Crude methanol extracts of fifteen ethnopharmacological plants were prepared by simple maceration procedure for studying their phytochemical and antimicrobial properties, cytotoxicity, and protein kinase inhibition activity. Among all, the maximum gallic acid equivalent (GAE) total phenolic content and quercetin equivalent (QE) total flavonoid content were found in Ficus carica (8.6 and 7.1 μg/mg DW, respectively). F. carica also exhibited significantly higher (P < 0.05) antiradical (DPPH) potential (IC50 = 12.6 μg/mL). Mentha piperita exhibited maximum total antioxidant capacity and reduction potential when expressed as equivalent to ascorbic acid (12.82 and 5.11 μg/mg DW, respectively). Ricinis communis was found more susceptible to Staphylococcus aureus, followed by Cannabis sativa against Salmonella typhi and Calotropis procera against Micrococcus luteus. A remarkable degree of cytotoxicity against brine shrimps was exhibited by Euphorbia helioscopia, with 50% mortality at 57.74 μg/mL. Convolvulus arvensis showed noteworthy protein kinase inhibitory activity with 20 ± 1.2 mm zone of inhibition (ZOI) analyzed by Streptomyces hyphae formation inhibition. It is concluded that methanol extracts of F. carica and M. piperita can be considered as potential sources of antioxidants, while R. communis, E. helioscopia and C. arvensis could provide potent antimicrobial and cytotoxic agent and oncogenic kinase inhibitor, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural world is the paramount healer of various ailments since antiquity. Plant-derived remedies play incredibly vital role in traditional medicine systems, such as Chinese, Ayurveda, and Egyptian, which accounts for their frequent use till today [1]. WHO estimates that approximately 80% of the world population relies on traditional medicine for their primary health care. The use of herbs for primary, complementary or alternative therapies is attributed to the economic affordability, cultural acceptability, lesser side effects and high therapeutic index [2]. Phytochemistry has created rapid progress in the recognition of herbal plants constituents. Despite the rapid development in the field of medicine/chemistry, the indiscriminate use of drugs against various infectious diseases, the emergence of resistance against antimicrobials and over-expression of reactive oxygen species necessitate the development of advanced scientific approaches and further research for the establishment of novel therapeutic values. For few last decades, there has been increasing interest around the globe to explore the hidden and novel potentials of traditional herbal remedies [3].
Pakistan is blessed with diverse flora and botanical wealth. About 6000 taxa of flowering plants have been reported in Pakistan, mostly in the northern and western seashore, deserts, and mountain regions [4]. Located between North and East longitudes, Mardan is a district with an area of about 1632 km2 and estimated population of about 14 millions. Like any other developing country, inhabitants of the Mardan district prefer the traditional utilization of medicinal plants as a significant cultural element. To the best of our knowledge, most of the traditional counter-measures in this locality are not yet characterized to illustrate their phytochemical and biological potential. Therefore, the current study was focused mainly on in vitro pharmacological profiling of 15 flowering plants from the Mardan district, selected on the basis of their reported ethnomedicinal importance. The current screening involved the use of battery of cost effective and bench top scientific approaches with an attempt to detect therapeutic potency of each selected plant.
Materials and Methods
Collection of Plant Materials
Plants were collected from the peripheries of district Mardan KPK, Pakistan, during the months of April and May, 2013. The plants were identified by Dr. Muhammad Zafar, Department of Plant Sciences, Quaid-i-Azam University (QAU), Islamabad, Pakistan. The authentic voucher specimen of each plant was deposited at the Herbarium of Medicinal Plants, QAU, Islamabad. The name of selected plants along with respective herbarium numbers are as follows:
Brassica campestris Linn (HMP-477); Calotropis procera (Willd) R. Br. (HMP-472); Coriandrum sativum Linn (HMP-474); Cannabis sativa Linn (HMP-468); Cichorium intybus Linn (HMP-483); Convolvulus arvensis Linn (HMP-480); Euphorbia helioscopia Linn (HMP-467); Ficus carica L (HMP-481); Mentha longifolia (Linn) Huds (HMP-482); Mentha x piperita L (HMP-473); Rumex dentatus L (HMP-466); Ricinis communis L (HMP-475); Silene conoidea L (HMP-484); Solanum nigrum L (HMP-471); Medicago denticulata Willd (HMP-469).
Chemicals and Reagents
The study involved the use of analytical grade solvents including methanol, trichloroacetic acid, and DMSO purchased from Sigma (Sigma-Aldrich, United States). The utilized reference standards/reagents, i.e., gallic acid, ascorbic acid, aluminum chloride, quercetin, potassium acetate, diphenyl-1-picrylhydrazyl (DPPH), Folin–Ciocâlteu reagent, ammonium molybdate, sodium phosphate, potassium ferricyanide and culture media (nutrient agar, Sabouraud dextrose agar, Mueller-Hinton broth, and ISP4) were purchased from Merck (Merck KGaA, Germany). Cefixime, clotrimazole, doxorubicin, and surfactin were purchased from Sigma (Sigma-Aldrich United States).
Preparation of Crude Extracts
Extraction was carried out by simple maceration procedure. Briefly, whole plants, except leaves of F. carica and R. communis, were air dried under shade at room temperature for 3 – 4 weeks and subsequently comminuted roughly to coarse powder by commercial miller. Weighed amount of each plant sample was soaked separately in methanol (25% w/v) for three days with mixing in an ultrasonic bath five times a day. After three days, the extracts of all plant samples were filtered through Whatman filter paper. The resultant filtrates were centrifuged at 4000 rpm for 10 min. The supernatant was concentrated to dryness in rotary evaporator (Buchi, Switzerland) under reduced pressure to obtain crude extract of each sample.
Phytochemical Analysis
Total phenolic and flavonoid content. Folin-Ciocâlteu reagent protocol was employed for the determination of total phenolic content (TPC) [5]. The absorbance of reaction mixtures containing 20 μL of each sample (20 mg/mL DMSO), 90 μL of the diluted Folin-Ciocâlteu reagent, and 90 μL of 6% sodium carbonate solution was recorded at 715 nm by using photodiode array spectrophotometer (PDA 8354 Agilent Technologies, Germany). Gallic acid was maintained as standard and the resultant TPC was expressed as μg GAE/mg DW.
Aluminum chloride based colorimetric method was employed for total flavonoid content (TFC) quantification, where quercetin was used as a standard [5]. Briefly, the reaction mixture containing 20 μL sample (20 mg/mL DMSO), 10 μL of 1 M of potassium acetate, 10 μL of 10% aluminum chloride, and 160 μL of distilled water was incubated at room temperature for 30 min. Subsequently, spectrophotometric absorbance of the reaction mixture was taken at 415 nm and TFC was expressed as μg QE/mg DW.
Biological Evaluation
Total antioxidant and reducing power potential. The method used to determine the scavenging potential of the samples involve the use of 2,2-diphenyl-1-picrylhydrazyl (DPPH 3.92 mg/100 mL methanol) as described previously [6]. DPPH aliquot of 180 μL was added to 20 μL of each sample (20 mg/mL DMSO) and incubated at 37oC for 1 h. Positive control included ascorbic acid, while DPPH solution was used as negative control. Spectrophotometric absorbance was taken by using microplate reader at 515 nm and ascorbic acid equivalent (AAE) results were recorded after triplicate analysis. Each experiment was performed in triplicate and the final scavenging percentage was calculated by the following formula:
where Abs indicates the absorbance of DPPH solution with sample extract while Abc is the absorbance of DPPH solution without sample extract. IC50 (μg ∙ mL-1) of each sample was calculated from the scavenging percentage plotted against concentration of extract.
The phosphomolybdenum based total antioxidant capacity determination of the samples was accomplished by incubating a mixture containing 1 mL of reagent (0.6 M sulfuric acid, 4 mM ammonium molybdate, and 28 mM sodium phosphate) and 0.1 mL of sample (20 mg/mL DMSO) for 90 min at 95°C. Subsequently, the absorbance was measured at 695 nm using a microplate reader (Elx 800, Biotech, United States). Standard solutions of ascorbic acid (25 – 125 μg/mL) were prepared to construct the calibration curve, and the resultant antioxidant potential was expressed as μg AAE/mg DW [7].
The procedure used to evaluate the reducing power of plant extracts involved the mixing of 200 μL of each sample with 500 μL each of 0.2 M phosphate buffer and 1% potassium ferricyanide, followed by incubation at 50oC for 20 min. Subsequently, 500 μL of trichloroacetic acid was added and the resultant mixture was centrifuged for 10 min at 3000 rpm. Finally, 100 μL of supernatant layer was transferred to 96-well plate and mixed with 0.1% of ferric chloride and 20 μL of distilled water. Spectrophotometric absorbance was taken at 630 nm on microplate reader, and AAE values were determined after triplicate analysis [8].
Antimicrobial activity. The sensitivity assay against bacterial and fungal strains (Gram positive strains of Staphylococcus aureus (ATCC# 6538), Bacillus bronchiseptica (ATCC# 6633), and Micrococcus luteus (ATCC# 10240); Gram negative strains of Pseudomonas aerogensa (ATCC# 9721) and Salmonella typhi (ATCC# 14028); and fungal strains of Mucor specie (FCBP# 0300), Aspergillus fumigatus (FCBP# 66), Aspergillus flavus (FCBP# OO64), Aspergillus niger (FCBP# 0198) and Fusarium solani (FCBP# 0291) was accomplished by the disk diffusion method [9].
The stored bacterial and fungal stock cultures were revived by streaking onto Petri dishes containing solidified nutrient agar and trypton soy medium, respectively. The plates were subsequently incubated for 18 – 24 h at 37°C for bacterial cultures and 28°C for fungal strains. The inoculum of each bacterial strain was swabbed smoothly on the surface of the nutrient agar plates. The spores of fungal strains were harvested in 0.02% Tween 20 solution and their turbidity was adjusted according to McFarland 0.5 turbidity standard. Then 100 μL of each harvested fungal spores was swabbed on plates containing SDA. Stock solution of each crude sample was prepared in DMSO (20 mg/mL), and 5 μL (100 μg) of each sample was impregnated on filter paper discs. Discs were put on their respective places in Petri dishes. DMSO impregnated discs were used as negative control. Positive control included discs impregnated with cefixime (4 mg/mL) for bacterial strains and clotrimazole (4 mg/mL) for fungal strains. Subsequently, each plate was incubated at 37°C for bacterial and 28°C for fungal strains. After 24 h incubation period, the zone of inhibition (ZOI, mm) was measured by using vernier caliper. Samples with ZOI ≥ 10 mm were serially diluted in 96-well microtitre plate with Mueller-Hinton broth to obtain 100, 50, 25, 12.5 and 6 μg/mL samples. Minimum inhibitory concentration (MIC) was determined according to standard microbroth dilution methodology [9].
Cytotoxicity assay: brine shrimp lethality method. The lethality of brine shrimps larvae was evaluated according to the documented protocol [5] with slight modification. Artemia salina/shrimp eggs (Ocean Star, United States) were hatched in a bipartitioned dish (22 × 32 cm) separated by a perforated wall and filled with artificial sea water (34 g/L sea salt). The larger compartment was covered with aluminum foil while the smaller compartment was illuminated with a light source. After 24 – 48 h incubation period, mature naupli was harvested and transferred to glass vials. Each sample (100 mg/mL DMSO) was initially tested at concentrations of 1000, 100 and 10 μg/mL. The corresponding volume of each concentration was transferred to respective glass vials. Doxorubicin (4 mg/mL) and DMSO were used as positive and negative control, respectively. After 24 h incubation, the percentage of killed larvae was calculated and LC50 (μg/mL) value was determined using table curve 2D Ver.4
software.
Protein kinase inhibition assay. The following assay inculpated the receptivity against Streptomyces 85E strain, which utilizes kinases for hyphae formation development. Prior to the testing, the strain was inoculated into tryptic soy broth and allowed to refresh for 24 – 48 h. Subsequently, revived culture was swabbed over mineral ISP4 media. Sample infused filter paper discs (5 μL of 20 mg/mL DMSO) were placed in the seeded plates and labeled accordingly. Surfactin (sporulation inhibitor) and DMSO impregnated discs were maintained as positive and negative controls, respectively. The plates were incubated for hyphae development at 28°C for 48 – 72 h and clear or bald zone of growth inhibition around discs was measured by using vernier caliper. Each sample with ≥ 12 mm bald zone of hyphae formation inhibition was considered to be significant kinase inhibitor [10].
Data Analysis
Each experiment has been conducted in triplicate and values are presented as mean ± standard deviation. Table curve 2D Ver.4 software was utilized to calculate LC50 values. Mean values determined for various treatments were subjected to analysis of variance and Post Hoc Tukey’s HSD (Honest significant difference) test was employed for the comparison of extracts. SPSS (IBM SPSS Statistics Ver. 20) software was used to determine the significance at 5% level (95% confidence interval).
Results and Discussion
Fifteen flowering plants selected based on ethnomedicinal values were collected from Mardan KPK district for phytochemical and biological screening. Taxonomical classification and ethnopharmocological utilization of these selected plants are summarized in Table 1.
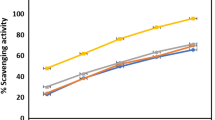
The highest phenolic content was quantified in F. carica leaves (8.6 ± 0.43 μg GAE/mg DW) followed by M. piperita (7.5 ± 0.12 μg GAE/mg DW), M. longifolia (7 ± 0.62 μg GAE/mg DW), C. intybus (6.8 ± 0.51 μg GAE /mg DW) and R. dentatus (6.6 ± 0.1 μg GAE /mg DW). While the highest amount of quercetin equivalent flavonoid content was observed in F. carica leaves (7.1 ± 1.05 μg QE/mg DW) followed by M. longifolia (4.05 ± 0.98 μg QE/mg DW), M. piperita (3.88 ± 0.93 μg QE/mg DW) and S. nigrum (3.23 ± 0.62 μg QE/mg DW). Phenolics and flavonoids are the group of organic aromatic compounds which confer scavenging potential owing to the presence of methoxy, hydroxyl, ketone group or double bond conjugation in the molecule [11]. The given TPC and TFC in methanol extract of F. carica leaves are significantly (P < 0.05) higher than the contents quantified in aqueous leaves extract of same plant [12]. The previously reported affirmatory connectivity of phenols and polyphenols with antioxidant ability in biological system illustrates the current interest in determining antioxidant efficiency. Antioxidant capability of a sample cannot be predicted by any single method. Therefore, in the current study, a battery of antiradical/antioxidant evaluation methods was acquired, each manifesting a different mechanism such as peroxide decomposition, chain termination, or free radical scavenging,. The DPPH quenching determination is based on the discoloration of deep visible purple colored 2,2-diphenyl-2-picryl-hydrazyl by antioxidant. The existence of an odd electron in the DPPH gives the absorbance at 515 nm. Acceptance of an electron from donor antioxidant possibly decolorizes DPPH radical which can be quantitatively measured from changes in absorbance [13]. From the obtained results, the IC50 values of M. piperita and M. longifolia on DPPH radical are in good agreement with the previous findings [14] depicting 50% scavenging potential at 12.12 – 14.64 μg/mL for M. piperita and 21.34 – 27.14 μg/mL for M. longifolia. A positive correlation was confirmed between TPC, TFC and radical scavenging potency of F. carica, M. longifolia and M. piperita (Fig. 1). This correlation strongly reinforces the foregoing consideration that the presence of hydroxyl group on aromatic hydrocarbon of a phenolic molecule is responsible for conferring free radical scavenging potential. Similarly, flavonoids also exhibit antioxidant property by quenching/scavenging singlet or triplet oxygen and free radicals [15].
RSA(radical scavenging activity, %), TPC (μg GAE/mg DW), and TFC (μg QE/mg DW) of methanol extracts of selected plant species. Values are presented as mean ± standard deviation from triplicate investigation. Columns with similar alphabets are not significantly different at P < 0.05. Bc: B. campestris, Cs: C. sativum, Cp: C. procera, Csa: C. sativa, Ca: C. arvensis, Ci: C. intybus, Eh: E. helioscopia, Fc: F. carica, Ml: M. longifolia, Mp: M. piperita, Rd: R. dentatus, Rc: R/ communis, Sn: S. nigrum, Md: M. denticulata.
Phosphomolybdenum based total antioxidant capacity assessment involves the reduction of Mo(VI) to Mo(V) and the subsequent formation of green colored phosphomolybdate complex. Phenolic components, such as phenolic acids or phenolic diterpenes, have been shown to be promising contributors towards antioxidant potential in various medicinal plants [16]. Among all studied samples, M. piperita was found to exhibit highest antioxidant potential (12.82 ± 1.21 μg/mg DW) followed by R. dentatus (12.13 ± 1.32 μg/mg DW), F. carica (10.92 ± 0.22 μg/mg DW) and R. communis (10.12 ± 1.1 μg/mg DW). The given phosphomolybdenum based antioxidant capacity results (Fig. 2) are in good accordance with previously postulated statement that the leaves of F. carica constitute considerably higher contents of phenol and anthocyanin (Cyanidine-3-O-rutinoside) which may equilibrate the homeostasis impaired by oxidative stress [17]. Similarly, the result strongly reinforces the previously reported positive correlation between TPC and antioxidant activity of M. piperita [18] which might be attributed to the presence of rosmarinic acid (phenolic compound) or eriocitrin and hesperidin (flavonoids). The redox property of a compound has a significant role in neutralizing and absorbing free radicals which is manifested by the transformation of Fe3+ to Fe2+ in the presence of extract samples. In the present study, M. piperita exhibited sufficiently high reduction potential (5.11 ± 0.14 μg/mg DW) followed by R. communis and M. longifolia (with 4.99 ± 0.68 and 4.93 ± 0.32 μg AAE/mg DW, respectively) (Fig. 2). The observed reduction potential of R. communis and F. carica are significantly higher than those reported in aqueous extracts (2.6 mg/g for R. communis and 3.9 mg/g for F. carica) [19]. Various researchers observed direct correlation between the reducing power and antioxidant activity of various medicinal plants. Reductones are generally associated with the reducing properties of agents known to exert antioxidant action either by reacting with peroxide and preventing its formation or by donating a hydrogen atom to free radical and reducing it to non-reactive species [20]. The given reducing capacity of samples suggests that reductone associated compounds terminate radical chain reaction by acting as electron donor and transforming free radical into a stable product.
RSA (radical scavenging activity, IC50μg/ml], TAC (total antioxidant capacity, μg AAE/mg DW) and TRP (total reducing power, μg AAE/mg DW) of methanol extracts of selected plant species. Values are presented as mean ± standard error from triplicate investigation. Bc: B. campestris, Cs: C. sativum, Cp: C. procera, Csa: C. sativa, Ca: C. arvensis, Ci: C. intybus, Eh: E. helioscopia, Fc: F. carica, Ml: M. longifolia, Mp: M. piperita, Rd: R. dentatus, Rc: R. communis, Sn: S. nigrum, Md: M. denticulata.
The results of susceptibility testing for all samples against bacterial strains under investigation are given in Table 2. Among all evaluated plant samples, the most promising activity was manifested by R. communis against S. aureus (ZOI, 17.3 ± 2.03 mm; MIC, 12.5 μg/mL) followed by S. nigrum (ZOI, 12.8 ± 0.7 mm; MIC, 50 μg/mL). Extract of C. sativa exhibited relatively higher inhibitory activity against S. typhi (ZOI, 15.33 ± 0.75 mm; MIC, 25 μg/mL). In comparison to the other samples active against M. luteus, the maximum activity was shown by C. procera (ZOI, 13 ± 1.4 mm; MIC, 50 μg/mL), while M. longifolia and M. piperita were found to exhibit MIC of 100 μg/mL. Extract of S. nigrum exhibited remarkable activity against P. aeruginosa (ZOI, 15.33 ± 1.5 mm; MIC, 25 μg/mL) followed by C. sativa (ZOI, 12.6 ± 1.52 mm; MIC, 50 μg/mL). Extract of R. communis (leaves) shows increased susceptibility pattern against S. aureus as compared to reported data [21]. Similarly, the susceptibility results for C. sativa against S. typhi and S. nigrum against P. aeruginosa are in accordance with previous findings [22]. The development of microbial resistance to antibiotics and the global interference with control of infectious diseases are major problems encountered at present. It is quite difficult to attribute the antimicrobial effect of active samples to one or few active principles because of different chemical composition of each extract. In addition to the major constituents, minor components may also make a significant contribution to the biological activity of plant extracts. Following the given results, we could infer that the antimicrobial effects of given plant extracts might be strictly connected to the synergistic/antagonistic effects of multiple phytochemicals in sample compositions. In addition, these results confirmed the previously reported substantiation that methanol is a better solvent for more efficient extraction of antimicrobials from medicinal plants as compared to other solvents [23]. Thus, all extracts were significantly active against tested bacterial strains, but none of them showed activity against fungal strains.
The cytotoxicity evaluation provides an efficient preliminary approach to determine antitumor, antimicrobial, antimalarial and insecticidal activities. Brine shrimp is a simple, convenient and an efficient probe to determine the cytotoxicity level of all samples. In the present investigation, the degree of cytotoxicity was perceived to be concentration dependent.
Most of the extracts exhibited remarkable toxicity profile against shrimp larvae, the most potent being E. helioscopia followed by C. procera, with 50% mortality at 57.74 and 217 μg/mL, respectively. The other cytotoxic extracts manifested promising activity with LC50 in the range of 344 to ≥ 1000 μg/mL (Table 3). The results are in good agreement with previous literature, where lethality of the subject plants evaluated against various cancer cell lines was attributed to the presence of diterpenoids in E. helioscopia [24], lacticifer proteins in latex of C. procera [25], affinity purified lectin RCAII in R. communis [26], or some novel toxic constituents which requires bioactivity guided isolation. The results of the protein kinase enzymes inhibition assay are summarized in Table 3. Among efficacious samples, C. arvensis showed noteworthy activity with 20 ± 1.21 mm bald zone of inhibition. Other samples, including C. procera, C. sativa, E. helioscopia, and M. denticulate, were also ascertained as sufficiently effective kinase inhibitors. Protein kinase inhibitors reflect a noteworthy class of compounds contemplated to be active against oncogenic kinases. Because of deregulated phosphorylation, protein kinases are considered to be a promising inhibitory target for the treatment of cancer. Streptomyces 85E strain utilizes kinases for hyphae formation development. The pharmacological inhibition of specific kinases strongly pursues the hypothesized statement that Streptomyces hyphae formation inhibitors might obstruct the procreation of cancer. The allosteric binding potential of an extract/compound to active or inactive site of a kinase might lead to the development of new tool for chemopreventive measures [27]. Recently reported increased kinase mediated p53 posttranscriptional regulation in cancer cell line by a glycoside extract of C. arvensis and subsequent induction of apoptosis [28] requires assessment of C. arvensis and other active species against several cancer cell lines to ascertain their complete cytotoxic potential.
Conclusion
The current study concludes that all of the screened plant samples are found to possess sufficient antioxidant activity that might be attributed to the presence of significant total phenolic and flavonoid contents. Extracts of R. communis, S. nigrum, C. sativa and C. procera exhibited promising antibacterial activity against various bacterial strains. Among all analyzed samples, E. helioscopia exhibited remarkable cytotoxic activity when assessed against brine shrimps larvae, while C. arvensis proved to be a remarkably effective protein kinase inhibitor when assessed against Streptomyces 85E strain, which is indicative of their chemopreventive and chemotherapeutic potential. The obtained results are significantly in accordance with previously reported ethnopharmacological importance and necessitate further pharmacological characterization and bioactivity guided isolation of components responsible for the observed activities.
References
D. Satyajit, Z. Latifand, and A. I. Gray, Natural Products Isolation, 2nd ed., Humana Press Inc. (2006), pp. 31 – 34.
C. Jiang, M. Chang, C. Wen, et al., J. Food Drug Anal., 14, 346 – 352 (2006).
Y. Bibi, S. Nisa, F. M. Chaudhary, et al., BMC Comp. Alt. Med., 11, 52 (2011).
R. U. Rehman, M. F. Chaudhary, K.M. Khawar, et al., Biologia, 69, 341 – 349 (2014).
I. U. Haq, A. Mannan, I. Ahmed, et al., Pak. J. Bot., 44, 1487 – 1490 (2012).
G. Bibi, I. U. Haq, N. Ullah, et al., Afr. J. Pharm. Pharmacol., 5(2), 252 – 258 (2011).
I. U. Haq, B. Mirza, S. Kanwal, et al., Iran J. Pharm. Res., 11(1), 241 – 249 (2012).
L. Jafri, S. Saleem, I. U. Haq, et al., Arab. J. Chem., (2014) in press.
Z. F. Rizvi, R. Mukhtar,M. F. Chaudhary, et al., Pak. J. Bot., 45, 275 – 278 (2013).
G. Yao, F. M. Sebisubi, L. Y. C. Voo, et al., J. Braz. Chem. Soc., 22, 125 – 1129 (2011).
F. H. Afshar, A. Delazar, H. Nazemiyeh, et al., Pharm. Sci., 18(3), 165 – 170 (2012).
M. Krishnaveni, K. Lavanya, P. Magesh, et al., World J. Pharm. Pharm. Sci., 3, 765 – 775 (2014).
P. Prieto, M. Pineda, and M. Aguilar, Anal. Biochem., 269, 337 – 341 (1999).
B. Nickavar, A. Alinaghi, A. Kamalinejad, Iran J. Pharm. Res., 7, 203 – 209 (2008).
A. Yildirim, A. Mavi, M. Oktay, et al., J. Agric. Food Chem., 48, 503 (2000).
H. J. Kim, F. Chen, X. Wang, et al., J. Agric. Food Chem., 54, 7263 – 7269 (2006).
B. Joseph and S. J. Raj, Int. J. Pharm. Tech. Res., 3, 8 – 12 (2011).
D. L. McKay and J. B. Blumberg, Phytother. Res., 20, 619 – 633 (2006).
M. Krishnaveni, S. Durairaj, P. Madhiyan, Int. J. Pharm. Sci. Res., 4, 3659 – 3662 (2013).
H. Wang, X. D. Gao, G. C. Zhou, et al., Food Chem., 106, 888 – 895 (2008).
M. Sharma, M. I. Mir, and M. Y. Malla, J. Nat. Prod. Plant Resour., 3, 72 – 75 (2013).
S. Nasrullah, K. Rahman, M. Ikram, et al., Int. J. Pharm. Sci. Rev. Res., 4, 25 – 29. (2012).
S. Kordali, R. Kotan, A. Mavi, et al., J. Agric. Food Chem., 53, 9452 – 9458 (2005).
Z. Q. Lu, J. Nat. Prod., 71, 873 – 876 (2008).
J. S. D. Oliveira, D. P. Bezerrab, C.D. T. D. Freitasa, et al., Toxicol. In Vitro., 21, 1563 – 1573 (2007).
G. Nicolson, M. Lacorbiereand, and T. R. Hunter, Cancer Res., 35, 144 – 155 (1975).
L. A. Smyth and I. Collins, J. Chem. Biol., 2, 131 – 151 (2009).
A. B. A. L. Asaad, D. K. Sukerand, and K. K. Hassan, Int. J. Med. Sci. Clin. Invent., 1, 203 – 209 (2014).
S. Bai, A. Malik, L. Seasotiya, et al., Int. J. Recent Adv. Pharm. Res., 4, 15 – 24 (2014).
A. Pandey, P. Bigoniya, V. Raj, et al., J. Pharm. Bioallied. Sci., 3, 435 – 441 (2011).
S. S. Tiwari, P. Tripathi, R. S. Singh, et al., Int. J. Adv. Pharm. Res., 5, 1783 – 1790 (2014).
M. Kaur and A. N. Kalia, Int. J. Pharm. Pharm. Sci., 4, 38 – 40 (2012).
A. S. Renee, J. Sidana, and G. Prinsloo, J. Evid. Based Complement. Altern. Med., 13 (2013).
W. S. Feng, L. Gao, X. K. Zheng, Y. Z. Wang, Chin. Chem. Lett., 20, 181 – 183 (2009).
I. T. A. Peixoto, V. F. Furlanetti, P. C. Anibal, et al., Rev. Ciênc. Farm. Básica. Apl., 30, 235 – 239 (2009).
N. Fatima, M. Zia, R. Rehman, et al., Afr. J. Biotechnol., 8, 6945 – 6951 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, S., Ur-Rehman, T., Mirza, B. et al. Antioxidant, Antimicrobial, Cytotoxic and Protein Kinase Inhibition Activities of Fifteen Traditional Medicinal Plants From Pakistan. Pharm Chem J 51, 391–398 (2017). https://doi.org/10.1007/s11094-017-1620-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-017-1620-5