Abstract
Biofilm formation of Chlorococcum sp. and mixotrophic algae was examined on different supporting materials, which were placed in horizontal and vertical position. Chlorococcum sp. and mixotrophic algae were cultivated in modified BG-11 and primary settled wastewater, respectively, for a period of 16 days. The highest attachment of Chlorococcum sp. was 49.5 and 32.0 g/m2 for the patterned plexiglas, in horizontal and vertical orientations, respectively. In case of mixotrophic algae, biomass attachment was 15.4 g/m2 with patterned plexiglas in horizontal position and 38.7 g/m2 with plexiglas in vertical position. The protein content of attached Chlorococcum sp. in horizontal orientation was increased from 35.8 to 69.3%. Physicochemical properties of the materials and microalgae were also examined. In all cases, the plexiglas or the engraved plexiglas performed better, despite the difference of the hydrophobicity of the two microalgae tested. Orientation of materials also played a crucial role in the attachment process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microalgae are photosynthetic microorganisms with a variety of biotechnological applications such as wastewater treatment, production of biofuels, and bioproducts [1, 2]. Algae can assimilate nitrogen and phosphorus, which are found in wastewater, minimizing their consumption in water ecosystems [3, 4]. They are also plenty of lipids, proteins, and carbohydrates making them promising for biofuel production, feedstock, fertilizers, and cosmetics [5,6,7]. Due to the above properties, efficient methods for algal culturing and processing are receiving increasing attention. Attached microalgae cultivation can overcome the high cost and energy requirements arising from conventional suspended systems [8].
In such systems, the supporting material is immersed in a container that promotes the growth of microalgae [9, 10]. Attached microalgal systems can be classified according to their position in horizontal and vertical–oriented [5]. de Assis et al. (2019) [11] tested horizontally placed cotton, nylon, and polyester surfaces in reactors and reported an algal productivity of 50.12 g/m2 with the polyester, after 32 days. Coral velvet gave a productivity up to 8.1 g/m2.d in a vertical algal biofilm raceway pond using wastewater [12], while cotton cording in a rotating algal biofilm reactor (RABR) system treating wastewater achieved 31 g/m2.d [13].
Until today, various types of supporting materials have been studied such as metals (e.g., stainless steel, titanium), polymeric materials (e.g., plexiglas, PVC) [10, 14], natural polymers (e.g., cotton, cork) [15], and lignocellulosic materials (pine sawdust, rice husk) [16]. All the above materials have different textures, roughness, and surface properties. In the literature, the prevailing opinion is that hydrophobic materials are the ones that will have the best performance in terms of biomass adhesion and that micropatterning surface can boost their performance [17, 18].
The attachment mechanism for each organism group differs from one to another; however, the whole process can be summarized in two steps: initial algal cell adhesion and biofilm thickening. During the first step, surface properties such as hydrophobicity, surface texturing, and surface energy play important role [19, 20]. The second step is more connected to the properties of algal cells, which can either actively move towards the conditioned surface by mobility or they can be transported gravimetrically or conventionally. After the transport, cells can spontaneously be attached on the surface of the material. The initial attachment is irreversible, if not influenced by exogenous factors. Exopolymeric substances (EPS) are secreted, aiding the binding of biofilm layers [21, 22]. Biofilm formation is mainly based on the initial adhesion of cells to a surface [5]. However, the whole process of biofilm formation can be dramatically affected by environmental and operational factors.
In addition to substrate type, the strain of microalgae has a crucial role in the whole process. Algal species present different characteristics and behaviors. For some algae, solid supports are more favorable for cultivation, while others prefer to grow suspended in the liquid [23]. For example, the best surface, among different materials tested, for Botryococcus braunii and Scenedesmus rubescens adhesion was the plexiglas yielding biofilm production after 16 days of 35.0 and 28.3 g/m2, respectively [15, 24]. In the literature, there are only few reported cases for the comparison of supporting materials using wastewater as substrate and mixotrophic algae [12, 25,26,27].
The objective of this study was to enhance the understanding of the factors influencing the formation and development of algal biofilm, in order to facilitate the scale up of biofilm reactors. More specifically, the biofilm formation of Chlorococcum sp. was cultured in modified BG-11 medium and mixtrophic algae cultured in primary treated wastewater using various supporting materials. Eleven materials were tested for Chlorococcum sp. and eight for mixtrophic algae. The coupons were placed in vertical and horizontal position, grown in synthetic medium and wastewater. Both microalgae were examined for their biocompounds like total proteins, carbohydrates, and lipid.
2 Materials and methods
2.1 Algal cultures
Chlorococcum sp. (SAG 22.83) was obtained from the Sammlung von Algenkulturen der Universität Göttingen (Culture Collection Algae at Göttingen University) bank (SAG). Algal precultures were prepared with modified 1/3 N BG-11 medium (Blue Green-11 enriched with one third times the nitrates concentration) in 1-L Erlenmeyer flasks. The flasks were illuminated by fluorescent lights (22 μmol/m2s) with constant aeration (3.5 L/min) and were placed in a walk-in incubator room under controlled environmental conditions at 20 °C. Mixotrophic microalgae were collected from a tank, which was periodically fed with raw wastewater. A sample was transferred in a 20-L bottle, fed with sewage, and then placed in a walk-in incubator room. Identification of microalgae was carried out by microscopic analysis, using an optical microscope (model DMLB, Leica Microsystems GMbH, Germany). The classification of species was based on taxonomic observations by Canter-Lund (1996) [28] and the work on taxonomix character by Temponeras et al.(2000) [29].
2.2 Biofilm reactors
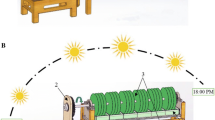
The experiments were carried out using materials placed in horizontal and vertical orientation. The horizontal system consisted of six rectangular reactor vessels (26.5 × 18.4 × 4.5 cm L × W × H each). The vertical system consisted of two vessels (28 × 22 × 10 cm L × W × H each) (Fig. 1). Eleven materials, stainless steel, silicone rubber, plexiglas, glass, denim, sponge towel, cork, geotextile, and three different-patterned plexiglas (plexiglas 1 to 3) were used for the examination of Chlorococcum sp., while for the mixotrophic cultures, eight materials were used and were selected based on their low cost, availability, and diversity in texture. Plexiglas coupons were etched in three different ways. Plexiglas 1 had horizontal parallel grooves with 1 mm depth and 1 mm width, plexiglas 2 had horizontal and vertical grooves, and plexiglas 3 had horizontal and diagonal grooves. More details can be found in an earlier work of our team [24]. The various surfaces tested were cut in rectangular coupons (7.4 \(\times\) 2.4 \(\times\) 0.1 cm, L \(\times\) W \(\times\) H). After the coupons were rinsed with deionized water and placed into the oven at 44 °C for 2 days, the coupons were weighted. Eight coupons of each material were placed into the bioreactors and two coupons removed each sampling day. Chlorococcum sp. inocula was prepared from stock cultures by appropriately diluting them with 1/3 N NO3 BG-11 to obtain an initial total suspended (TSS) concentration of 240 mg/L. The suspension was then poured on the top of the coupons. Every 4 days and for a period of 16 days, coupons were removed, using tongs and rinsed by gently shaking on the spot. Mixotrophic inocula was prepared from a 20-L bottle, placed in the walk in incubator, diluting with primary settled wastewater in order to obtain an initial TSS concentration of 30 mg/L. This suspension was then transferred into the reactors. The liquid of the reactors was recirculated at a flow rate of 2 mL/min using a peristaltic pump (Masterflex L/S 7519–85, Cole Pamer Instrument, Co., USA). Two fluorescent lamps were placed above the reactors providing illumination of 100 μmol/ m2s. In each experiment, a blank reactor without coupons was used.
2.3 Monitoring
Every 4 days, a total sample of 100 mL was collected from the reactors with Chlorococcum sp. and 250 mL from the reactors containing mixotrophic algae, in order to perform characterizations. In the first case, the liquid removed was replaced with 100 mL distilled water, and in the second, with 250 mL of sewage. To characterize the algal biomass in the suspended culture, TSS and chlorophyll-a (chl-a) were determined according to standard methods [30] using a 0.45-μm filter. pH was measured with a pH-meter (pH 300/310, Oakton Instruments, Singapore). The optical density of algal cultures was measured at 650 nm with a UV–Vis spectrophotometer (U-1100, Hitachi, Japan). Turbidity was measured by the nephelometric method with a laboratory turbidimeter (2100AN IS, HACH Company, USA). In addition, the free algal cell concentration was determined with a Neubauer hemocytometer (0.1 mm, 0.0025 mm2, Optic Labor, Germany) after algae staining with Lugol’s solution in order to separate dead from live algae. Anion concentration was determined using ion chomatography (Metrohm 850 Professional IC, Metrohm AG, Switzerland). Total phosphorus (Total-P) was determined by the ascorbic acid method after digestion of the sample with ammonium pelsulfate. The absorbance was measured at 880 nm with a spectrophotometer (U-1100, Hitachi, Tokyo, Japan) [30]. Chemical oxygen demand (COD) was measured by the open reflux method. The soluble fractions of COD (sCOD) and Total-P (sTotal-P) were determined by passing the sample through a 0.45 μm pore size membrane filter. Ammonia nitrogen (NH3-N) was determined by the titrimetric method using the macro-Kjeldahl procedure [30].
The experiments were conducted in duplicate, and the data were shown as mean ± SD (standard deviation). Data were analyzed and plotted by IGOR Pro (WaveMetrics, Inc., USA).
2.4 Wastewater
The wastewater were collected from the wastewater treatment plant (WWTP) of the University of Patras Campus at Rio, Greece. After sampling, wastewater was allowed to settle for 24 h. Then, the samples were stored in polyethylene vessels and stored in a freezer at − 4 °C. The wastewater were defrozen before each experiment. The mean concentration of main physicochemical characteristics of settled wastewater is presented in Table 1.
2.5 Characterization of biomass
The algal biofilm productivity (g/m2) was calculated as follows:
where Mt and M0 are the dried mass of the tested coupons harvested at days (t) and before cultivation, respectively, and As = 0.00177 m2 is the surface of each tested coupon.
The procedure for measuring total proteins was followed according to a modified Lowry method, using bovine serum albumin (BSA) as the standard [31, 32]. The optical density (OD) of samples was measured at 540 nm. The total carbohydrate content was determined by the phenol–sulfuric method [33], and the absorbance was measured at 484 nm using a glucose standard curve. To determine the biofilm or EPS production (proteins and carbohydrates), on the coupons, the harvested cells were washed with de-ionized water and then biomass was placed into an oven at 44 °C. The above procedure was only applied to coupons in horizontal orientation for Chlorococcum sp., as they were the only ones with sufficient attached biomass. The lipid content of the dry algal biomass was measured by the modified method of Folch et al. (1957) [34, 35]. The zeta potential of the algal cells was determined using a Zetasizer (Nano ZS, Malvern, UK). A volume of 1 mL of the algal culture was used, and zeta potential measurements were performed for at least three times. The pH of the cell suspensions was adjusted by the addition of HNO3 or NaOH.
2.6 Surface properties and hydrophobicity determination
The hydrophobicity of the materials was estimated by the sessile drop test. First, it is necessary to make three independent contact angle measurements with three probe liquids whose surface energy components are known. Using the three equations below, surface energy can be calculated:
where, θ is the measured contact angle. The subscripts of s and l refer to the solid surface and probe liquid respectively. The Lifshitz-van der Waals/acid–base method is expressed as Eq. (2) and in this approach, surface free energy is decomposed into Lifshitz-van der Waals component (γLW) and Lewis acid–base component (γΑΒ) that is consisted of a Lewis acid componenent (γ+) and a Lewis base component (γ−) [36,37,38].
The contact angle of algal cells was measured in the same way. In order to create flat layers, highly concentrated suspensions were filtered through a nitrate cellulose membrane, (0.45 μm pore size, 47 mm diameter, Whatman). The methodology has been described in detail in previous work [15]. According to the extended DLVO theory, the degree of hydrophobicity of materials is also determined via free energy cohesion (ΔGcoh), using the water surface tension parameters according to van Oss et al. (1988) [39], shown in the following equation.
A negative value of ΔGcoh indicates hydrophobicity and a positive indicates the opposite (Hao et al. 2017) [37].
2.7 Scanning electron microscopy
The formation of Chlorococcum sp. and mixotrophic algae biofilm on the surface of coupons was studied by scanning electron microscopy (SEM) analysis (microscope JEOL 6300, JEOL Ltd.). A small surface of coupon (1 × 1 cm2) with attached algae (dehydrated in an oven) was glued to SEM stubs with colloidal silver and sputter-coated with gold–palladium using a gold ion sputter coater (JEOL, JFC1100 Fine Coat). The samples were examined with SEM operating at 20 kV. For each sample, at least four fields were observed at different magnifications between 250 and 1000 × .
3 Results and discussion
3.1 Microalgae identification
The microalgae identified in mixotrophic cultures in the horizontal and vertical reactors are presented in Table 2. The algal species involved varied with the two orientation schemes examined, and this was probably due to the prevailing conditions. Microalgae derived from the stock culture were identified befrore each culture and found out that the starting species were the same for both horizontal and vertical culture. According to Palmer (1969), the species with the most frequency of occurrence in wastewater are Chlorella, Ankistrodesmus, Scenedesmus, Euglena, Chlamydomonas, Oscillatoria, Micractinium, and Golenkinia [40]. In our stock, the microalgae species of Chlorella sp., Chlorella vulgaris, Scenedesmus obliquus, Chlamydomonas sp., Euglena gracilis, and Oscillatoria were identified.
Generally, Scenedesmus obliquus is unicellular green algae, while Scenedesmus quadricauda commonly occurs in four-cell colonies that approach 100 μm in diameter [41]. According to Prescott (1973), Scenedesmus quadricauda is characterized by its oblong cylindrical cells usually in one series and outer cells with long curved spines at each pole and the inner cells without spines [42]. The cell shapes and arrangement, cell length and width, details of the outer cells, and the shape and length of the spines were mainly used as the main diagnostic features in species delineation. Microalgal cells were observed also, by SEM analysis (data not shown). On the other hand, Chlorella sp. concern Chlorella species, which is represented by 60 different strains. The characteristic bristles are important species-specific characteristics of the genus Chlorella vulgaris [43]. The recognition was done during the whole period of 16 days. This means that Chlorella may have been found in the beginning of the experiment and in the last few days or in the meantime Chlorella vulgaris may have also been found.
Over time, the species that dominate into biofilm bioreactors, changed into macroalgae, in which many protozoa and crustaceans also coexisted. For example, in vertical cultures, the protist Paramecium caudatum was spotted, while in horizontal cultures, Euplotes sp. and Amoeba proteus were identified. Some of the microalgae detected in stock cultures did not continue their growth in the bioreactors with coupons or in the blank reactors. Additionally, we can observe different species grown in horizontally and vertically oriented biofilm reactors compared to blank reactors. The explanation behind is that in biofilm reactors, which contained materials, microalgae in order to get attached, they had to secrete EPS in synergy with bacteria.
So the conditions were different from the blank containers and the development of other microalgae was proportional. Numerous studies employing different culturing techniques with the same strain, giving different results [44]. In the case of horizontal coupons, many diatoms made their appereance (Table 2).
3.2 Biomass growth and nutrient removal
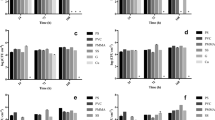
Figure 2 shows the culture parameters, such as pH, OD650, and cell concentration during the cultivation period. Prior to sampling, the liquid of the reactors was gently stirred. The pH values fluctuate over time from 8.6 to 10.9 for Chlorococcum sp. and from 7.9 to 10.1 for mixotrophic algae (Fig. 2a, b). The pH of the culture medium is greatly affected by the dissolved inorganic carbon and vice versa. Also, the pH of the liquid in mixotrophic cultures was lower compared to the pH of Chlorococcum sp. cultures. This was due to the neutral pH of wastewater (pH = 7), and specifically in the beginning, where the concentration of mixotrophic microalgae was low (30 mg/L). It has been also reported that pH may also vary, depending on the the type of the substratum materials [1].
Cultivation of Chlorococcum sp. and mixotrophic algae in different oriented surfaces. Variation of a pH of Chlorococcum sp., b pH of mixotrophic algae, c OD650 of Chlorococcum sp., d OD650 of mixotrophic algae, e cell concentration of Chlorococcum sp., and f cell concentration of mixotrophic algae in the liquid of reactors. The values represented the mean ± standard deviation (SD) (n = 2)
The optical density showed an increasing trend in all cultures, with the smallest change being observed in the vertical-oriented mixotrophic culture (Fig. 2c, d). The same trend was also repeated with the cell concentration; however, there was a higher increase in blank containers and this is justified, as biomass was adhered to surfaces (Fig. 2e, f). Nitrogen and phosphorus compounds are known to be the main nutrient sources for algal growth. Figure 3 shows the concentration of nutrient during cultivation. In Chlorococcum sp. cultures, nitrates were gradually consumed, while in mixotrophic cultures, nitrate concentration was already low in the beginning of the experiment. EPS secretion is affected directly by the nutrient availability, aiding the enhancement of algal attachment. In the case of Chlorococcum sp., the increase of biomass is greater due to higher nitrate concentration in the medium.
Phosphates were gradually consumed during the cultivation of Chlorococcum sp. in horizontal orientation, while in the other cases (Chlorococcum sp. in vertical orientation and mixotrophic algae in both orientations) reached zero values by day 4 (Fig. 3c, d). Total-P concentration in the liquid was below 5 mg/L, while in the 1/3 N BG-11, it was below 8 mg/L (Fig. 4a, b), while sTotal-P concentration was below 2.6 mg/L.
The COD concentration of the primary settled wastewater was around 200 mg/L. In the case of blank, the assimilation of COD was lower, because in addition to microorganisms, which exist in the wastewater, algae that are attached on coupons aid the process. Zhang et al. [12] reported that the removal of total nitrogen and phosphorus was enhanced by the action of heterotrophic microorganisms. When COD was sharply decreased, the concentration of total nitrogen and total phosphorus was also rapidly dropped. After the stabilization of COD concentration, the removal of nutrients, as well as the growth of biofilm on coral velvet with synthetic wastewater, slowed down. COD, total nitrogen, and total phosphorus removal reached values of 86.6%, 73.7%, and 89.9%, respectively [12]. In our experiment, the sCOD concentration reached values close to 55 mg/L for all cases in mixotrophic cultures on the 16th day.
Algal cells commonly prefer NH3+-N for growth, because less energy is needed during ammonium assimilation and this may affected the high decrease in NH3-N in both cases of mixotrophic cultures by 4th day (Fig. 5a, b). This was also observed by Ge et al. [45].
Concentration of NH3-N and COD in the liquid of mixotrophic cultures. Variation of a NH3-N of mixotrophic algae horizontal b NH3-N of mixotrophic algae vertical, c COD of mixotrophic algae in horizontal and vertical orientation d sCOD of mixotrophic algae in horizontal and vertical orientation. The values represented the mean ± standard deviation (SD) (n = 2)
Figure 6 presents the concentration of chlorophyll-a in the liquid of reactors. During Clorococcum sp. cultivation, the chl-a concentration is similar for both orientations and on the 16th day, it is 452 and 552 mg/m3 for horizontal and vertical reactors, respectivelly. Mixotrophic algae presented lower chlorophyll-a values and on the 16th day, chl-a was around 320 mg/m3 in both orientations.
3.3 Biomass characteristics
The lipid content increases during cultivation, reaching 13.0% in both orientations for Chlorococcum sp. and 14.3% (horizontal) and 13.1% (vertical) for mixotrophic algae on day 16 (Fig. 7). The content of total proteins in the liquid is higher in Chlorococcum sp. in both cases, while in mixotrophic algae, the protein content is lower (Fig. 8a). Zang et al. reported higher lipid content (23.3%) and lower protein content (36.7%) testing a mixed microalgal culture in a biofilm photobioreactor, using pine sawdust as a substrate and synthetic water [6]. Total proteins were increased during cultivation, reaching up to 82% for Chlorococcum sp. in horizontal position, while mixotrophic algae, in horizontal position reached up to 37.3% (Fig. 9a) by day 16. As far as it concerns the total carbohydrates content, Chlorococcum sp. begun with 6.6% in both orientations and reached 3 and 2% for vertical and horizontal orientation, respectively, on day 16. On the contrary, mixotrophic algae contained 1.1% at the beginning and at the end of cultivation the content reached 2.7% for both orientations (Fig. 9b). The total protein content of the suspended biomass was found to be significantly higher than the content of total carbohydrates and lipids. The algal concentration in mixotrophic cultures was low compared to Chlorococcum sp. cultures. As the mixotrphic cultures contained bacteria and protozoa, which have lower lipid content compared to algae, a lower lipid content of mixotrophic cultures is expected [6].
Biofilm is a well structured microalgal community, in which cells are entrapped in a matrix of self-produced EPS. This is a process which is species-dependent and complicated [21]. Cells and solid surfaces are binding together via biofilm, which consists mainly of proteins, carbohydrates, and lipids. Between the four cases examined, the ability to recover sufficient biomass, in order to analyze total proteins and carbohydrates, was possible only in the case of Chlorococcum sp. from few materials tested in horizontal orientation. Biomass was only recovered from stainless steel, plexiglas, plexiglas 1, plexiglas 2, and plexiglas 3 materials. We observed an increase of total protein content during time. More specifically, with plexiglas total proteins on day 8, started at 35.8% and reached on day 16, 69.3% (Fig. 9a), while stainless steel started from 17.4% and reached 55.9% after 16 days. The best material in terms of biomass adhesion was plexiglas 1, and the content in total proteins was 65.3% (Fig. 9a). The total carbohydrate content of algal cells was decreased over time for all plexiglas materials (Fig. 9b). Plexiglas and plexiglas 3 started both from 5.9% and on day 16 reached 2%. Stainless steel started from 2.7% in the beginning and gave 3.2% at the end of cultivation. Compared to the suspended culture, the biofilm had lower content in proteins and carbohydrates. However, the total protein and carbohydrate contents were greater in Chlorococcum sp. cultures, where BG-11 medium was used, in contrast to mixotrophic cultures, using wastewater. Shen et al. examined the EPS concentration of Botryococcus braunii in wastewater and in modified basal medium (MB) on polyethylene foam at day 16 and found out 2936 mg/m2 for the first medium and 3770 mg/m2 for the latter [46]. While examining single-species biofilm, it is easier to adjust the parameters of the cultivation such as the culture period, the culture volume, pH, and initial concentration of total nitrogen [21]. Inversely, while examining multiple-species biofilm, the study becomes more complex, as the interaction between microalgae and bacteria prevailing from mutualism to parasitism is governed by the secretion of organic matter that is released [47]. This is also why we observe a big difference in mixotrophic algae cultivated on horizontal and vertical surfaces (Fig. 10).
3.4 Biomass productivity on different surfaces
The algal biofilm productivity with different materials as substratum is shown in Fig. 11 and Table 3. The small standard deviation demonstrated a good stability and reproducibility of the results. Vertical biofilm systems for algal cultivation are proposed, which have a smaller footprint compared to horizontal [48]. Accoding to Sukačová et al. the footprint area determined for a geotextile-based (vertical system) and concrete slab biofilm system (horizontal system) ranges between 2.3 and 2.6 m2 and 2.9 to 3 m2 per person equivalent, respectively [26].
Comparing biomass production between the two tested species, it is observed that Chlorococcum sp. presented higher productivity in both orientations. As it is seen, the nitrogen concentration significally affected cell growth (Figs. 2 and 3). More specifically, on day 16, plexiglas 1 had the highest productivity up to 49.5 g/m2, followed immediately by plexiglas with 48.4 g/m2 in horizontal orientation. In vertical position, at the end of cultivation, again, plexiglas 1 was the most efficient material, giving 32.1 g/m2, while in the next three in order materials were the two engraved plexiglas and the simple plexiglas, with very close values (29.1 to 31.2 g/m2). The worst material in terms of performance was the cork in both orientations, while denim and steel did not sustain the attachment at the end of cultivation. The production of microalgae in the horizontal coupons was slightly higher and this is because gravity helped the cells to be adhered on surfaces, without this meaning that microalgae were not selective for some specific materials. This was also the reason why we obseved variation of the attachment in all materials, while in vertical orientation, some material did not show any adherence at all. Orfanos and Manariotis (2019) tested cotton textile and polyethylene in an open pond using secondary effluent wastewater and reached 1.38 g/m2.d and 0.49 g/m2.d, respectivelly [25]. In the case of mixotrophic algae, fewer materials were tested. The best-performing materials, both in horizontal and vertical orientations, were the plexiglas and the engraved plexiglases. In horizontal orientation, plexiglas 3 presented the highest productivity with 15.4 g/m2 after 16 days, while in the vertical orientation, it reached up to 30.9 g/m2. In vertical orientation, the highest attachment was observed by plexiglas reaching 38.7 g/m2. Although in horizontal orientation, algal biomass was shown to adhere on the surface of silicone rubber, cork, and spongue towel, a positive difference in weight was not measured on day 16. This is also shown in the SEM images (Figs. S1, S2) and does not mean that microalgae were not attached onto surfaces. Either the material was corroded and the fibers were lost or degraded by microorganisms. This phenomenon is intense in the case of denim in both orientations, where there is no value of biofilm productivity but the SEM illustations indicate the opposite (Figs. S1, S2). By day 4, the formation of micro-colonies is obvious. Unlike Chlorocococcum sp., in mixotrophic algae, the vertical orientation proved to be better in terms of productivity. This is justified, because the sample contains many heterotrophic microorganisms, where they take advantage of the shadow between the coupons surface to grow up.
3.5 Physicochemical properties and cell interactions
The surface properties were determined using the approach of van Oss et al. (1988), where the contact angle, which was used for the determination of hydrophobicity, was that of water [15]. Table 3 shows the water contact angles of all materials and microalgae used. Based on the measured angle values, the surface energy was also calculated. It is observed that hydrophobic materials have a lower energy surface, while hydrophilic ones have a higher value. Indicatively, the surface energy of plexiglas was 35.0 mJ/m2 and of the sponge towel 70.9 mJ/m2. The binding capacity of a surface is greatly affected by its characteristics such as proton-active carboxylic, phosphoric, phosphodiester, hydroxyl, and amine functional groups on cell surfaces [49]. More information about values of contact angles, Lifshitz-van der Waals component, and Lewis acid and base component are given in our previous work [15, 24].
Additionally, the free energy of a material immersed in water is expressed as ΔG. Higher contact angles were measured for microorganisms presenting more hydrophobic surfaces (ΔG < 0) [20]. When ΔG > 0, surfaces are hydrophilic, like denim, sponge towel, and glass (Table 3). Between the Chlorococcum sp. and mixotrophic cultures, we observe that Chlorococcum sp. is quite hydrophobic in contrast to mixotrophic algae that tend to be hydrophilic. Hydrophobicity is considered one of the most important factors of cellular surface properties. Μaterials with high hydrophobicity have been reported to enhance algal attachment.
As shown in Table 4, both cultures are negatively charged with zeta potential ranging from − 38.0 mV on day 0 to − 28.0 mV on day 16 for Chlorococcum sp., while for mixotrophic algae, zeta potential ranged from − 13.5 to − 19.7 mV for days 0 and 16, respectively. Functional groups like hydroxyl (-OH), carboxyl (-COOH), and amine (-NH2) influence the surface charge of the material. The latter combined with the pH of the culture media affects zeta potential values. When cells are exposed to low pH values, functional groups are protonated and on the contrary, when cells are exposed to high pH values, functional groups are deprotonated. Since the pH of the medium measured when the samples were collected was high, propably, the functional groups on the microorganisms surface were deprotonated, so this is the reason why negative values are observed [50]. The mean diameter of algal cells is also presented in Table 4. Indicatively, the variation of zeta potential at different levels of pH was examined for mixotrophic algae for days 0 and 16 (Fig. 10). We did not observe any significant difference between different pH or between 0 and 16 days. Zeta potential values less than − 15 mV typically represent the beginning of particle agglomeration.
The strains examined in this study proved that in all cases, plexiglas or engraved plexiglas was the best adhesive material. The whole process is also affected by the hydrophobicity of the microalgae. However, as we can observe in Table 2, Chlorococcum sp. as an hydrophobic algae should have been adhered to a hydrophilic material and, on the contrary, mixotrophic algae should have been adhered to a hydrophobic. According to the literature, during their initial attachment, the strains should choose materials contrary to their hydrophobicity to minimize the contact between the cells and the water. Indeed, mixotrophic algae attached better on hydrophobic materials, and so Chlorococcum sp. did, although it is hydrophobic. However, in the case of vertical coupons of Chlorococcum sp., all plexiglas materials had better performance, but the next best material was sponge towel among the rest hydrophobic materials.
Although in previous research work, hydrophobicity has been considered one of the main factors which contribute to biofilm formation, it is not enough to derive certain results for the performance of a material. Many abiotic factors such as pH, temperature, or CO2 supply affect the process, as well as biotic factors such as the type of strain involved, the involvement of microorganisms, and the production of EPS. One reason that plexiglas was more effective in biofilm production in various microalgae strains may be the light permeability. It seems that the refraction of radiation favors the increase in biomass production. However, in addition to the light permeability on the surface, roughness seems to also play an important role in the retention and maturation of the biofilm. For example, in a previous study, we had found that Botryococcus branii adhered quite satisfactorily on a glass surface reaching 50 g/m2 on the 8th day, but in the process, the biomass was detached due to the zero roughness of the glass, which was not able to hold the algal cells [24].
4 Conclusion
Attachment to various materials, placed in different orientations (horizontally and vertically), was examined by Chlorococcum sp. and mixotrophic algae, and the results showed that microalgae kind and orientation affected the whole process. Chlorococcum sp. was more productive in horizontal orientation, while mixotrophic algae were in vertical one. Among all materials tested, plexiglas and engraved plexiglas were the best in algal attachment. More specifically, engraved plexiglas 1 was the best material in terms of biomass productivity for Chlorococcum sp. (49.5 g/m2 in horizontal and 32 g/m2 for vertical position). In the case of mixotrophic algae, the productivity reached up to 15.4 g/m2 with engraved plexiglas 3 in horizontal orientation and 38.7 g/m2 in vertical orientation with plexiglas 1. The content of total proteins was much higher than of total carbohydrates and increased during the cultivation period, while total carbohydrates tended to decrease. Mixotrophic algae completely removed ammonia and phosphates by the end of cultivation and this implies that microalgae can effectively remediate nutrient from wastewater. The selection of plexiglas can be considered a supporting material in scale up biofilm reactors for wastewater treatment and algal biomass production.
References
Rosli SS, Amalina Kadir WN, Wong CY et al (2020) Insight review of attached microalgae growth focusing on support material packed in photobioreactor for sustainable biodiesel production and wastewater bioremediation. Renew Sustain Energy Rev 134:110306. https://doi.org/10.1016/j.rser.2020.110306
Zhao G, Chen X, Wang L et al (2013) Ultrasound assisted extraction of carbohydrates from microalgae as feedstock for yeast fermentation. Bioresour Technol 128:337–344. https://doi.org/10.1016/j.biortech.2012.10.038
Sukačová K, Trtílek M, Rataj T (2015) Phosphorus removal using a microalgal biofilm in a new biofilm photobioreactor for tertiary wastewater treatment. Water Res 71:55–63. https://doi.org/10.1016/j.watres.2014.12.049
Mustafa H, Hayder G, Jagaba A (2021) Microalgae : A sustainable renewable source for phytoremediation of wastewater and feedstock supply for biofuel generation by Hauwa Mustafa. Biointerface Res Appl Chem 11:7431–7444
Wang JH, Zhuang LL, Xu XQ et al (2018) Microalgal attachment and attached systems for biomass production and wastewater treatment. Renew Sustain Energy Rev 92:331–342. https://doi.org/10.1016/j.rser.2018.04.081
Zhang Q, Liu C, Li Y et al (2017) Cultivation of algal biofilm using different lignocellulosic materials as carriers. Biotechnol Biofuels 10:1–16. https://doi.org/10.1186/s13068-017-0799-8
Oliveira CYB, Nader C, Silva MFO et al (2020) Integrated use of microalgal biomass of Choricystis minor var. minor: a promising model for production of biodiesel and aquafeeds. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01091-4
Gross MA (2015) Development and optimization of biofilm based algal cultivation. Iowa State University, Ames
Hoh D, Watson S, Kan E (2016) Algal biofilm reactors for integrated wastewater treatment and biofuel production: a review. Chem Eng J 287:466–473. https://doi.org/10.1016/j.cej.2015.11.062
Fatima N, Kumar V, Rawat BS, Jaiswal KK (2020) Enhancing algal biomass production and nutrients removal from municipal wastewater via a novel mini photocavity bioreactor. Biointerface Res Appl Chem 10:4714–4720. https://doi.org/10.33263/BRIAC101.714720
de Assis LR, Calijuri ML, Assemany PP et al (2019) Evaluation of the performance of different materials to support the attached growth of algal biomass. Algal Res 39:101440. https://doi.org/10.1016/j.algal.2019.101440
Zhang Q, Yu Z, Zhu L et al (2018) Vertical-algal-biofilm enhanced raceway pond for cost-effective wastewater treatment and value-added products production. Water Res 139:144–157. https://doi.org/10.1016/j.watres.2018.03.076
Christenson LB, Sims RC (2012) Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotechnol Bioeng 109:1674–1684. https://doi.org/10.1002/bit.24451
Gross MA, Henry W, Michael C, Wen Z (2013) Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour Technol 150:195–201. https://doi.org/10.1016/j.biortech.2013.10.016
Tsavatopoulou VD, Manariotis ID (2020) The effect of surface properties on the formation of Scenedesmus rubescens biofilm. Algal Res 52:102095. https://doi.org/10.1016/j.algal.2020.102095
Zhang Q, Wang L, Yu Z et al (2020) Pine sawdust as algal biofilm biocarrier for wastewater treatment and algae-based byproducts production. J Clean Prod 256:120449. https://doi.org/10.1016/j.jclepro.2020.120449
Zeriouh O, Reinoso-Moreno JV, López-Rosales L et al (2017) Biofouling in photobioreactors for marine microalgae. Crit Rev Biotechnol 37:1006–1023. https://doi.org/10.1080/07388551.2017.1299681
Roostaei J, Zhang Y, Gopalakrishnan K, Ochocki AJ (2018) Mixotrophic microalgae biofilm: a novel algae cultivation strategy for improved productivity and cost-efficiency of biofuel feedstock production. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-018-31016-1
Palmer J, Flint S, Brooks J (2007) Bacterial cell attachment, the beginning of a biofilm. J Ind Microbiol Biotechnol 34:577–588. https://doi.org/10.1007/s10295-007-0234-4
Ozkan A, Berberoglu H (2013) Cell to substratum and cell to cell interactions of microalgae. Colloids Surfaces B Biointerfaces 112:302–309. https://doi.org/10.1016/j.colsurfb.2013.08.007
Cheah YT, Chan DJC (2021) Physiology of microalgal biofilm: a review on prediction of adhesion on substrates. Bioengineered 12:7577–7599. https://doi.org/10.1080/21655979.2021.1980671
Devi ND, Tiwari R, Goud VV (2021) Cultivating Scenedesmus sp. on substrata coated with cyanobacterial-derived extracellular polymeric substances for enhanced biomass productivity: a novel harvesting approach. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01432-x
Katarzyna L, Sai G, Avijeet Singh O (2015) Non-enclosure methods for non-suspended microalgae cultivation: literature review and research needs. Renew Sustain Energy Rev 42:1418–1427. https://doi.org/10.1016/j.rser.2014.11.029
Tsavatopoulou VD, Aravantinou AF, Manariotis ID (2021) Comparison of botryococcus braunii and neochloris vigensis biofilm formation on vertical oriented surfaces. Biointerface Res Appl Chem 11:12843–12857
Orfanos AG, Manariotis ID (2019) Algal biofilm ponds for polishing secondary effluent and resource recovery. J Appl Phycol 31:1765–1772. https://doi.org/10.1007/s10811-018-1731-8
Sukačová K, Vícha D, Dušek J (2020) Perspectives on microalgal biofilm systems with respect to integration into wastewater treatment technologies and phosphorus scarcity. Water (Switzerland) 12:2245. https://doi.org/10.3390/w12082245
Garbowski T, Pietryka M, Pulikowski K, Richter D (2020) The use of a natural substrate for immobilization of microalgae cultivated in wastewater. Sci Rep 10:1–9. https://doi.org/10.1038/s41598-020-64656-3
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Tan L, Zhao F, Han Q et al (2018) High correlation between structure development and chemical variation during biofilm formation by Vibrio parahaemolyticus. Front Microbiol 9:1–10. https://doi.org/10.3389/fmicb.2018.01881
American Public Health Association (APHA) (1998) American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 19th edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/s0021-9258(19)52451-6
Waterborg JH, Matthews HR (1994) The Lowry method for protein quantitation. Methods Mol Biol 32:1–4
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Folch J, Lees M, Sloane SG (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509. https://doi.org/10.1016/S0021-9258(18)64849-5
Aravantinou AF, Theodorakopoulos MA, Manariotis ID (2013) Selection of microalgae for wastewater treatment and potential lipids production. Bioresour Technol 147:130–134. https://doi.org/10.1016/j.biortech.2013.08.024
van Oss CJ, Chaudhury MK, Good RJ (1988) Interfacial Lifshitz—van der Waals and Polar interactions in macroscopic systems. Chem Rev 88:927–941. https://doi.org/10.1021/cr00088a006
Hao W, Yanpeng L, Zhou S et al (2017) Surface characteristics of microalgae and their effects on harvesting performance by air flotation. Int J Agric Biol Eng 10:125–133. https://doi.org/10.3965/j.ijabe.20171001.2698
Ji C-c, Zhou H, Deng SK et al (2021) Insight into the adhesion propensities of extracellular polymeric substances (EPS) on the abiotic surface using XDLVO theory. J Environ Chem Eng 9:106563. https://doi.org/10.1016/j.jece.2021.106563
van Oss CJ, Good RJ, Chaudhury MK (1988) Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 4:884–891. https://doi.org/10.1021/la00082a018
Palmer CM (1969) A composite rating of algae tolerating organic pollution. J Phycol 82:78–82. https://doi.org/10.1007/s10295-007-0234-4
Ozyurt CE, Yesilcimen HO, Mavruk S et al (2017) Assessment of some of the feeding aspects and reproduction of s. undosquamis distributed in the İskenderun Bay. Turkish J Fish Aquat Sci 17:51–60. https://doi.org/10.4194/1303-2712-v17
Prescott GW (1973) Algae of the Western Great Lake area. W:M.C. Brown Company. Brown Company Publishers, Dubuque
Jo SW, Do JM, Kang NS et al (2020) Isolation, identification, and biochemical characteristics of a cold-tolerant chlorella vulgaris knua007 isolated from king george island, antarctica. J Mar Sci Eng 8:1–11. https://doi.org/10.3390/jmse8110935
Mohsenpour SF, Hennige S, Willoughby N et al (2021) Integrating micro-algae into wastewater treatment: a review. Sci Total Environ 752:142168. https://doi.org/10.1016/j.scitotenv.2020.142168
Ge S, Champagne P, Wang H et al (2016) Microalgae recovery from water for biofuel production using CO2-switchable crystalline nanocellulose. Environ Sci Technol 50:7896–7903. https://doi.org/10.1021/acs.est.6b00732
Shen Y, Zhang H, Xu X, Lin X (2015) Biofilm formation and lipid accumulation of attached culture of Botryococcus braunii. Bioprocess Biosyst Eng 38:481–488. https://doi.org/10.1007/s00449-014-1287-1
Mühlenbruch M, Grossart HP, Eigemann F, Voss M (2018) Mini-review: phytoplankton-derived polysaccharides in the marine environment and their interactions with heterotrophic bacteria. Environ Microbiol 20:2671–2685. https://doi.org/10.1111/1462-2920.14302
Hu Y, Xiao Y, Liao K et al (2021) Development of microalgal biofilm for wastewater remediation: from mechanism to practical application. J Chem Technol Biotechnol 96:2993–3008. https://doi.org/10.1002/jctb.6850
Hadjoudja S, Deluchat V, Baudu M (2010) Cell surface characterisation of Microcystis aeruginosa and Chlorella vulgaris. J Colloid Interface Sci 342:293–299. https://doi.org/10.1016/j.jcis.2009.10.078
Gonçalves AL, Ferreira C, Loureiro JA et al (2015) Surface physicochemical properties of selected single and mixed cultures of microalgae and cyanobacteria and their relationship with sedimentation kinetics. Bioresour Bioprocess 2:. https://doi.org/10.1186/s40643-015-0051-y
Acknowledgements
The authors would like to thank Kyriakos Bourikas from Hellenic Open University for providing the zeta meter, Katerina Govatsi from the University of Patras for recording SEM images, and Andriana Aravantinou for conducting microalgae identification.
Author information
Authors and Affiliations
Contributions
Vasiliki D. Tsavatopoulou: conceptualization, experimental work, writing — original draft preparation. Ioannis Manariotis: conceptualization, supervision, writing — reviewing and editing.
Corresponding author
Ethics declarations
Consent to participate
No conflicts, informed consent, or human or animal rights are applicable to this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsavatopoulou, V.D., Manariotis, I.D. Chlorococcum sp. and mixotrophic algal biofilm growth in horizontal and vertical–oriented surfaces using wastewater and synthetic substrate. Biomass Conv. Bioref. 14, 4743–4758 (2024). https://doi.org/10.1007/s13399-022-02752-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02752-2