Abstract
Stone fruit processing produces almost 20–30% of waste as seed kernels, which are rich source of valuable compounds like protein that can be used in various food formulations. So, the present study aimed to isolate protein from Meghalayan cherry kernel and study the effect of pH and dephenolization on biochemical, functional, and nutritional characteristics of protein isolate from the Meghalayan cherry kernel. Alkali (pH, 8.5–12.5) extraction and isoelectric precipitation were used to isolate kernel protein. Based on yield, purity, and color, pH 10.5 was most suitable for extracting protein. Dephenoized protein isolate (MCKPI-DP) showed higher protein content (88.81 ± 0.03%) as compared to natural protein isolate (MCKPI-N) (83.50 ± 0.01%). The significantly higher solubility, dispersibility, foaming properties, emulsion stability, and capacity were seen in dephenolized protein isolate, which increased with increased pH. Water binding capacity was higher for MCKPI-N than MCKPI-DP and further increased with an increase in pH. Meghalayan cherry kernel protein isolate was a rich source of essential amino acids except tryptophan required by infants and adults (FAO). MCKPI-DP showed better protein efficiency ratio (2.67 ± 0.03(PER1), 2.49 ± 0.02(PER2), 2.14 ± 0.04(PER3)), essential amino acid index (78.82 ± 0.85), EAA/TAA % (41.76 ± 0.01), nutritional index (65.82 ± 0.71), and biological value (74.22 ± 0.93) indicating the presence of good-quality proteins. As per the FAO standard, the amino acid score showed that all essential amino acids are in excess, thus helping fulfill essential amino acid requirements and being a good protein supplement source in food products. Moreover, this work helps better utilize agro-wastes as low-cost substrates for protein isolation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
For many years, human protein nutrition has emphasized animal products as a source of protein. Animal protein is referred to as a complete protein source since it contains all necessary amino acids. The exponential rise of the human population raises demand for animal protein, which, in turn, restricts its supply. The scarcity of resources to meet demands gives rise to an alternate source of protein, which could provide the same health benefits and helps in eliminating animal-based product safety issues. Plant-based edible protein is the upcoming solution for such concerns [1]. Animal husbandry is also not much regarded as an environmentally friendly practice, and thus, it could be replaced by plant-based protein as these are cost-effective sources [2]. Agro-industrial by-products like fruit seeds are a rich source of proteins and other nutrients that humans can consume. Currently, these massive wastes are discarded without being recycled. Protein recovery and utilization for human consumption can help prevent environmental pollution and contribute to the industry’s profit by lowering waste treatment costs.
Meghalayan cherry (Prunus nepalensis), locally called sohoing, belongs to the Rosaceae family in the Khasi and Jaintia hills of Meghalaya. It is consumed as raw and may be used to manufacture juices, jam, preserves, assorted wine, squash, etc. [3, 4]. With the industrial application, by-product generation also comes into consideration which causes disposal problems in the industry. The cherry kernels (approx. 30% of whole fruit) are a prominent source of protein to fulfill the industrial demand as an economical raw material for the replacement of expensive protein sources. Direct utilization of the kernel is limited as its sensory qualities are not appealing due to its dark color and bitter taste. The kernel of the fruit seed is a rich source of polyphenols which gives it different organoleptic properties [5]. The presence of polyphenols in kernel hinders the protein quality by reducing its digestibility, imparting unwanted brown color and structure alteration, which also affects the functional characteristics of protein in the different food systems [5]. Polyphenols are more prone to oxidation due to polyphenol oxidase and extraction conditions for protein extraction. The oxidation of polyphenols results in the production of compounds like o-quinones and o-dihydroxy structure, which are highly reactive and covalently attached to thiol and amino groups of protein. These bondings impart green and dark brown color to protein meal and isolates [6]. Hence, for the utilization as a source of protein, seed kernels must be first treated to remove the polyphenols.
The high quality and quantity of kernel proteins determine their good nutritional and functional properties. Apart from being a good source of essential amino acids, protein’s performance as a good ingredient is determined by its functional qualities in various processing conditions, such as pH, temperature, and processing time. It has been observed that the functional characteristics of protein vary according to the change in pH. Thus, it is important to study the effects of pH on the functional characteristics of protein isolates as it can alter the properties of the end product [7]. Solubility is the crucial property of protein isolates, which helps determine other functional characteristics. Polyphenols significantly impact protein solubility as they enhance the internal cross-linking of protein, which reduces the net surface charge on protein molecules. This results in a reduction of protein solubility and ultimately affects the behavior of other functional properties.
The limited literature on nutritional and functional characteristics of Meghalayan cherry kernel protein and considering kernel proteins as an alternative source of protein to handle ever-increasing food security problems, the present study was intended to study the effect of dephenolization and pH on the biochemical and functional properties of the Meghalayan cherry kernel protein isolate. Furthermore, amino acid profiling, nutritional properties, and amino acid score for infants and adults according to FAO (2013) were also investigated.
2 Material and methods
2.1 Procurement of raw materials
Mature Meghalayan cherry fruits were collected during September 2019 from Shillong market, Meghalaya (India). The kernel was collected by cracking the seed shell and dried in a tray drier (SICO House, Patiala, India) at 45 ± 2 °C. Dried kernels were then milled in a laboratory mill (Agrosaw Pvt. Ltd. Haryana, India), and to obtain uniformity in particle size, kernel powder was sieved through a 60 mesh size sieve. Meghalayan cherry kernel powder was kept in airtight containers at 4 °C till analysis. Analytical grade reagents were used for the analysis and were procured from Sigma-Aldrich, Mumbai, India.
2.2 Sample preparation
The defatting of kernel powder was done by soxhlet extraction using hexane as a solvent for 5 h. The dried defatted kernel powder was subjected to detoxification by soaking (1:10 w/v) in water for 12 h, heating at 60 °C for 30 min, drained and dried in an oven at 45 ± 2 °C. The defatted and detoxified kernel powder was kept in airtight containers at 4 °C till analysis.
2.3 Phenol compound extraction
The conventional solvent extraction method was used to extract phenolic compounds from Meghalayan cherry kernel. The detoxified sample was dispersed in 60% (v/v) methanol in the ratio of 1:20 (w/v) and kept for 2 h with continuous stirring. The suspension obtained was filtered, and the extraction process was repeated four times. The dephenolized kernel powder thus obtained was then dried in a hot air oven overnight at 45 ± 2 °C. The two portions of cherry kernel powder with polyphenols and cherry kernel powder without polyphenols were obtained.
2.4 Isolation of protein and process standardization
Cherry kernel protein isolation was done by an alkaline extraction process followed by isoelectric precipitation [8]. Five different pH (8.5, 9.5, 10.5, 11.5, and 12.5) solutions of cherry kernel powder with polyphenols and cherry kernel powder without polyphenols were made with 2 N NaOH. The ratio of powder to solvent was kept at 1:10. The produced slurries were heated at 40 °C temperature with continuous stirring in a hot water bath for 2 h. After heating, the protein-containing slurry was centrifuged (Eppendorf, India) at 8000 × g for 20 min, and the supernatant was collected. The protein precipitation was carried out by inducing the collected supernatant at its isoelectric pH of 4.5 with 2 N HCl. The precipitated protein was collected by centrifugation process at 8000 × g for 20 min, followed by freeze-drying (using freeze dryer (Allied frost, India) of the collected sample and kept in storage at 4 °C until further analysis. The protein isolated from the cherry kernel with polyphenols and without polyphenols was designated as MCKPI-N and MCKPI-DP. The standardization of protein isolate was done based on yield, purity, and color as many food manufacturers request protein isolates with high yield and purity with light color.
2.5 Determination of protein yield and purity
The yield of MCKPI-N and MCKPI-DP extracts acquired at various pH was calculated as per the method of Kaushik et al. [9] as follows:
where “P” is the weight (g) of protein isolate powder acquired after the extraction process and freeze-drying and “S” is the weight (g) of seeds powder obtained for the protein extraction. For determining the purity of protein isolates, the AOAC [10] method was adopted.
2.6 Color measurement
The color of protein isolates, MCKPI-N and MCKPI-DP, was measured by Hunter colorimeter, Model D25, optical Sensor (Hunter Associates Laboratory Inc., Reston, VA., USA) on account of CIE L*, a*, and b* values. A cell of glass carrying protein isolates was put above the source of light covered with a white plate and L*, a*, and b* color values were noted down.
2.7 Characterization of protein isolates
2.7.1 Chemical characteristics of protein isolates
To determine moisture, protein (nitrogen conversion factor is 6.25) and ash content of MCKPI-N and MCKPI-DP, the standard methods of AOAC [10] were used. The total phenolic content (TPC) was determined by Folin-Ciocalteu reagent method [11]. Gallic acid was taken as standard and absorbance was taken at 765 nm. The total phenol content was expressed as mg of gallic acid per g (mgGAE/g) of extract.
2.7.2 Wettability of protein isolates
Wettability of MCKPI-N and MCKPI-DP was ascertained by the static wetting test used by Mir et al. [8] with some modifications. A 250-ml beaker carrying 100 ml of distilled water was used and a glass funnel with its open end blocked by a test tube was kept above the beaker with the help of a ring stand. Protein isolate sample (1 g) was put in the funnel, and a test tube was taken out to allow the protein powder sample to fall inside the beaker and a stopwatch was used to record the time. Time was noted down for the powder sample to become thoroughly wet (when all the powder particles crept into the water’s surface).
2.7.3 Effect of dephenolization and pH on functional properties of Meghalayan cherry kernel protein isolates
2.7.3.1 Solubility
The solubility of MCKPI-N and MCKPI-DP was determined by Achouri et al. [12]. Protein isolate dispersions (2 g/100 ml) were prepared with distilled water at five different pH (3.5–11.5) and mixed for 1 h using a magnetic stirrer. The solution was then centrifuged at 3000 × g for 30 min. The soluble protein contents present in the supernatant were measured by Kjeldhal method, and the following equation calculated the solubility:
2.7.3.2 Emulsifying activity and emulsion stability
Emulsifying activity (EA) and emulsion stability (ES) of MCKPI-N and MCKPI-DP were determined by the method of Lawal et al. [13] with slight modifications. Briefly, 5 ml of 2% (w/v) protein solution at different pH (3.5–11.5) was homogenized with 5 ml soybean oil. The emulsions were then centrifuged for 5 min at 3500 × g. The height of emulsified layer and that of the total contents in the tube was measured. The emulsifying activity (EA) was calculated as:
For the estimation of emulsion stability, the samples were heated at 80 °C for 30 min. It was then centrifuged at 3500 × g for 5 min and emulsion stability was calculated as:
2.7.3.3 Water-binding capacity
The procedure of Malik and Saini [14] based on the principle of quantitative indicators of the amount of water retained/entrapped within a protein matrix, was applied to estimate the water-binding capacity of MCKPI-N and MCKPI-DP, with slight changes. Protein isolate (1 g) was mixed with 10 ml of distilled water, and the desired pH was adjusted to 3.5–11.5, followed by stirring for 5 min. The mixture was left to stand for 30 min and then centrifuged at 5000 × g for 30 min. The supernatant was disposed of, and the tube was upturned for 25 min at 45 °C to siphon off the remaining water from the protein sediment. The following equation calculated the water-binding capacity:
where “a” is the weight (g) of the tube with the protein isolate and absorbed water. “b” is the weight (g) of the tube with protein isolate. “c” is the weight (g) of the protein isolate.
2.7.3.4 Dispersibility
The procedure of Kullarni et al. [15] was applied to calculate the dispersibility of protein isolated with slight changes. Three grams each of MCKPI-N and MCKPI-DP were dispersed distinctly in 30 ml of distilled water in measuring cylinders, and pH (3.5–11.5) was adjusted either with 0.5 N NaOH or 0.5 N HCl. The mixtures were vigorously stirred and allowed to settle for 2 h. The volumes of settled particles acquired were measured, and dispersibility was calculated as per the following equation:
2.7.3.5 Foaming capacity and foam stability
Foaming capacity and stability were calculated as per the procedure of Fekria et al. [16]. Briefly, 3 g of MCKPI-N and MCKPI-DP were dispersed in 100 ml distilled water and the solution pH was altered from 3.5 to 11.5. The solution was then whipped for 5 min using high-speed mixer blender (Remi, India Pvt. Ltd.). The whipped solution was replaced in a measuring cylinder and foam volume was instantly measured to determine the foaming capacity. The foam volume was measured after 15, 30, 45, and 60 min to calculate the volume change with time. The percentage foam volume that remained after each time interval depicted the foam stability after that time interval.
2.8 Amino acid composition
The hydrolysis of MCKPI-N and MCKPI-DP was done at 110 °C in 6 M HCl for 24 h and then allowed to cool to room temperature. The visible sediments in the hydrolysate were removed by filtration, and the dried residue was dissolved in citrate buffer after flash evaporation. A known sample volume was injected into an automatic amino acid analyser (Biochrom, USA) to estimate the amino acid profile in the samples. The analysis was carried out in triplicate, and the amino acid composition was reported in g/kg protein.
2.9 Estimation of nutritional quality of Meghalayan cherry kernel protein isolate
The composition of amino acids of MCKPI-N and MCKPI-DP were used for the estimation of various nutritional parameters studied as follows:
2.9.1 Protein effeciency ratio (PER)
Protein efficiency ratio (PER) was calculated by following three regression equations given by Alsmeyer et al. [17]:
2.9.2 Essential amino acid index
Essential amino acid index (EAAI) was calculated based on amino acid composition standard of whole egg protein using the method of Oser [18] as per the equation:
where “a” is the amino acid in test sample and “b” is the amino acid in reference protein sample.
2.9.3 BV PER1
Biological value was estimated as per the equation of Oser [18].
2.9.4 Nutritional Index
Nutritional index (NI) was estimated as a function of essential amino acid index and total protein, as used by Ijarotimi [19]:
2.9.5 Amino acid score (chemical scoring)
Amino acid score was estimated as the ratio of observed value of essential amino acid to the reference pattern as provided by FAO [20].
2.10 Statistical analysis
A two-way analysis of variance test (ANOVA) was performed using the software (Statistica-7 (M/s. Stat Soft Inc., OK, USA) to evaluate the effect of pH and dephenolization on functional characteristics of cherry kernels protein isolates. Duncan’s test determined significant differences between means at the 5% significance level. Data reported are mean ± SD of triplicate observation.
3 Results and discussion
3.1 Isolation of protein and process standardization
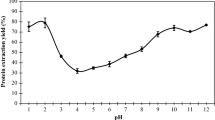
Table 1 shows the parameters used for the process standardization of protein isolate from Meghalayan cherry kernel. Protein isolate from Meghalayan cherry kernel without polyphenols showed higher yield and purity of protein isolate than cherry kernel with polyphenols. This might be related to the interaction of polyphenols with proteins at basic pH during the extraction process, which caused the decrease of protein content in the extracted protein isolates [17]. Similar results were found by Malik and Saini [14] and Subhashi et al. [21]. The yield of protein isolate for both MCKPI-N and MCKPI-DP increased with an increase in pH (8.5–12.5) and varied from 9.82 ± 0.02 to 15.21 ± 0.02% and 10.37 ± 0.04 to 16.08 ± 0.03%, respectively. This was because of the fact that protein isolate’s solubility gets raised towards higher alkaline conditions of the isolation medium. In higher alkaline conditions, the proteins tend to become more negatively charged because of the ionization of the carboxylic groups and de-protonation of the amine group, which increases the electrostatic repulsion between negatively charged proteins. Thus, increases the solubility of proteins with increase in pH values [22]. Given outcomes agrees with those of Mir et al. [8]. Even so, the purity of protein isolate decreased from 87.03 ± 0.02 to 77.25 ± 0.03% and 91.71 ± 0.04 to 80.15 ± 0.02% for MCKPI-N and MCKPI-DP, respectively, with an increase in pH. The protein content of MCKPI-N and MCKPI-DP was comparable to that of other protein isolates, i.e., 83.96% in cranberry bean protein isolate [23], 89.7% in soyabean protein isolate [24], and 89.42% in sunflower kernel protein isolate [14].
A significant difference was seen in color values for both MCKPI-N and MCKPI-DP and also with change in pH of solution. MCKPI-N showed lower L* value but higher a* and b* value at all the pH for MCKPI-DP. This was because the interaction of phenols-protein and oxidation of phenolic components to o-quinones at basic pH during protein extraction imparts darker color to protein isolate. Whereas, increase in pH for both MCKPI-N and MCKPI-DP showed the highest value of L* (78.30 for CKPI-N and 81.09 for CKPI-DP) at pH 10.5 decreased further. The value of a* and b* varied from 2.01–4.02 and 14.22–19.72 for MCKPI-N and 2.12–3.97 and 10.36–17.15 MCKPI-DP, respectively. Similar results were reported by Salgado et al. [25] and Xu et al. [26] in case of sunflower protein concentrate and isolate and canola protein isolate, respectively. pH 10.5 was found to be suitable for extracting protein from Meghayalan cherry kernel based on desirable yield, purity of protein, and adequate color.
3.2 Characterization of protein isolate
3.2.1 Chemical characteristics of natural and dephenolized Meghalayan cherry kernel protein isolate
Table 2 shows the chemical composition of protein isolate from MCKPI-N and MCKPI-DP. The extraction of Meghalayan cherry kernel with methanol significantly reduced the phenolic content from 3.86 ± 0.04 mgGAE/g DW to 0.19 ± 0 mgGAE/g DW, which showed 95% decrease in total phenolics. MCKPI-N (83.50 ± 0.01%) had lower protein content than MCKPI-DP (88.81 ± 0.03%) due to the interaction of polyphenols with proteins at basic pH 10.5 during extraction. Similar results were reported by Subasi et al. [21] and Malik and Saini [17] in case of sunflower kernel. Significant difference was also observed in the case of ash in which MCKPI-N showed higher values than MCKPI-DP.
3.2.2 Wettability of natural and dephenolized Meghalayan cherry kernel protein isolate
The dispersion of protein powders to make aqueous beverages depends on their wettability. Fluid can disperse or adhere to a solid surface surrounded by other fluids. Density, porosity, particle size, surface charge, surface area, and presence of amphipathic substances between the surface of the powder and penetrating fluid affects the wettability. Higher wettability was observed in the case MCKPI-DP (22 ± 0.90 min) as compared to MCKPI-N (18 ± 0.81 min). It might be because reducing polyphenols reduces the hydrophobicity of the protein, which results in proteins wetting faster [17].
3.2.3 Effect of dephenolization and pH on functional properties of protein isolate of Meghayalan cherry kernel
Solubility
Solubility of protein is an important factor for food systems as it impacts the other functional characteristics such as emulsifying and foaming capacity and works as a convenient indicator of denaturation and interactivity of proteins. The solubility of a protein depends on pH, ionic strength, temperature, concentration, and the presence of other molecules [27]. A significant difference was seen in protein solubility for both MCKPI-N and MCKPI-DP with a change in pH. The lowest protein isolate solubility was seen at pH 5.5 near the isoelectric point and increased steadily on both sides of the pH. It might be because of no net charge (neutral charge) on protein at its isoelectric point, which results in that there is not any repulsive force between proteins which leads to protein–protein interaction and hence protein aggregates [28]. This aggregation of proteins leads to lesser solubility at isoelectric pH. At higher alkaline pH, the protein solubility increases as seen in Table 3. This occurred due to increased negative charge which will lead to repulsive electrostatic interactions between protein molecules which hinder their aggregation and hence increased solubility. Malik and Saini [14] also showed higher protein isolate solubility at pH 11 and lowest at pH 5 in the case of sunflower seed and kernel protein isolate.
In the case of MCKPI-N and MCKPI-DP, the protein isolate solubility was seen higher in cherry kernel without polyphenols than cherry kernel with polyphenols. After interactivity with phenolic components, the protein denaturation can be accredited to the decreasing solubility of modified proteins by phenols [29]. Moreover, the reaction of protein with phenolic components goes along with a corresponding blocking of hydrophilic groups and introducing an aromatic ring structure, resulting in the hydrophobic nature of protein and subsequent reduction in protein solubility [30].
Emulsion activity and stability
During emulsion formation, the proteins are adsorbed in a tightly packed layer at the surface of oil droplets [31]. The emulsion activity of the protein is represented by absorption of protein on the oil–water interface, whereas its ability to form stable emulsion without flocculation represents the emulsion stability. The effect of dephenolization and pH on the emulsion activity and emulsion stability is seen in Table 3. Increase in the pH showed increased emulsion activity, with the highest value for MCKPI-N and MCKPI-DP was 41.17 ± 0.53% and 47.88 ± 0.49%, respectively, at pH 11.5. Similar increase in emulsion activity with pH were shown by Xu et al. [32] and Ulloh et al. [33]. The same trend was seen in emulsion stability where emulsion stability varied from 36.59 ± 0.37 to 46.55 ± 0.47% for MCKPI-N and 37.66 ± 0.58 to 53.89 ± 0.49% for MCKPI-DP. The change in emulsion activity and emulsion stability of protein isolate with change in pH is due to a change in hydrophilic-lipophilic balance directly connected to emulsion properties [34]. Additionally, increased pH also increases the columbic interactions between neighboring droplets coupled with hydration of charged molecules, thus increasing the emulsifying properties [8]. The emulsion activity was comparable with sunflower kernel protein isolate (48.86–53.35%) [14] and sour cherry kernel protein isolate [35] whereas lower emulsion activity was reported as compared to Chenopodium album protein isolate (55.09–64.70%) [8].
MCKPI-DP showed higher emulsion activity and emulsion stability as compared to MCKPI-N. This is because of a decrease in solubility and hydrophobicity of protein isolate due to phenolics. As increased solubility and hydrophobicity is the precondition for a good emulsifier. The decreased solubility blocks the fast movement of peptides to the oil–water interface. The reduction in the surface hydrophobicity of protein isolate may decrease the capacity of protein to localize at the oil–water interface. Phenolic compounds also enhance protein aggregation, which reduced the hydrophobicity and thus reduce the unfolding at the interface [14].
Water-binding capacity
Table 3 shows the effect of dephenolization and pH on the water holding capacity of cherry kernel protein isolate. The protein isolate (MCKPI-DP) formed after phenolic removal showed lower WBC compared to protein isolate (MCKPI-N) with phenolics. This occurred at all the pH values. It might be because of the reduction in hydrophobicity of protein upon interaction with phenolics. Also, the phenolics present in the protein isolate have many binding sites for water, where it gets through hydrogen bonding and enhances the water binding capacity [14].
The pH had a significant effect on the WBC of protein isolate in both cases MCKPI-N and MCKPI-DP. With the increase in pH, the WBC increases from 202.86 ± 0.80 to 242.28 ± 0.59% for MCKPI-DP and 214.65 ± 0.59 to 258.54 ± 0.56% for MCKPI-N. Similar outcomes were acquired by Mir et al. [8] and Malik and Saini [14]. At isoelectric pH, the lowest WBC was seen in both cases because of strong intermolecular interactions to form aggregates, leading to less interaction and less WBC [36]. The different WBC at different pH might be because of conformational characteristics in protein structure, which influences the binding sites for water. Cherry kernel protein isolate showed higher WBC than pea and soy protein isolate [37], chenopodium album [8], and sunflower meal [14] protein isolate. Higher WBC of cherry kernel protein isolate proposes its appropriateness for enhancing the viscosity of food products.
Dispersibility
Dispersibility is the reconstitution capacity of proteins and is mainly affected by the temperature, ionic composition, pH, and the degree of agitation of solvent. Increased value of dispersibility amplifies other functional characteristics like emulsifying and foaming ability. A significant increase in dispersibility was seen with the increase in pH from 3.5 to 11.5 for MCKPI-N and MCKPI-DP. The dispersibility value for MCKPI-N and MCKPI-DP varied from 65.44 ± 0.80 to 81.13 ± 0.66% and 66.83 ± 0.63 to 86.74 ± 0.55%, respectively, with the highest value was reported at pH 11.5. As solubility is directly connected to dispersibility, it increased with increased pH as seen in the case of solubility. Similarly, dispersibility increases with phenol removal because solubility increases with the removal of phenols.
Foam capacity and stability
Foam formation and characteristics are affected by the type of protein, preparation procedure, composition, solubility, concentration, pH, salts present, and hydrophobic interactions. Foaming capability depends on the diffusion of soluble proteins to the air–water interface, faster conformational modifications and rearrangement at the interface [27]. Proteins with more foaming capability and foam stability are needed in many food systems and are mainly used in food applications for aeration and whipping. A significant difference was seen between foaming capacity and foam stability in the case of both MCKPI-N and MCKPI-DP (Table 4). With the increase in pH the foam capacity increased significantly, and the highest value was obtained at pH 11.5 for both MCKPI-N and MCKPI-DP, i.e., 51.33 ± 0.36% and 57.80 ± 0.53%, respectively. Higher foaming capacity was observed in MCKPI as compared to sour cherry kernel protein isolate (35 ± 3.4%) [35], and sunflower kernel protein isolate (57.31 ± 0.50%) [14]. Whereas similar increase in foam capacity with pH was seen by Das et al. [38] and Mir et al. [8]. Aluko and Yada [39] showed that at increased pH values, the total charge on proteins is raised, which effects in weakening of hydrophobic interactivity but enhances the flexibility of proteins. Higher protein flexibility helps protein diffuse rapidly to the air–water interface to encapsulate air, resulting in increased foam formation. The lowest foam capacity and stability after 60 min were found at pH 5.5 due to protein behavior at isoelectric pH. Damodaran [27] reported that only soluble protein fractions are involved in the foam formation and since near the isoelectric point, the concentration of these soluble fractions are very low and thus amount of foam formation will be less. Similarly, like foam capacity, the foam stability also increased with an increased pH, but as time progresses stability of foam decreases significantly for both MCKPI-N and MCKPI-DP. Similar results were obtained for sour cherry kernel, sunflower seed protein isolate [17, 35].
The presence of polyphenols directly affects the foaming capability and stability due to impaired solubility and hydrophobicity of proteins which decreases its foaming properties, and thus, natural protein isolate has lower foam capacity compared to dephenolized protein isolate.
3.3 Amino acid profiling of protein isolates
Table 5 present the amino acids profile of protein isolates from natural and dephenolized Meghayalan cherry kernel. Removing phenolics caused significant differences in amino acids composition in MCKPI-N and MCKPI-DP. This might be due to polyphenols (mainly phenolic acids and flavonoids) blocking amino acid residues and decreasing their availability. For example, phenolic compounds in alkaline conditions react with oxygen to form phenolate ions, leading to the formation of semiquinones. These semiquinones react with an amino group of lysine and decrease their availability. Similarly, the indole structure of tryptophan is involved in the reaction with polyphenols [40].
Results showed that both MCKPI-N and MCKPI-DP are a rich sources of essential amino acids where sulfur-containing amino acids like methionine and cysteine are low in an amount, which may be because of solubility of albumins in water and sulfur-containing amino acids are partially removed due to washing of kernel powder before alkaline treatment [41]. Higher essential amino acids were found in the case of MCKPI-DP. Furthermore, higher content of both aspartic and glutamic acid (i.e., 38.9 and 49.8 g/kg and 172.1 and 181.5 g/kg) was found in MCKPI-N and MCKPI-DP, respectively. Higher content of these amino acids indicates the acidic properties of both protein isolates. Leucine, valine, and isoleucine are considered as essential branched amino acids and are required for building the muscles and both MCKPI-N and MCKPI-DP exhibited higher content of all these amino acids. The leucine, valine, and isoleucine content in MCKPI-N and MCKPI-DP was 74.6, 89.6, and 25.6 g/kg and 71, 81.9, and 30.1 g/kg, respectively. Some of the amino acids present in Meghayalan cherry kernel protein isolate like tyrosine, methionine, histidine, and lysine have been shown to play a specific role in improving the antioxidant property of peptides [42]. The threonine content in MCKPI-N and MCKPI-DP was 38.1 and 40.1 g/kg, respectively, which helps maintain the positive nitrogen balance in body as it helps in lowering nitrogen excretion [43].
3.4 Nutritional quality of protein isolates
The nutritional profile of protein isolate based on the composition of amino acids is presented in Table 6. The protein quality is assessed as per the basis of its amino acid composition as compared to reference pattern of amino acids. The nutritional quality of protein is measured as per the basis of amino acid score, protein efficiency ratio (PER), biological value, nutritional index, essential amino acid index, and ratio of essential amino acid to total amino acid (E/T %). The theoretical protein efficiency ratio that bears a good relationship to real PER values was calculated depending upon the concentration of leucine and proline (PER1), leucine and tyrosine (PER2), and methionine, leucine, histidine, and tyrosine (PER3). Generally, a PER value less than 1.5 approximately shows lower protein quality; a value between 1.5 and 2.0 as medium quality, while a value more than 2.0 is of good quality [44]. The Meghayalan cherry protein isolate has a PER value higher than 2 for both MCKPI-N and MCKPI-DP and is considered high-quality protein. The present result showed higher PER value in Meghayalan cherry protein isolate than cowpea (1.21), pigeon pea (1.82), and peanut (1.45–1.76) [45]. However the results were comparable with pumpkin seed protein isolate (2.70) [46]. Protein efficiency ratio is directly connected to the leucine contents; therefore, any change in the leucine content will affect the protein efficiency ratio. The percentage of E/T for MCKPI-N and MCKPI-DP was seen to be 37.63 ± 0.01% and 41.76 ± 0.01%, respectively, which is almost similar to lotus and pumpkin seed proteins as well as soya protein [46, 47]. Higher E/T % was found in dephenolized Meghalayan cherry kernel protein isolate because of the increased amount of essential amino acid present (Table 6).
Biological value (BV) is the percentage of the protein which is used as the proteins of the human body. The biological value of better nutritional quality protein foods is found to be in between 70 and 100% [18]. The biological value has given a measurement of the body's efficiency to utilize the protein consumed in the diet. A food with more BV value connected to the good supply of EAAs [48]. Our study showed that dephenolized protein isolate have a higher biological value (74.22 ± 0.93) than natural protein isolate (66.06 ± 0.84) and thus can be incorporated in efficient protein diets. The nutritional index is the function of essential amino index and protein content of the sample. It is known that higher the protein content in the sample more will be the nutritional index. The nutritional index of MCKPI-N and MCKPI-DP was 63.35 and 65.82, respectively.
Egg was the first to be assigned a chemical score as it was considered nutritionally complete [49]. The EAAI may be defined as the geometric mean value of EAA content in a protein compared with a corresponding amount in the reference whole egg protein. The protein quality rating was observed when all of the individual chemical scores were used to calculate EAA (Table 5). For the essential amino acid index, it has been observed that protein-based food products are of good quality when their essential amino acid index is greater than 90 and are considered useful when their essential amino acid index ranges between 70 and 80. Also, the nutritional quality of a food is entitled as insufficient when its essential amino acid index is below 70 [34]. Both natural and dephenolized protein isolate of Meghalayan cherry kernel have EAAI higher than 70, i.e., 71.34 ± 0.77 and 78.82 ± 0.85, respectively, and are considered as useful protein isolates. Higher EAAI was found dephenolized protein isolate due to higher essential amino acids.
The branched-chain amino acids (BCAA) (e.g., valine, leucine, and isoleucine) are involved in promoting the anabolic processes in protein metabolism, helpful in preventing proteins from degradation, associated with protein synthesis and also promoting muscle growth after physical activity [50]. An increase in the leucine content improves protein synthesis and or retardation in muscle degradation. Therefore, diets containing more leucine to isoleucine ratio can provide more endurance than natural diets. Higher leucine to isoleucine ratio was observed in dephenolized protein isolate (2.91 ± 0.03) as compared to natural protein isolate (2.37 ± 0.02).
3.5 Amino acid score for protein quality evaluation
The protein quality is based on its capacity to provide an appropriate patterns of dietary indispensable amino acids [20]. The amino acid score was computed based on its composition to know the limiting amino acid. The amino acid score for infants and adults was shown in Fig. 1. The corresponding amino acid is considered as limiting amino acid if its amino acid score is less than 100. Based on the amino acid score, isoleucine has been reported as limiting amino acid for both MCKPI-N and MCKPI-DP in infants, whereas for adults, only MCKPI-DP showed isoleucine as the limiting amino acid. The MCKPI-N and MCKPI-DP presented higher protein quality than commercial protein supplements from pea protein isolate as methionine + cysteine, which are sulfur containing amino acids with powerful antioxidant properties, present in higher amounts in Meghalayan cherry protein isolate as compared to legumes which lack sulfur-containing amino acids. These results showed that Meghalayan cherry protein isolate can be utilized as a protein supplement source.
4 Conclusion
The significant effect of dephenolization was seen in the functional, physicochemical and nutritional properties of Meghayalan cherry kernel protein. Increased pH increases the yield of protein isolate, but the purity (protein content) of protein isolate decreased significantly. The color of protein isolate was also improved after the removal of phenolics from kernel powder. The significant increase in functional properties for both MCKPI-N and MCKPI-DP was seen with increase in pH. However, MCKPI-DP showed improved functional properties as compared to MCKPI-N. In terms of amino acid composition, the significant difference was observed between MCKPI-N and MCKPI-DP with a higher value of essential amino acids found in the case of MCKPI-DP. However, both MCKPI-N and MCKPI-DP showed good amount of essential amino acids but based on amino acid score, both the protein isolate were limiting in isoleucine. The nutritional values (PER, EAAI, BV, and nutritional index) of MCKPI-DP were higher than MCKPI-N and are considered as good quality useful proteins. On the basis of amino acid score for adult and infants, isoleucine was found to be limiting amino acid for both MCKPI-N and MCKPI-DP. These finding showed that dephenolized Meghayalan cherry kernel proteins isolate has improved functional properties with good balance of essential amino acids. Thus, it could be utilized as a novel protein source in food systems as well as to formulate various health foods. In the future, the effect on dephenolization on in vitro protein digestibility, thermal and structural properties of MCKPI need to be explored in order to better utilize it in various food products.
References
Henchion M, Hayes M, Mullen AM, Fenelon M, Tiwari B (2017) Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Foods 6(7):53
Deng Y, Huang L, Zhang C, Xie P, Cheng J, Wang X, Li S (2019) Physicochemical and functional properties of Chinese quince seed protein isolate. Food Chem 283:539–548
Kashyap P, Riar CS, Jindal N (2021) Optimization of ultrasound assisted extraction of polyphenols from Meghalayan cherry fruit (Prunus nepalensis) using response surface methodology (RSM) and artificial neural network (ANN) approach. J Food Meas Charact 15(1):119–133
Kashyap P, Riar CS, Jindal N (2022) Polyphenol bio-accessibility and antioxidant activity of in vitro digested ultrasound-assisted Meghalayan cherry (Prunus nepalensis) pomace extract. . Biomass Convers Biorefnery: 1–15
González-Pérez S, Vereijken JM (2007) Sunflower proteins: overview of their physicochemical, structural and functional properties. J Sci Food Agric 87(12):2173–2191
Sabir MA, Sosulski FW, Kernan JA (1974) Phenolic constituents in sunflower flour. J Sci Food Agric 22(4):572–574
Chavan UD, McKenzie DB, Shahidi F (2001) Functional properties of protein isolates from beach pea (Lathyrus maritimus L). Food Chem 74(2):177–187
Mir NA, Riar CS, Singh S (2019) Effect of pH and holding time on the characteristics of protein isolates from Chenopodium seeds and study of their amino acid profile and scoring. Food chem 272:165–173
Kaushik P, Dowling K, McKnight S, Barrow CJ, Wang B, Adhikari B (2016) Preparation, characterization and functional properties of flax seed protein isolate. Food Chem 197:212–220
AOAC (2006) Official methods of analysis, 18th edn. Association of Official Analytical Chemists, Arlington, VA
Kazemi M, Karim R, Mirhosseini H, Hamid AA (2016) Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: punicalagin and hydroxybenzoic acids. Food Chem 206:156–166
Achouri A, Nail V, Boye JI (2012) Sesame protein isolate: fractionation, secondary structure and functional properties. Food Res Int 46:360–369
Lawal OS, Adebowale KO, Adebowale YA (2007) Functional properties of native and chemically modified protein concentrates from bambarra groundnut. Food Res Int 40:1003–1011
Malik MA, Saini CS (2017) Polyphenol removal from sunflower seed and kernel: effect on functional and rheological properties of protein isolates. Food Hydrocoll 63:705–715
Kullarni DK, Kullarni DN, Ingle UM (1991) Sorghum malt-based weaning food formulation: preparation, functional properties and nutritive value. Food Nutr Bull 13:324–327
Fekria AM, Isam AMA, Suha OA, Elfadil EB (2012) Nutritional and functional characterization of defatted seed cake flour of two Sudanese groundnut (Arachis hypogaea) cultivars. Int Food Res J 19:629–637
Alsmeyer RH, Cunningham AE, Happich ML (1974) Equations predicting PER from amino acid analysis. Food Technol 28:34–40
Oser BL (1959) An integrated essential amino acid index for predicting the biological value of proteins. In: Albanese AA (ed) Protein and amino acid nutrition. Academic Press, New York, pp 295–311
Ijarotimi OS (2012) Influence of germination and fermentation on chemical composition, protein quality and physical properties of wheat flour (Triticum aestivum). J Cereals OilSeeds 3:35–47
FAO (2013) Findings and recommendations of the 2011 FAO Expert Consultation on protein quality evaluation in human nutrition. In: Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation. FAO Food and Nutrition Paper 92. Food and Agriculture Organization of the United Nations, Rome, Italy, chapter 4, 29
Subaşı BG, Casanova F, Capanoglu E, Ajalloueian F, Sloth JJ, Mohammadifar MA (2020) Protein extracts from de-oiled sunflower cake: structural, physico-chemical and functional properties after removal of phenolics. Food Biosci 38:100749
Gao Z, Shen P, Lan Y, Cui L, Ohm JB, Chen B, Rao J (2020) Effect of alkaline extraction pH on structure properties, solubility, and beany flavor of yellow pea protein isolate. Food Res Int 131:109045
Rui X, Boye JI, Ribereau S, Simpson BK, Prasher SO (2011) Comparative study of the composition and thermal properties of protein isolates prepared from nine Phaseolus vulgaris legume varieties. Food Res Int 44(8):2497–2504
Tang CH (2008) Thermal denaturation and gelation of vicilin-rich protein isolates from three Phaseolus legumes: a comparative study. LWT-Food Sci Technol 41(8):1380–1388
Salgado PR, Molina Ortiz SE, Petruccelli S, Mauri AN (2011) Sunflower protein concentrates and isolates prepared from oil cakes have high water solubility and antioxidant capacity. J Am Oil Chem Soc 88(3):351–360
Xu L, Diosady LL (2002) Removal of phenolic compounds in the production of high-quality canola protein isolates. Food Res Int 35(1):23–30
Damodaran S (1997) Food proteins: an overview. In: Damodaran S, Paraf A (eds) Food proteins and their applications. Marcel Dekker, New York, pp 1–24
Singh N, Kaur M, Sandhu KS (2005) Physicochemical and functional properties of freeze-dried and oven dried corn gluten meals. Dry Technol 23:975–988
Ali M, Homann T, Khalil M, Kruse HP, Rawel H (2013) Milk whey protein modification by coffee-specific phenolics: effect on structural and functional properties. J Agric Food Chem 61(28):6911–6920
Rohn S, Rawel HM, Röber M, Kroll J (2005) Reactions with phenolic substances can induce changes in some physico-chemical properties and activities of bromelain–the consequences for supplementary food products. Int J Food Sci Technol 40(7):771–782
Dickinson E (2010) Flocculation of protein-stabilized oil-in-water emulsions. Colloids Surf B: Biointerfaces 81(1):130–140
Xu D, Li C, Zhuo Z, Ye M, Fu B, Pu B (2020) Physicochemical and emulsifying properties of protein extracted from Zanthoxylum armatum seed kernel. Iranian J Sci Technol Trans A: Sci 44(1):65–73
Ulloa JA, Villalobos Barbosa MC, Resendiz Vazquez JA, Rosas Ulloa P, Ramírez Ramírez JC, Silva Carrillo Y, González Torres L (2017) Production, physico-chemical and functional characterization of a protein isolate from jackfruit (Artocarpus heterophyllus) seeds. CyTA-J Food 15(4):497–507
Sathe SK, Deshpande SS, Salunkhe DK (1982) Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrates. J Food Sci 47(2):491–497
Çelik M, Güzel M, Yildirim M (2019) Effect of pH on protein extraction from sour cherry kernels and functional properties of resulting protein concentrate. J Food Sci Tech 56(6):3023–3032
Deng Q, Wang L, Wei F, Xie B, Huang F, Huang W, Shi J, Huang Q, Tian B, Xue S (2011) Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem 124:1458–1465
Fernández-Quintela A, Macarulla MT, Del Barrio AS, Martínez JA (1997) Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum Nutr 51(4):331–341
Das D, Mir NA, Chandla NK, Singh S (2021) Combined effect of pH treatment and the extraction pH on the physicochemical, functional and rheological characteristics of amaranth (Amaranthus hypochondriacus) seed protein isolates. Food Chem 353:129466
Aluko RE, Yada RY (1995) Structure-function relationships of cowpea (Vigna unguiculata) globulin isolate: influence of pH and NaCl on physicochemical and functional properties. Food Chem 53(3):259–265
Kroll J, Rawel HM (2001) Reactions of plant phenols with myoglobin: influence of chemical structure of the phenolic compounds. J Food Sci 66(1):48–58
Pastor-Cavada E, Juan R, Pastor JE, Alaiz M, Vioque J (2010) Protein isolates from two Mediterranean legumes: Lathyrus clymenum and Lathyrus annuus Chemical composition functional properties and protein characterisation. Food Chem 122(3):533–538
Udenigwe CC, Aluko RE (2012) Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci 77(1):R11–R24
Sibian MS, Saxena DC, Riar CS (2017) Effect of germination on chemical, functional and nutritional characteristics of wheat, brown rice and triticale: a comparative study. J Sci Food Agric 97(13):4643–4651
Anand TP, Chellaram C, Chandrika M, Rajamalar CG, Parveen AN, Shanthini F (2013) Nutritional studies on marine mollusk Pleuroploca trapezium (Gastropoda: Fasciolariidae) from Tuticorin coastal waters. J Chem Pharm Res 5(4):16–21
Salunkhe DK, Kadam SS (1989) Handbook of world food legumes: nutritional chemistry, processing technology, and utilization. CRC Press, Boca Raton, Flourida
Vinayashree S, Vasu P (2021) Biochemical, nutritional and functional properties of protein isolate and fractions from pumpkin (Cucurbita moschata var Kashi Harit) seeds. Food Chem 340:128177
Zeng HY, Cai LH, Cai XL, Wang YJ, Li YQ (2013) Amino acid profiles and quality from lotus seed proteins. J Sci Food Agric 93(5):1070–1075
Hoffman JR, Falvo MJ (2004) Protein–which is best? J Sports Sci Med 3(3):118
Babji AS, Fatimah S, Abolhassani Y, Ghassem M (2010) Nutritional quality and properties of protein and lipid in processed meat products–a perspective. Int Food Res J 17(1):35–44
Pietrysiak E, Smith DM, Smith BM, Ganjyal GM (2018) Enhanced functionality of pea-rice protein isolate blends through direct steam injection processing. Food Chem 243:338–344
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kashyap, P., Singh Riar, C. & Jindal, N. Effect of dephenolization and pH on functional properties, amino acid profile, and nutritional characteristics of protein isolate from Meghalayan cherry (Prunus nepalensis) kernel. Biomass Conv. Bioref. 14, 4883–4895 (2024). https://doi.org/10.1007/s13399-022-02740-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02740-6