subtracted. The mass concentration of fuse oil is given in terms of the equivalent amount of absolute alcohol, mg dm−3. The measurement range is 2–15 mg/dm3 of anhydrous alcohol. The final measurement result is taken as the arithmetic mean of the results of two parallel measurements.
2.2.3 Determination of mass concentration of aldehydes
In alcohol production, the determination of the mass concentration of aldehydes is carried out by the photoelectron colorimetric method. The method is based on measuring the color intensity of the analyzed solution formed after aldehyde’s reaction present in alcohol with resorcinol in a sulfuric acid medium using a photoelectron colorimeter [16]. The mass concentration of aldehydes in alcohol is determined by the optical density of the analyzed solution, colored in light yellow, proportional to the mass concentration of aldehydes [21]. The measurement range is 2–10 mg/dm3 of anhydrous alcohol [23]. The final measurement result is taken as the arithmetic mean of the results of two parallel measurements.
The content of ethyl alcohol with a concentration of 87–90% from plants of the Asteraceae family was determined in the scientific laboratory of the A. B. Bekturov Institute of chemistry “Agilent 5973 N” by gas -chromatography–mass spectrometric method.
3 Results and discussion
Inulin content is higher in a large burdock root, dahlia, and Jerusalem artichoke, while the amount of pectin substances is higher in Jerusalem artichoke (Table 1). The pectin content was also examined for the four plants. Our findings revealed that all the four plants from the Asteraceae family found in Khazakstan contained at least 12% of inulin. Inulin is a polymer consisting of several fructose residues (from 10 to 36) in the form of furanose [24].
Partial inulin hydrolysis was performed by an addition of wheat milk before fermentation. Inulinases exhibited activity during fermentation after optimal treatments. After the fermentation, the concentration of the first distilled raw alcohol is 5–12%. The concentrations of ether, fusel oil, and aldehyde are shown in Figs. 2, 3, and 4. The content of esters, fusel oils, and aldehydes in the first distilled raw alcohol ranged between 80 and 87 mg dm−3 without differences between the four plant species (Fig. 2). The first distilled raw alcohol is adsorbed with activated carbon, zeolite, then the content of esters, fusel oils, and aldehydes decreased to 65 mg dm−3. Raw alcohol is adsorbed once with calcium oxide, then distilled.
After distillation, the concentration of raw alcohol from 20% increases by 40–56%, and the content of esters, fusel oils, and aldehydes in the alcohol decreases to 38–60 mg dm−3. The concentration of activated carbon and zeolite treated and distilled alcohol increased by 40%, and the amount of esters, fusel oils, and aldehydes decreased from 65 to 25 mg dm−3.
When this alcohol is adsorbed once with calcium oxide, and after distillation, its alcohol concentration increases to 40–68%. Consequently, the content of esters, fusel oils, and aldehydes in its composition is within 60–38 mg dm–3. When adsorbed with calcium oxide and after distillation 2–3 times, the alcohol concentration increased by 58–76%, and the content of esters, fusel oils, and aldehydes from 35 to 45 mg dm−3 decreases to 15–19 mg dm−3. When adsorbed for the fourth time with calcium oxide, the alcohol concentration increases by 87–90%, the content of esters, fusel oils, and aldehydes decreases by 8–15 mg dm−3.
Chuprova and Ryazanova [25] studied the chemical composition of the vegetative part of Jerusalem artichoke to find ways to use it as a raw material for biochemical processing. Later on, Kovalev et al. [26] confirmed that the biomass of Jerusalem artichoke serves as a raw material for biochemical production, as it serves as a good source of fermentable sugars. Enzymatic hydrolysis of Jerusalem artichoke and fermentation of the resulting hydrolysate showed its suitability to produce bioethanol. The current study showed that Jerusalem artichoke and chicory, dahlia, and burdock are capable of producing similar amounts of alcohol after fermentation and distillation.
Nowadays, both researchers and the industry seek to identify abundant sources of biomass from crops that more efficient in terms of nutrient requirements, land, and water. The current view is that the definition of the most efficient biomass source is region-dependent. Therefore, it is essential to identify plant species suitable to local cultivation conditions to increase the economic viability of biomass production [27,28,29]. Additionally, there are significant changes in the contents of the medicinal plants depending on the region and climatic conditions [30].
In that context, Zhukova and Mezenova [31] proposed an environmentally safe technology for obtaining bioethanol from wheat straw, which provides a combination of physical and biotechnological processing methods to isolate cellulose from the complex with lignin and hemicellulose and its subsequent hydrolysis to glucose and fermentation to ethanol. Our approach allowed to obtain alcohol from biological resources. Therefore, our study contributed to the development of ethyl alcohol production process that uses alternative biological resources locally abundant. We also showed that high-quality alcohol can be obtained by successive distillations. Altogether, the current work can reduce the Kazakhstan dependence on wheat to produce bioethanol and ultimately reduce the costs of bioethanol production.
4 Conclusions
We have shown the possibility of obtaining bioethanol from four different plant species from the Asteraceae. We also confirmed the presence of aldehydes, fusel oil, and esters in ethyl alcohol via the photocolorimetric method. Additionally, our results showed that distillation, followed by rectification with activated carbon and calcium oxide, can reduce the concentration of those waste products. Based on that, the alcohol obtained from plants of the Asteraceae family corresponds to Gost 5962–2013 rectified ethyl alcohol from food raw materials.
We provided a pre-processing of raw materials at atmospheric pressure, combining the stages of enzymatic hydrolysis and alcoholic fermentation to obtain bioethanol from inulin-rich Asteraceae plants that can later be distilled and rectified. Our findings confirmed the potential of Jerusalem artichoke to ethanol production.
References
Zhukova YA, Naydanova MV, Mezenova OY (2011) Obtaining bioethanol from polysaccharide-containing raw materials of the Kaliningrad region. News of higher educational institutions. Food Technol 5(6):51–54
Chemical Encyclopaedical Dictionary (2005) – p. 792
Shoaib M, Shehzad A, Omar M, Rakha A, Raza H, Sharif HR, Shakeel A, Ansari A, Niazi S. Properties, health benefits and food applications. Carbohydrate Polymers 147:444–454
Apolinário AC, Damasceno BPGL, Beltrão NEM, Pessoa A, Converti A, da Silva JA (2014) Inulin-type fructans: a review on different aspects of biochemical and pharmaceutical technology. Carbohydr Polym 101:368–378
Abou-Arab AA, Talaat HA, Abu-Salem FM (2011) Physico-chemical properties of inulin produced from Jerusalem artichoke tubers on bench and pilot plant scale. Aust J Basic Appl Sci 5(5):1297–1309
Prangviset K, Songpim M, Yodsuwan N, Wannawilai S, Dejsungkranont M, Changlek P, Sirisansaneeyakul S (2018) Fructose production from Jerusalem artichoke using mixed inulinases. Agric Nat Resour 52(2):132–139
Viriyasuthee W, Jogloy S, Saksirirat W, Saepaisan S, Gleason ML, Chen RS (2019) Biological control of Alternaria leaf spot caused by Alternaria spp. in Jerusalem artichoke (Helianthus tuberosus L.) under two fertilization regimes. Plants 8(11):463
Mu Y, Gao W, Lv S, Li F, Lu Y, Zhao C (2021) The antioxidant capacity and antioxidant system of Jerusalem artichoke (Helianthus tuberosus L.) tubers in relation to inulin during storage at different low temperatures. Ind Crops Prod 161:113229
Paixão SM, Alves L, Pacheco R, Silva CM (2018) Evaluation of Jerusalem artichoke as a sustainable energy crop to bioethanol: energy and CO2eq emissions modeling for an industrial scenario. Energy 150:468–481
Dzhanaev KI, Gobeev VN (2012) Perspective of using the biomass of Jerusalem artichoke of the Skorospelka variety for bioethanol production. News Gorsky State Agrar Univ 1–2:396–398
Ananyina NA, Andreeva OA, Oganesyan ET (2008) Polysaccharides of Dahlia single l. Chemistry of vegetable raw materials. 2:135-136
Umarova FA, Khabibullayeva NH, Kychkorova UO, Mansurov AH (2019) Development of tincture technology from the burdock plant Arctium lappa L. Sci Time 5(65):86–89
Babaeva EY, Burova AE, Voroshilov AI (2015) On the quality of the roots of burdock (Arctium lappa L.) depending on the modes of drying. Vestnik PFUR, Med Ser 1:113–114
Dyakova NA (2015) Development and validation of express methods of quantitative determination of water-soluble polysaccharides in the roots of burdock (Arctium lappa L.). Chemicopharm J 49(9):35–38
Chirkova YN, Arkhipov IV (2018) Rational use of fusel oil - waste of bioethanol production. ICNS "Science and Education" Best student article 2018 collection of articles of the XVIII International research competition. 68–70
Azimbaeva GE, Kudaibergenova GN, Butin BM, Sharipova SA (2016) Method of inulin production from Dahlia variabilis. Bull Sci Educ Dev 2:6–10
Holt R (1954) Volumetric determination of pectin as calcium pectate. Analyst 79(943):623–627
Kamysbaeva AK, Azimbaeva GE (2021) Biologically active substances of some species of the genus Asteraceae. Bull Natl Eng Acad Repub Kazakhstan 1:62–69
Moldes AB, Alonso JL, Parajo JC (2001) Strategies to improve the bioconversion of processed wood into lactic acid by simultaneous saccharification and fermentation. J Chem Technol Biotechnol 76(3):279–284
Yarovenko VL, Marinchenko VA, Smirnov VA (2002) Technology of alcohol Moscow: Kolos, Kolos-press, 3–10
GOST 5964–93 (2005) Ethyl alcohol. Acceptance rules and analysis methods. Interstate Council for standardization. Metrol Certif Minsk 2–6
Kalashnik O, Remisova N, Rachinska Z (2016) The study of quality and safety parameters of the special vodka of native production. EUREKA: Life Sci (3):8–13. https://doi.org/10.21303/2504-5695.2016.00142
GOST 5962–2013 (2014) Group H74 rectified ethyl alcohol from food raw materials. Interstate standard Moscow, 6–8
Serbaeva ER, Yakupova AB, Magasumova YR, Farhutdinova KA, Akhmetova GR, Kuluev BR (2020) Kuluev. Inulin: natural sources, features of metabolism in plants and practical application. Biomica 12(1):57–79
Chuprova NA, Ryazanova TV (2010) Obtaining bioethanol from the vegetative part of Jerusalem artichoke. Chem Plant Raw Mater 2:49–52
Kovalev AA, Ryazanova TV, Chuprova NA (2016) Complex processing of Jerusalem artichoke to obtain bioethanol. Federal state budgetary educational institution of higher education Siberian state University of science and technology named after academician M. F. Reshetnev (Krasnoyarsk) Reshetnev Read 2:312–313
Akhmedov HM, Partoev K, Tashbaev GA (2015) Chemical composition, biological and economic productivity of Jerusalem artichoke. Proceedings of the Academy of Sciences of the Republic of Tajikistan. Dep Phys Math Chem Geol Tech Sci 3(160):124–131
Lim S-H, Ryu J-M, Lee H, Jeon JH, Sok D-E, Choi E-S (2011) Ethanol fermentation from Jerusalem artichoke powder using Saccharomyces cerevisiae KCCM50549 without pretreatment for inulin hydrolysis. Biores Technol 102(2):2109–2111
Matías J, Encinar JM, González J, González JF (2015) Optimisation of ethanol fermentation of Jerusalem artichoke tuber juice using simple technology for a decentralised and sustainable ethanol production. Energy Sustain Dev 25:34–39
Güneş A, Kordali Ş, Turan M, Bozhüyük AU (2019) Determination of antioxidant enzyme activity and phenolic contents of some species of the Asteraceae family from medicinal plants. Ind Crops Prod 137:208–213
Zhukova YuA, Mezenova OY (2011) Technology for production of bioethanol from wheat straw. Vestn Int Acad Refrig 4:21–25
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Azimbayeva, G.E., Kamysbayeva, A.K., Sagimbayeva, A.E. et al. Production of ethyl alcohol from plants of the Asteraceae family. Biomass Conv. Bioref. 13, 9775–9781 (2023). https://doi.org/10.1007/s13399-022-02840-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02840-3