Abstract
In order to reduce the cost of microalgae harvesting, biofilm algal cultivations have received attention as a potential platform for algal biomass production and wastewater treatment. Two 50-L ponds containing vertically oriented geotextiles, cotton textiles, and polyethylene sheets were fed secondary effluent to examine the growth of algal biofilms. The removal of total phosphorus, PO43−-P and NO3−-N ranged from 52 to 97%, 59 to 93%, and 0 to 99%, respectively. The highest biomass productivity was 1.4 and 0.5 g m−2 day−1, and the lipid content of the attached biomass was quite low, 0.36 and 0.48%, in the cotton textile and polyethylene-baffled pond, respectively. The lipid content of the suspended biomass of the cotton textile and polyethylene pond was very low and similar (0.5%), but it increased to 13.8 and 3.4%, respectively, after a starvation period of 13 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The application of microalgae in wastewater treatment has been extensively studied in natural systems (Oron et al. 1979; Buhr and Miller 1983; Santiago et al. 2013). The majority of natural wastewater treatment systems consists of suspended algal biomass and waste stabilization ponds, which are used worldwide as an effective and low-cost technology (Babu et al. 2010; Craggs et al. 2015). Wastewater treatment is based on the symbiotic action of microalgae and bacteria (Schumacher and Sekoulov 2002; Aravantinou et al. 2017). Microalgae consume carbon dioxide, as carbon source, nutrients and light as energy source, to produce oxygen that is consumed by the bacteria for the aerobic heterotrophic degradation of organic matter (Boelee et al. 2014a). The main disadvantages of these systems include the escape of microalgae in the effluent and the cost demand for algal cells harvesting (Davis et al. 2011; Gross et al. 2015; Vergini et al. 2016; Wang et al. 2018). It is estimated that 60 to 90% of the biological oxygen demand in the effluent of a wastewater pond is attributed to microalgae (Schumacher and Sekoulov 2002), while the biomass harvesting cost alone accounts for 21% of the total capital cost of an open pond cultivation system (Davis et al. 2011).
As an alternative to suspended culture systems, algal biofilm cultures have drawn the interest in recent years. The biomass produced is much more concentrated and harvesting and dewatering cost could be significantly reduced (Schnurr and Allen 2015; Wang et al. 2018). The growth of algal biofilms is affected by several factors such as supporting material, light intensity, temperature, nutrients, substratum, extracellular polymeric substances, and species interactions (Kesaano and Sims 2014; Schnurr and Allen 2015).
Depending on the movement of the supporting material, two main types have been reported in the literature, the rotating and the stationary algal biofilms (Gross et al. 2015). In rotating biofilms, the supporting material is partially submerged in a pond and brings the biofilm in contact with the air and liquid medium. The algal biomass is harvested by scraping the supporting material (Blanken et al. 2014; Gross and Wen 2014). In stationary reactors, the biofilm grows on horizontally, vertically, or inclined placed flat surfaces, and a thin layer of liquid flows over the biofilm (Wilkie and Mulbry 2002; Boelee et al. 2011; Naumann et al. 2013; Shi et al. 2014; Sukačová et al. 2015). Another option is to submerge the supporting material vertically in a pond to promote biofilm growth (Liu et al. 2013; Genin et al. 2014). The vertically oriented support drastically reduces the land required, when compared to the horizontally oriented material. Furthermore, higher light penetration is expected in ponds with vertical supporting material, due to the significant chance of the light to strike on the biofilm surface (Gross et al. 2015).
A few studies have been carried out to investigate the nutrient removal and biomass productivity performance of algal biofilms in wastewater treatment. Christenson and Sims (2012) designed a rotating cylindrical drum for the tertiary treatment of wastewater and tested different growth substrata on cord or sheet arrangement to study the biofilm formation. The most effective substratum appeared to be the cotton cord. Fica and Sims (2016) used rotating cylinders with cotton rope, partially submerged in dairy wastewater, in order to determine the effect of temperature and organic carbon concentration on biofilm biomass productivity and nutrient uptake. Shi et al. (2014) applied a twin-layer photobioreactor for N and P removal from primary- and secondary-treated municipal wastewater by immobilized microalgae. Genin et al. (2014) designed a parallel plate air lift reactor to examine the growth kinetics of mixed culture algal biofilms grown on various materials (acrylic, glass, polycarbonate, polystyrene, and cellulose acetate). Boelee et al. (2014b) examined the effect of harvesting on biomass production and nutrient removal in phototrophic biofilm reactors. A vertical and a horizontal reactor were employed, which treated synthetic wastewater. In the vertical reactor, the biofilm was grown on a polyethylene-based woven geotextile, which was placed on the top of a nonwoven geotextile, and both layers were fixed to a polypropylene support plate. Su et al. (2016) investigated the effect of the wall-attached biofilm for nutrient removal in an open photobioreactor during long-term operation and reported that the reactor wall-attached biofilm supported high phosphorus and nitrogen removal.
The scope of this study was to investigate the further removal of nutrients from secondary-treated wastewater in laboratory-scale open ponds as well as the attachment of algal biomass on different supporting surfaces. The ponds contained vertically oriented geotextiles, cotton textiles, and polyethylene sheets to facilitate the development of algal biofilm. The behavior of the ponds, in batch or continuous flow experiments, and the lipid content of the suspended and attached biomass was evaluated.
Materials and methods
Experimental system
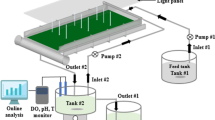
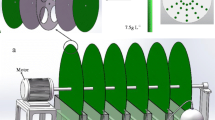
Two 50-L (80 × 24 × 30 cm, L×W×H) open ponds made of glass were employed, using the arrangement shown in Fig. 1. Four baffles were vertically placed in each open pond to facilitate the development of algal biofilm. Cotton and polyethylene (PE) sheets were selected as supporting materials and were attached on four plexiglass baffles placed along the pond. In another configuration, four nonwoven (67 × 15 cm, L×H) geotextile sheets (code 250CNW, Thrace Plastics Co S.A., Greece) were vertically immersed in a same open 50-L pond, which was operated in batch mode.
Illumination was continuously provided by two 36-W fluorescents lamps (cool daylight) placed above each pond’s surface. The photosynthetic irradiance was measured by a light meter (Lightscout, Quantum Light Meters) 5 cm above the liquid surface of the ponds. The ponds were placed in a constant temperature room. A variable speed peristaltic pump (Masterflex, Cole Pamer Instrument, Co., USA) activated by a timer for appropriate periods was used to feed the units. Air supply, when necessary, was provided by air pumps to improve CO2 mass transfer in the pond.
Ponds start-up and operating conditions
Microalgal polyculture, cultivated in dilute primary settled wastewater, was used to inoculate the pond with geotextiles. The pond was fed with secondary effluent from the wastewater treatment plant (WWTP) of the University of Patras. A batch experiment was conducted, which lasted for 56 days, and the operating conditions of the pond are presented in Table 1. Samples were taken from the pond after careful mixing of the liquid content. At the end of the operation period, the geotextiles were removed and dried in an oven at 60 °C for at least 24 h. Then, to determine the total suspended biomass, samples were taken from the liquid content of the pond, after careful mixing. Τhe attached biomass was harvested from the surface of the geotextiles with a spatula to determine the dry weight and lipid content. The pond was operated at room temperature (20.5 ± 0.5° C). The photosynthetic irradiance was 120 μmol photons m−2 s−1. A continuous supply of air was provided by three air pumps (each of 3.2 L min−1).
The cotton and PE baffled ponds were inoculated with Chlorococcum sp. preculture, instead of using an algal polyculture. This procedure was chosen in order to have more controlled conditions at the start-up of the ponds. Chlorococcum sp. SAG 22.83 was obtained from the SAG Culture Collection of the University of Göttingen. The preculture was cultivated in a 2-L Erlenmeyer flask with \( \raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$3$}\right. \) N BG-11 (BG-11 enriched with one third times the nitrates), according to Aravantinou et al. (2015), at 26° C, before being used for the inoculation of the reactors. Secondary-treated wastewater from the WWTP of the University of Patras was used to feed the ponds. The secondary effluent COD, TSS, NH4+-N, NO3−-N, and total-P concentration during the entire period of operation (geotextile, cotton, and PE ponds) was 10.7 ± 7.8, 3.5 ± 2.1, 0, 14.6 ± 5.6, and 3.2 ± 0.6 mg L−1. To avoid the presence of zooplankton, wastewater was filtered through cheesecloth. The ponds were filled with a mixture of tap water, wastewater and 250 mL of Chlorococcum preculture at a final volume of 48 L (Table 2). During the first 5 days of operation, the ponds were operated in batch mode and samples were taken from the liquid medium of the ponds after careful mixing. Thereafter, the two ponds were fed by a peristaltic pump at hydraulic retention times (HRT) from 12 to 48 days (Table 2). Samples were taken from the effluent of the ponds by a siphon arrangement. The growth of microalgae occurred in two phases. The residual colonies on the supporting materials were used as the inoculum for the second growth phase. At the end of the first and the second phase, the liquid of the ponds was removed using a siphon, and the suspended biomass was measured. After the draining of the ponds, the attached biomass was harvested from the surface of the supporting materials with a spatula. The photosynthetic irradiance was 85 μmol photons m−2 s−1. The experiments were conducted under room temperature of 24 ± 3° C. During the first period, air was continuously supplied by three air pumps, while during the second growth phase air was not supplied.
Analytical methods
Total suspended solids (TSS) were measured by a gravimetric method (APHA et al. 2012). Chemical oxygen demand (COD) was measured by the open reflux method (the alternate low COD procedure was used when appropriate) according to standard methods (APHA et al. 2012). The soluble fraction of COD (SCOD) was determined by passing the sample through a 0.45 μm membrane filter. Total phosphorus (total-P) was determined using the persulfate method and ascorbic acid colorimetric technique (APHA et al. 2012). Ammonia nitrogen (NH4-N) was determined by the titrimetric method after a preliminary distillation step and total Kjeldahl nitrogen (TKN) was measured using the macro-Kjeldahl procedure (APHA et al. 2012). Other nutrients (nitrates, phosphates) were determined by ion chromatography (DX-500, Dionex Corporation, USA). The lipid content of the dry algal biomass was measured by the modified method of Folch et al. (1957), and details can be found in Aravantinou et al. (2013).
Algal biofilm was periodically sampled and observed by scanning electron microscopy (SEM) (microscope JEOL 6300, JEOL Ltd.). For each sample, at least three fields were observed at different magnifications (× 2500 to × 14000). Algal biofilms were also observed by an optical microscope at × 100, × 400, and × 1000 magnification.
Results and discussion
Pond with vertically oriented geotextiles
In the pond with vertically oriented geotextiles, low TSS and COD values were observed during the entire operation period in the influent and the liquid medium of the pond (Table 3). During the first days of the experiment, microalgae were attached on the geotextiles and growth started. As a consequence, there was not much suspended biomass in the liquid medium. The concentration of TSS in the influent and the liquid medium of the pond ranged from 6 to 7 mg L−1 and 5 to 19 mg L−1, respectively and the corresponding COD values ranged from 0 to 20 mg L−1 and 0 to 46 mg L−1 (Fig. 2a). The COD values in the influent were expected to be low as the substrate was secondary effluent. The observation of the algal growth was quite difficult because of the black color of the supporting material. When the concentration of the attached biomass became higher, microalgae were more easily observed by the naked eye.
The total-P concentration in the effluent was below 1.0 mg L−1 (Fig. 3a) and its removal ranged from 52.2 to 91.1% and. The removal of PO43−-P exceeded 84% over the operation period and the PO43−-P concentration in the effluent remained below the detection limit of 0.5 mg L−1. Nitrate nitrogen concentration in the effluent of the pond ranged from 9 to 17 mg NO3−-N L−1 (Fig. 4a). TKN and NO3−-N removal was up to 90 and 48%, respectively. Su et al. (2016) reported total nitrogen and PO43−-P removal efficiencies of 43 and 98%, respectively, in an open photobioreactor (stainless steel pot) with wall attached microalgal biofilm. In another study, Boelee et al. (2014b) achieved low PO43− concentration in the effluent of a vertical phototrophic biofilm reactor (below 0.15 mg P L−1) and the NO3−-N concentration in the effluent was generally below 2.2 mg L−1.
The overall attached and suspended algal productivity was based on the measurement of biomass at the last day of the experiment. The harvested attached biomass was 74% of the total biomass (attached and suspended) produced in the pond (Table 4), corresponding to a ratio of about 3 to 1 (attached to suspended biomass). A higher percentage would be observed if the biomass between the pores of the geotextiles could be taken into account. The harvested attached biomass from the surface of the geotextiles corresponded to an algal biofilm productivity of 0.16 g m−2 day−1 (Table 4). The attached biomass footprint area productivity of the reactor was also calculated and was 0.65 g m−2 day−1. Higher biomass productivity on woven geotextile (7 g m−2 day−1) was observed by Boelee et al. (2014b), where harvesting occurred every 2, 4, or 7 days and the first harvests were not taken into consideration. In that study, the biofilm was grown on a polyethylene-based woven geotextile, which was placed on the top of a nonwoven geotextile, and both layers were fixed to a polypropylene support plate. In the present study, the sheets are separate, and this configuration (baffled ponds) provides the potential of increasing the attached biomass footprint area productivity, if more sheets are placed in the ponds. The lipid content of the harvested biomass was 1%, lower than that of the suspended biomass (6%). This difference may be due to higher bacterial content of the attached biomass.
Ponds with cotton textile and polyethylene sheet baffles
The growth of microalgae occurred in two phases, at the end of which the ponds were emptied and the attached biomass was harvested with a spatula to determine the dry weight and lipid content. During the first growth phase, low total suspended solids (TSS) and COD values were observed (Table 3), and microalgae did not show significant growth. The concentration of TSS in the influent and the liquid medium of both ponds ranged from 2 to 8 mg L−1 and 0 to 11 mg L−1, respectively (Fig. 2b). The COD values of the influent ranged from 6 to 20 mg L−1, while in the effluent they ranged from 4 to 31 mg L−1 and 2 to 31 mg L−1 in the cotton textile and polyethylene sheet pond, respectively. Although Chlorococcum sp. was used to inoculate ponds, at the 18th day of operation, except for Chlorococcum sp., other microalgal species were also observed in the attached biomass including diatoms and multicellular species.
After the first growth phase, and due to the fact that microalgae did not show significant growth, the ponds were left without wastewater feed, but with water supply, for 51 days. Then, the ponds were emptied and the liquid of the two ponds was mixed in order to achieve the same initial conditions for the next growth phase. The attached biomass was harvested and the ponds were refilled with the mixed medium for the beginning of the second growth phase.
At the second growth phase, higher algal growth rates were observed in both ponds (Table 3). In the cotton textile pond, TSS (Fig. 2c) and COD values of the effluent showed increasing trend during the entire operation period, with maximum values of 16 and 46 mg L−1, respectively. The corresponding values of the influent remained in lower and quite constant levels (1 to 4 mg L−1 for TSS and 8 to 25 mg L−1 for COD). In the polyethylene sheet pond, microalgae grew more intensely until the 12th day. At that day, the concentration of TSS and COD in the effluent was 38 and 62 mg L−1, respectively. In the following days of operation, zooplankton was observed in the pond. As a consequence, the concentration of the biomass in the liquid medium of the pond decreased rapidly. More specifically, the TSS and COD values decreased to 4 and 27 mg L−1 (very close to their initial levels), respectively. Zooplankton was also observed in the cotton textile pond, but at quite lower level, so the concentration of the biomass was less influenced.
Total-P concentration during the first growth phase was at about 0.1 mg L−1 in both ponds up to the 15th day of operation and thereafter increased up to 0.8 and 0.6 mg L−1 for cotton and PE ponds, respectively (Fig. 3b). During the second growth phase, total-P concentration exhibited similar behavior and ranged from 0.1 to 0.5 mg L−1 in both ponds (Fig. 3c). In the first growth phase, the average PO43−-P removal in both ponds was 81.5 ± 10%. The average removal of total phosphorus was 88.7 ± 10.5 and 89.7 ± 7.9% in the cotton textile and polyethylene sheet pond, respectively. Nitrate nitrogen concentration during the first growth phase was lower in the effluent of the PE pond, compared to the cotton pond, and was similar in both ponds during the second growth phase (Fig. 4b). The removal of TKN ranged from 0 to 100% and 66.7 to 100% and the NO3−-N removal ranged from 53.8 to 78.8% and 50.8 to 77.2% in the cotton textile and polyethylene sheet pond, respectively.
During the second growth phase, the average total phosphorus removal was 94.5 ± 2.2 and 91.6 ± 4.8% in the cotton textile and PE sheet pond, respectively, and the average removal rate was 0.26 ± 0.06 and 0.25 ± 0.06 g m−2 day−1. In the cotton textile pond, the average PO43−-P removal was 92.5 ± 0.8% and the average removal rate was 0.17 ± 0.016 g m−2 day−1, while in the PE sheet pond, the average removal and removal rate of PO43−-P were 92.1 ± 1.4% and 0.17 ± 0.016 g m−2 day−1, respectively. TKN removal ranged from 33.3 to 80% and 33.3 to 77.8% in the cotton textile and PE sheet pond, respectively, and the corresponding NO3−-N removal ranged from 15.7 to 99.3% and 10.2 to 99.3%. Phosphorus was removed via algal assimilation and precipitation at periods with high pH values (> 8.5). Due to the absence of ammonium in the nitrified secondary effluent, nitrates were the main source for algal growth. High PO43−-P removal (86%) from secondary effluent was also observed by Gao et al. (2015) in a lab-scale biofilm membrane photobioreactor where flexible fiber bundles were used as solid carriers, and the removal rate of PO43−-P was 0.35 g m−2 day−1. In another study, Shi et al. (2014) reported 73% PO43−-P removal, corresponding to an average daily uptake rate of 0.45 g m−2 day−1, and also 83% of the nitrate was removed from secondary settled wastewater. Wei et al. (2008) studied nutrient removal from simulated wastewater by an algal biofilm reactor, and total phosphorus and nitrogen removal reached 98 and 87%, respectively.
The biomass productivity of the two ponds at the first growth phase was quite low and thus a comparison between the supporting materials was not possible (Table 4). The attached biomass productivity was 0.031 and 0.096 g m−2 day−1 and the lipid content of the harvested biomass was 0.23 and 1.44% for the cotton textile and polyethylene sheet, respectively. In the second growth phase, higher attached biomass productivity of 1.4 g m−2 day−1 was observed with the cotton textile pond. The polyethylene sheet followed with biomass productivity of 0.5 g m−2 day−1. The reactors footprint area productivity was 2.7 and 1.0 g m−2 day−1 for the cotton textile and polyethylene sheet pond, respectively. These results indicated that the cotton textile was more suitable to support algal biofilm growth. In a comparison of the two supporting materials, higher percentage of the total biomass of the pond was attached on the cotton textile (Table 4) and, as a result, the harvested biomass from the cotton textile was about three times higher than the harvested biomass from the polyethylene sheet. Shi et al. (2014) reported biomass growth rates of 1.2 g m−2 day−1 and footprint biomass productivity of 6.3 g m−2 day−1 with a twin-layer photobioreactor treating primary and secondary municipal wastewater effluent. Zamalloa et al. (2013) reported footprint biomass productivity of 2.5 g m−2 day−1 in a roof-installed parallel plate microalgal biofilm reactor treating domestic wastewater. The lipid content of the harvested biomass was 0.36 and 0.48%, in the cotton textile and polyethylene baffled pond, respectively. The lipid content of the suspended biomass of the two ponds was similar (~ 0.5%), but it increased to 13.8 and 3.4% in the cotton textile and polyethylene pond, respectively, after no feeding for 13 days. In literature, algal biofilms have produced significantly lower lipid concentrations than algae grown planktonically (Schnurr and Allen 2015). Shayan et al. (2016) reported lipid accumulation of 20% on a rotating algal biofilm reactor for wastewater nutrient removal, and Gross et al. 2013, reported lipid content of 7.7% in a rotating algal biofilm reactor operating with Bold’s Basal medium.
The addition of solid surfaces or carriers into the liquid of an algal pond positively affects the algal biomass productivity and nutrient removal. Liu et al. (2013) reported that the algal productivity in a vertical supported material pond was 4 to 7 times higher compared to open ponds. Tao et al. (2017) reported that the addition of solid carriers suspended in the culture media resulted in higher volumetric algal biomass and lipids productivity by 51 and 73%, respectively, compared to conventional photobioreactors. Furthermore, they observed improved nutrient removal in the biofilm photobioreactors due to the higher algal biomass productivity. The improved photosynthetic activity of attached algal cultures may be due to the improved light availability compared to conventional ponds (Liu et al. 2013). However, the configuration of attached surfaces in the pond has a significant role on the dissipation of light on solid surfaces and the optimum liquid depth should be further investigated in combination with the pond geometry, and the distance between the baffles.
Conclusions
The attached biomass productivity was higher in the cotton textile pond, compared to the polyethylene sheet pond, indicating that the cotton textile was more suitable to support the algal biofilm growth. The lipid content of the harvested and suspended biomass was similar in the two ponds and the lipid content of the suspended biomass of both ponds increased to 13.8% in the cotton textile pond after a 13-day non-feeding period. The biomass productivity on the geotextiles was lower, but in terms of footprint productivity, this difference was proportionately decreased. The removal of PO43−-P and total-P exceeded 50% in all the ponds.
References
APHA, AWWA, WEF (2012) Standard Methods for the Examination of Water and Wastewater, 22nd edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC
Aravantinou AF, Theodorakopoulos MA, Manariotis ID (2013) Selection of microalgae for wastewater treatment and potential lipids production. Bioresour Technol 147:130–134
Aravantinou AF, Tsarpali V, Dailianis S, Manariotis ID (2015) Toxic effects of ZnO nanoparticles to freshwater and marine microalgae cultures. Ecotoxicol Environ Saf 114:109–116
Aravantinou AF, Barkonikou EF, Manariotis ID (2017) Microalgae biomass growth and lipid production using primary treated wastewater. Desalin Water Treat 91:228–234
Babu MA, Hes EMA, van der Steen NP, Hooijmans CM, Gijzen HJ (2010) Nitrification rates of algal-bacterial biofilms in wastewater stabilization ponds under light and dark conditions. Ecol Eng 36:1741–1746
Blanken W, Janssen M, Cuaresma M, Libor Z, Bhaiji T, Wijffels RH (2014) Biofilm growth of Chlorella sorokiniana in a rotating biological contactor based photobioreactor. Biotechnol Bioeng 111:2436–2445
Boelee NC, Temmink H, Janssen M, Buisman CJN, Wijffels RH (2011) Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res 45:5925–5933
Boelee NC, Temmink H, Janssen M, Buisman CJN, Wijffels RH (2014a) Balancing the organic load and light supply in symbiotic microalgal-bacterial biofilm reactors treating synthetic municipal wastewater. Ecol Eng 64:213–221
Boelee NC, Janssen M, Temmink H, Taparavičiūtė L, Khiewwijit R, Jánoska Á, Buisman CJN, Wijffels RH (2014b) The effect of harvesting on biomass production and nutrient removal in phototrophic biofilm reactors for effluent polishing. J Appl Phycol 26:1439–1452
Buhr HO, Miller SB (1983) A dynamic model of the high-rate algal-bacterial wastewater treatment pond. Water Res 17:29–37
Christenson LB, Sims RC (2012) Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotechnol Bioeng 109:1674–1684
Craggs R, Park J, Sutherland D, Heubeck S (2015) Economic construction and operation of hectare-scale wastewater treatment enhanced pond systems. J Appl Phycol 27:1913–1922
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531
Fica ZT, Sims RC (2016) Algae-based biofilm productivity utilizing dairy wastewater: effects of temperature and organic carbon concentration. J Biol Eng 10:18
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Gao F, Yang Z-H, Li C, Zeng GM, Ma DH, Zhou L (2015) A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour Technol 179:8–12
Genin SN, Aitchison JS, Allen DG (2014) Design of algal film photobioreactors: material surface energy effects on algal film productivity, colonization and lipid content. Bioresour Technol 155:136–143
Gross M, Wen Z (2014) Yearlong evaluation of performance and durability of a pilot-scale revolving algal biofilm (RAB) cultivation system. Bioresour Technol 171:50–58
Gross M, Henry W, Michael C, Wen Z (2013) Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour Technol 150:195–201
Gross M, Jarboe D, Wen Z (2015) Biofilm-based algal cultivation systems. Appl Microbiol Biotechnol 99:5781–5789
Kesaano M, Sims RC (2014) Algal biofilm based technology for wastewater treatment. Algal Res 5:231–240
Liu T, Wang J, Hu Q, Cheng P, Ji B, Liu J, Chen Y, Zhang W, Chen X, Chen L, Gao L, J C, Wang H (2013) Attached cultivation technology of microalgae for efficient biomass feedstock production. Bioresour Technol 127:216–222
Naumann T, Çebi Z, Podola B, Melkonian M (2013) Growing microalgae as aquaculture feeds on twin-layers: a novel solid-state photobioreactor. J Appl Phycol 25: 1413–1420
Oron G, Shelef G, Levi A, Meydan A, Azov Y (1979) Algae/bacteria ratio in high-rate ponds used for waste treatment. Appl Environ Microbiol 38:570–576
Santiago AF, Calijuri ML, Assemany PP, Calijuri MC, dos Reis AJD (2013) Algal biomass production and wastewater treatment in high rate algal ponds receiving disinfected effluent. Environ Technol 34:1877–1885
Schnurr PJ, Allen DG (2015) Factors affecting algae biofilm growth and lipid production: a review. Renew Sust Energ Rev 52:418–429
Schumacher G, Sekoulov I (2002) Polishing of secondary effluent by an algal biofilm process. Water Sci Technol 46:83–90
Shayan SI, Agblevor FA, Bertin L, Sims RC (2016) Hydraulic retention time effects on wastewater nutrient removal and bioproduct production via rotating algal biofilm reactor. Bioresour Technol 211:527–533
Shi J, Podola B, Melkonian M (2014) Application of a prototype-scale twin-layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour Technol 154:260–266
Su Y, Mennerich A, Urban B (2016) The long-term effects of wall attached microalgal biofilm on algae-based wastewater treatment. Bioresour Technol 218:1249–1252
Sukačová K, Trtílek M, Rataj T (2015) Phosphorus removal using a microalgal biofilm in a new biofilm photobioreactor for tertiary wastewater treatment. Water Res 71:55–63
Tao Q, Gao F, Qian C-Y, Guo X-Z, Zheng Z, Yang Z-H (2017) Enhanced biomass/biofuel production and nutrient removal in an algal biofilm airlift photobioreactor. Algal Res 21:9–15
Vergini S, Aravantinou AF, Manariotis ID (2016) Harvesting of freshwater and marine microalgae by common coagulants and magnetic microparticles. J Appl Phycol 28:1041–1049
Wang JH, Zhuang LL, Xu XQ, Deantes-Espinosa VM, Wang XX, Hu HY (2018) Microalgal attachment and attached systems for biomass production and wastewater treatment. Renew Sust Energ Rev 92:331–342
Wei Q, Hu Z, Li G, Xiao B, Sun H, Tao M (2008) Removing nitrogen and phosphorus from simulated wastewater using algal biofilm technique. Front Environ Sci Eng 2:446–451
Wilkie AC, Mulbry WW (2002) Recovery of dairy manure nutrients by benthic freshwater algae. Bioresour Technol 84:81–91
Zamalloa C, Boon N, Verstraete W (2013) Decentralized two-stage sewage treatment by chemical-biological flocculation combined with microalgae biofilm for nutrient immobilization in a roof installed parallel plate reactor. Bioresour Technol 130:152–160
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orfanos, A.G., Manariotis, I.D. Algal biofilm ponds for polishing secondary effluent and resource recovery. J Appl Phycol 31, 1765–1772 (2019). https://doi.org/10.1007/s10811-018-1731-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1731-8