Abstract
Ficus benjamina (FB) is a perennial plant that serves ornamental purposes. Its fruits are nonedible and considered ‘waste’ with no defined application. This paper discusses the valorisation and identification of the potential of Ficus benjamina fruits as a suitable biofuel feedstock. The whole fruit was characterised by scanning electron microscopy (SEM), energy dispersive X-ray (EDS), thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and bomb calorimeter. In addition, the proximate and ultimate analyses were performed to determine their physical, thermal, and chemical properties for potential biofuel application. Pulverised Ficus benjamina fruits (PFB) have a porous morphology that makes them less dense with a crystallinity index of 25.5%. The moisture, ash, volatile matter, and fixed carbon contents were 9.29, 6.26, 64.35, and 20.10%, respectively. The higher heating value (19.74 MJ/kg) and lower heating value (18.55 MJ/kg) are comparable to other biomass feedstocks. The results establish the possibility of using PFB as a solid biofuel.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Increasing global energy demand and climate goals make it necessary to seek sustainable and eco-friendly energy generation sources. Wood is currently the most common plant biomass used for heating purposes. However, it contributes to the health and environmental challenges [1]. Hence, the search for sustainable and efficient energy sources that make use of other plant biomass as an alternative to both fossil and wood fuel [2]. Among the various renewable energy sources, plant biomass holds a high possibility due to advancements in conversion technologies such as gasification and pyrolysis. The conversion of lignocellulosic biomass can generate solid biofuel (e.g. pellet and briquettes that can substitute for wood fuel), bio-oil, bioethanol, and biogas.
First-generation biofuel requires food crops and oilseeds, raising food security issues [3]. Second-generation biofuel relies on lignocellulose-based biomass, such as field- and process-based residues, which do not compete with food [4], though they contest with other applications such as livestock feed, mulching, and industrial purposes. Third-generation biofuel makes use of microalgae as feedstock. Although the latter has some prospects, it is not currently economical, and research is still at its relatively early stage. Fourth-generation fuel involves the genetic modification of plants and microalgae to reduce the lignin resistance during hydrolysis and increase CO2 sequestration. This type of biomass possesses tremendous opportunities in contributing to the energy supply chain but poses various problems [5]. In this study, we investigate the potentials of nonedible whole fruits as biomass feedstock for energy production. Nonedible fruits from ornamental and forest trees are practically wasted. Generally, despite their rich lignocellulose contents, they are neither characterised nor included as possible biomass feedstock for biofuel production. It is important to note that the use of these fruits avoids the overdependence on crop residues. Also, the trees producing such fruits do not require replanting, which makes them economical and sustainable. Weeping fig, botanically called Ficus benjamina (FB), is one of such trees. FB is native to Asia and Oceania, although it has adapted beyond its native range, spreading to other continents [6]. The wide range of adaptability allows it to flourish on fertile and moistened soils with sufficient sunlight [7]. It can tolerate drought, a wide range of soil types, and pH range from acidic to alkaline [8]. Due to its appealing properties as an easy-to-grow species and its high-density foliage and dimensions, FB has been introduced massively in urban areas all over the world, becoming a predominant species [9]. FB tree is generally used for ornamental and landscaping purposes [10] and not for their fruit. Nevertheless, they are capable of producing fruits more than two times a year [11]. FB fruits have no known application; hence they have no value and thus can be found as waste in parks, gardens, streets, highways, and river banks. In this regard, the novelty and priority of the present study are to understand the potential economic value that can be obtained from pulverised Ficus benjamina fruits (PFB). In order to achieve this, we performed fundamental physical, chemical, and thermal characterisations. From the preliminary results, it was discovered that PFB showed a very good prospect as a feedstock for biofuel application. This work intends to attract the attention of the scientific community and the government, such that appropriate programmes for the collection of this fruit will be initiated.
To the authors’ knowledge, this is probably the first paper that discusses the valorisation of Ficus benjamina (FB) fruits. Detailed information is provided to access its potential application. For this purpose, the physical, chemical, and thermal properties must be evaluated [12, 13] to allow suitable processing and policy decisions towards biofuel application.
As mentioned earlier, there are knowledge gaps with regard to the characterisation of FB fruits, especially as feedstock for biofuel production. Our motivation is to fill in some of the knowledge gaps by investigating the physicochemical and thermal properties. We also discuss the morphology, physical, and chemical compositions.
2 Materials and methods
2.1 FB fruit collection and preparation
FB fruits were collected from the grounds of AUST (African University of Science and Technology, Abuja, Nigeria). Figure 1 shows the oval-shaped FB fruits with yellow-orange colour.
The whole fruits collected were washed in clean water to remove impurities, sun dried, and pulverised in a blender (BLG-403, China). The pulverised FB fruit was labelled as PFB. The latter was sieved using a mesh of 425 µm to obtain particles with size ≤ 425 µm, then stored in clean, air-tight Ziploc bags for characterisation.
Figure 2 illustrates the schematic experimental process and flowchart for valorising FB fruits. The flowchart displays possible decisions that could be applied for further analysis.
2.2 Characterisation of PFB
2.2.1 Morphological analysis
The morphology of the FB fruit (internal and external surface) and PFB was observed using a scanning electron microscope (ZEISS, EVO LS10, USA). The samples were mounted on conductive adhesive carbon tape then coated with a thin layer of gold to prevent surface charging. Energy-dispersive X-ray (EDAX, USA) was used for the quantitative elemental analysis of the FB fruit.
The bulk density of PFB was determined using modifications of the method described by Stella Mary et al. [14]. An amount of PFB was added to a graduated glass cylinder (25 ml) and slightly tapped for 1–2 min to compact the content [15].
2.2.2 Proximate and ultimate analysis
The moisture and ash content of PFB was determined using the ASTM D7582 method [16]. The dry solid obtainable from PFB was determined following the approach by Singh et al. [17]. The volatile matter was calculated according to UNE 32,019 [18]. The fixed carbon content was estimated from the proximate analysis [17]. The carbon, hydrogen, and nitrogen contents of PFB were determined using a LECO CHN-2000 analyser, while the sulphur content was determined in a LECO S-144DR analyser. The oxygen content was estimated according to [17, 19].
The ether extractives yield of PFB was obtained following the Randall method [20]. In a Soxhlet apparatus, PFB (1.0 g) was added in the sample chamber and 250 ml of petroleum ether in the receiver flask, then placed on a heating mantle. After 7 h of extraction, the petroleum ether was recovered using a rotary evaporator. The resulting extract was left in the oven at 70 ℃ until constant weight, then the ether extractive yield was calculated using Eq. 1.
where W1 = weight of empty oil flask; W2 = weight of oil + flask after extraction; W = weight of PFB.
2.2.3 Fourier-transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) analyses
The functional groups in the PFB were characterised by means of FTIR spectroscopy (Thermo Fisher Scientific, USA). First, PFB was mixed with KBr in a ratio of 1:10 then compressed into a pellet. All spectra were recorded in the absorbance mode at the wavenumber range of 4000–400 cm−1.
The XRD analysis of PFB was carried out with Cu-Kα radiation of wavelengths 1.540598 Å generated at 40 mA and 45 kV (Empyrean). The crystallinity index (CrI) of the sample was estimated using the Ruland–Vonk method [21, 22]. This method is based on the ratio of the area of the crystalline profile to the total area (Eq. 2).
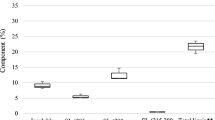
The lignocellulose content which comprises hemicellulose, lignin, and cellulose was determined gravimetrically [23]. The PFB (1.0 g) obtained after the ether extractives experiment (i.e. extractive-free PFB) was mixed with 10 ml of 0.5 mol/L of NaOH and heated at 80 ℃ for 4 h. The resulting mixture was washed till the pH became neutral then dried to a constant weight. The hemicellulose content is the difference between the initial (A) and final weights (B) (Eq. 3). For the lignin content determination, 1.0 g of extractive-free PFB was soaked overnight in 30 ml concentrated sulphuric acid (98%), after which, it was boiled at 100 ℃ for 1 h [24]. The resulting product was washed until no trace of sulphate ions was visible in the filtrate when tested with drops of barium chloride (10%). Afterwards, the residue was dried at 100 ℃ until constant weight was attained, then the lignin content was estimated (Eq. 4). Furthermore, the cellulose content was found by subtracting the ether extractives, hemicellulose, and lignin contents from 100 (Eq. 5).
where A = weight of extractive-free sample; B = weight of dried hemicellulose; wt = weight.
where A = weight of extractive-free sample; C = weight of dried lignin.
The calorific value of PFB was estimated as the higher heating value (HHV) and the lower heating value (LHV) using a bomb calorimeter (IKA C 4000). Moreover, the thermal behaviour was characterised using thermogravimetric analysis (TGA; PerkinElmer 4000, USA). The devolatilisation was investigated in the temperature range of 30–900 ℃ (10 ℃/min) in a nitrogen atmosphere with a flow rate of 60 ml/min.
3 Results and discussion
The biofuel potential of PFB is discussed based on their morphology, physical, chemical, and thermal properties.
3.1 Morphology
The morphology of the FB fruit revealed unique surface patterns, sizes, shapes, and orientations of the various parts (Fig. 3a–f). As shown in Fig. 3a–d, the internal structure has irregular patterns with several cavities, making it less dense. The endocarp (Fig. 3c) revealed a planar sheet-like structure, while the mesocarp (i.e. the region between the outer and the inner portion) showed pores (Fig. 3d). The lightweight and buoyancy of the dried FB fruit can be attributed to the less dense internal structure. In addition, the outer surface (epicarp) has a compact form with a uniformly distributed rough texture (Fig. 3e, f). For the PFB sample, the morphology (Fig. 3g) showed irregular shapes with the internal plant structure. This observation is different from that of pulverised unmodified Dikanut shell, which has a scattered orientation, few pores, and dense structure [25].
The bulk density of PFB is 0.32 g/ml. This can be related to the large granular particles from the epicarp (Fig. 3e, f) that create interparticle voids, leading to the low value obtained. The bulk density of PFB is lower than the rice kernels of various rice cultivars (0.77–0.87 g/cm3) [26]. The density of feedstocks has been reported to significantly influence their behaviour during the thermochemical/biological conversion process [27].
The analysis of different parts (outer and inner portions) of the FB fruit by energy-dispersive X-ray spectroscopy (EDS) showed carbon, oxygen, and potassium elements in high concentrations (see Table 1). In principle, biomass with low metallic elements is more suitable for energy generation by combustion [28]. The alkali metals (calcium and potassium) identified by EDS can affect thermochemical conversion processes during biofuel production because they lead to unwanted by-products (such as slag, sinter, and foul formation) in the boiler. However, it must be noted that calcium is only present in the outer portion of the fruit and that potassium concentration is much higher in this part than in the inner portion of the fruit. Therefore, it may require the removal of the outer part of the fruit for its use as biofuel. The EDS analysis of other nonedible fruit waste [29] has been included in Table 2 for comparison purposes. PFB presents a similar carbon content to banana peel or orange bagasse but a lower oxygen content and a much higher potassium content (Table 3). It is worth noting that potassium may play a vital role as a catalyst, thus increasing the rate of conversion of biomass [13].
3.2 Proximate and ultimate analysis
The moisture content of PFB, 9.29 wt.%, is lower than eucalyptus wood sawdust and comparable to that of wheat straw [38], suggesting its suitability for energy purposes (such as pellets and briquettes) (see Table 3). Hence, it is suitable for application in thermal conversion systems for rapid heat transfer and storage. Furthermore, the low moisture content of PFB is beneficial as little energy input may be needed to remove moisture, thus reducing the cost of processing the feedstock. Compared to the other biomasses included in Table 3, PFB has the lowest volatile matter content, 64.35 wt.%, although this is just below that of pinewood and wheat straw. Volatile matter is combustible organic matter that may contribute to heat energy generation. Generally, plant biomass is known to have high volatile matter [17, 34], making it readily reactive to oxygen. During pyrolysis, some liquid products (such as bio-oil) can also be obtained, in which case, less char will be generated from the feedstock. The fixed carbon of PFB is 20.10 wt.%, which is higher than that of eucalyptus sawdust [30], pinewood [32], grasses [34], seeds [13], and fruit peels [40], and slightly lower than that of a wheat stalk.
The ash content of PFB is 6.26 wt.%. This is well below the high limit of 10%, which indicates that it possesses a good potential for thermal utilisation [17, 45]. However, the ash content of PFB is higher than most of the biomass types considered in Table 3, except King grass. Moreso, the ash content is inversely proportional to the energy derived from the feedstock [46]. The formation of slag from the metallic elements in the ash presents operational challenges in boilers during thermal conversion at high temperatures. PFB may be suitable as briquettes and pellets for cooking and heating purposes in rural areas. Low ash content makes pyrolysis a suitable energy conversion route [29, 47]. The VM/FC ratio is an indicator of the quality of fuels. This ratio is lower for PFB than most of the biomass types included in Table 3 but comparable to pinewood and wheat straw.
The ultimate analysis showed a relatively high carbon content, of 50.56 wt.% (daf). Hydrogen, nitrogen, and sulphur contents are 5.82, 1.66, and 0.11 wt.% (daf), respectively, and the estimated oxygen content is 41.85 wt.% (daf). The comparison with other biomass feedstocks, presented in Table 3, shows the third-highest C content, the fifth-lowest H content, and the third-lowest O content for PFB. The sulphur content in PFB is low, as expected for biomass sources [19]. This finding is essential because a low oxide concentration of these elements will be formed during combustion. Therefore, PFB can be identified as eco-friendly during combustion and suitable for energy production via gasification as pollutants are limited [1].
3.3 Ether extractives
PFB produces low-yield ether extractives, 1.48 wt.% (Table 4). Since the latter is below 10%, its effect on the thermal conversion is negligible [47]. Therefore, it indicates that fewer liquid products (such as bio-oil) will be produced during pyrolysis. The extractives (majorly the oil content) are low and solidify even at room temperature, making them not appropriate for biodiesel production. This result agrees with the study on different walnut shells where the extractives were in the range of 1.4–1.7% [47].
3.4 van Krevelen diagram and biofuel reactivity
The atomic ratio of H/C and O/C defines the reactivity of biofuels. The H/C ratio reflects the degree of condensation and aromaticity in the plant material. The lower the H/C (high aromaticity), the higher the energy content. On the other hand, oxygen does not make any useful contribution to the heating value but makes it difficult for the transformation of biomass into liquid fuels [19]. In this study, PFB showed the second-lowest O/C ratio compared to all the lignocellulosic materials reviewed in Table 5, including coconut shells. Figure 4a shows the position of PFB in the van Krevelen diagram. The location of PFB is closer to fossil fuels than most of the biomass types included in this work, with the exception of that of pinewood and similar to that of coconut shell (Fig. 4a). It therefore implies that high energy density, stored as chemical energy, may be embedded in the C–C and C–O bonds [17].
The biofuel reactivity plot (Fig. 4b) revealed that the atomic ratios (H:C and O:C) of PFB are comparable to other biomass residues. The VM/FC ratio ranges from 3.20 to 6.64, and PFB was found in a similar position with the wheat straw and king grass. The VM/FC is higher than the atomic ratios, thus showing potential for biofuel, possibly solid biofuel [13].
3.5 Cellulose/hemicellulose ratio
The cellulose/hemicellulose ratio is indispensable in estimating ethanol yield [27]. Biomass feedstock with a high cellulose/hemicellulose ratio yields high ethanol. However, this ratio is low in PFB (0.57) due to the high hemicellulose content (Table 5). Therefore, the production of ethanol from PFB will require pretreatment and additional enzymes for hydrolysis.
3.6 X-ray diffraction analysis
In the XRD pattern of PFB (Fig. 5a), a broad peak was identified demonstrating its low crystallinity. The dominating amorphous nature may be a consequence of the rich carbon content. The latter could be linked to its lignocellulosic character, particularly its high hemicellulose content (48.30%), as shown in Table 5. The crystallinity index for PFB is 25.5%, which is similar to that of soy peels (25%) but lower than Açaí and coffee husk (30%) [30].
Generally, low crystallinity is associated with fast degradation. A narrower peak can also be identified in Fig. 5a. This corresponds to calcium oxalate hydrate oxide (C2Ca5O4.H2O), according to the International Centre for Diffraction Data (ICDD) reference card number 00–016-0379, which is identified as whewellite, with a monoclinic structure.
The XRD results are in good agreement with the EDS results, shown in Table 1, where Ca and C were identified. The presence of these elements makes PFB a suitable reinforcement agent in composites such as particleboards.
3.7 Fourier transform infrared spectroscopy (FTIR) analysis
In the FTIR spectra of PFB, shown in Fig. 5b, alkanes, aliphatic-primary amines, and hydrocarbons are identified (see Table 6). These groups provide binding sites for other elements in the fuel matrix [29]. The fingerprint region exhibits peaks that can be attributed to C–H, C–N, and S \(=\) O, amongst others. In the diagnosis region, N–H, C \(=\) O, C \(=\) C, and N–O have strong stretching and bending vibration bonds. The broad band related to the OH bond confirms the presence of alcohols or phenols in the carbohydrate and lignin contents. During thermal hydrolysis, the OH groups in the lignocellulose and the C \(=\) O bond from the carboxylic ends may be released. The stretching vibration of C–H could be related to the hemicellulose or the alkyl chain of the lipid content. However, the lignin decomposition might further generate the C \(=\) C bond. The existence of OH, C–O, C–H, and C \(=\) C functional groups in the structure of corn cob has been reported in the literature to facilitate the formation of condensable and non-condensable liquid with gaseous by-products [62].
3.8 Thermal analysis
The calorific value of biomass is an important parameter to measure its biofuel potential. For PFB, the higher heating value (HHV) and lower heating value (LHV) are 19.743 and 18.549 MJ/kg, respectively (dry ash-free basis). The HHV value is higher than most of the biomass feedstocks included in Table 5 and similar to wood sawdust. This relatively high value is connected to the chemical composition, specifically the extractives and lignin. Furthermore, the HHV results from the energy density associated with the C–C chemical bond. The high calorific value of PFB implies that it is suitable for solid biofuel application.
The thermochemical behaviour of PFB was investigated using TGA to determine the thermal parameters that influence gasification. Results are shown in Fig. 6. The high cellulose and hemicellulose contents enhance the thermal degradation of PFB as it decomposes at 200–700 ℃. Three stages of degradation can be observed in Fig. 6: in the first stage, moisture removal occurred with 8.98% mass loss; a moderate mass loss (28.72%) was identified between 279 and 367 ℃, at the second stage, accompanied by the release of gases from the volatile and other organic matters; finally, at 367–535 ℃, a high weight loss (42.39%) occurred due to the breakdown of cellulose, hemicellulose, and lignin with gaseous by-products, constituting the third stage of mass loss. The latter stage may be regarded as the active pyrolysis zone where the minor and major reactions take place. The thermal decomposition of PFB further affirms its chemical constituents. This result agrees with the lignocellulose components of biomass [29, 30].
3.9 Suitability of PFB as a biofuel feedstock and other applications
The moisture and ash contents of PFB are less than 10 wt.%, which makes it suitable for direct combustion or pyrolysis. In addition, the ether extractives of negligible value further support combustion. From our findings, PFB can favourably compete with the other documented biomass feedstock such as switchgrass and sawdust. Moreover, the rich calcium content makes it a suitable biomaterial for other applications, such as fillers in particleboard and biocomposite, while the high carbon content can be processed into bio-charcoal and activated carbon.
4 Conclusions
In this work, pulverised Ficus benjamina fruits (PFB) were extensively characterised for their potential biofuel application. The PFB are amorphous, rich in carbon, have negligible extractives, with low nitrogen and sulphur contents, which makes it eco-friendly as a solid biofuel. Also, the low bulk density and moisture content make PFB cost-effective to process into biofuel. The structural analysis by XRD showed a low crystallinity index value. Furthermore, PFB revealed a high heating value of 19.74 MJ/kg, thus having a high prospect as an alternative to sawdust and wood fuel. However, further research is needed prior to its application in local stoves and boilers, such as optimised pellet densification, analysis of the pellet’s combustion properties, and storage. From the EDS analysis results, the outer portion of the fruit holds all the calcium and most of the potassium present in the whole pulverised Ficus benjamina fruits. Considering the ether extractives content (1.48 wt.%) and the volatile matter (64.35 wt.%), these also have the potential to generate biogas via decomposition and should be further explored. Other envisaged applications of Ficus benjamina fruits that deserve attention, given their high carbon content and biogenic origin, may include biochar, activated carbon, biocomposite production for soil remediation and environmental sustainability.
Data availability
Data supporting the findings of this study is available upon request.
References
Tursi A (2019) A review on biomass: importance, chemistry, classification, and conversion. Biofuel Res J 6(2):962–979. https://doi.org/10.18331/BRJ2019.6.2.3
Alatzas S, Moustakas K, Malamis D, Vakalis S (2019) Biomass potential from agricultural waste for energetic utilization in Greece. Energies 12(6):1095. https://doi.org/10.3390/en12061095
Brinkman M, Levin-Koopman J, Wicke B, Shutes L, Kuiper M, Faaij A, van der Hilst F (2020) The distribution of food security impacts of biofuels, a Ghana case study. Biomass Bioenerg 141:105695. https://doi.org/10.1016/j.biombioe.2020.105695
Dey P, Pal P, Kevin JD, Das DB (2020) Lignocellulosic bioethanol production: prospects of emerging membrane technologies to improve the process a critical review. Int Rev Chem Eng 36(3):333–367. https://doi.org/10.1515/revce-2018-0014
Mat Aron NS, Khoo KS, Chew KW, Show PL, Chen W, Nguyen THP (2020) Sustainability of the four generations of biofuels – a review. Int J Energy Res. https://doi.org/10.1002/er.5557
Randall RP (2012) A global compendium of weeds. Perth, Australia: Department of Agriculture and Food Western Australia, 1124 pp. http://www.cabi.org/isc/FullTextPDF/2013/20133109119.pdf. Accessed 05 Oct 2021
Whistler WA (2000) Tropical ornamentals. Timber Press, Portland
Gilman EF, Watson DG (2007) Ficus benjamina, Fact Sheet ENH 410. Florida, USA: Environmental Horticulture Department, Institute of Food and Agricultural Sciences (IFAS), University of Florida. http://edis.ifas.ufl.edu/st251. Accessed 05 Oct 2021
Vargas-Garzón B, Molina-Prieto LF (2012) Ficus benjamina L. in the cities: high number of individuals, severe damages to infrastructure and expensive economic losses. Revista Nodo 13(7):93–101
Mahomoodally MF, Asif F, Rahman R, Nisar AIS (2019) A review of the pharmacological potential and phytochemical profile of Weeping Fig-Ficus benjamina L. Int J Chem Biochem Sci 16(2019):70–75
Lambert FR, Marshall AG (1991) Keystone characteristics of bird-dispersed ficus in a Malaysian lowland rain forest. J Ecol 79(3):793. https://doi.org/10.2307/2260668
De Jong W (2014) Biomass composition, properties, and characterization. In de Jong W, van Ommen JR (Eds.), Biomass as a sustainable energy source for the future: Fundamentals of conversion processes (pp. 36–68). John Wiley & Sons. https://doi.org/10.1002/9781118916643.ch2
Mishra RK, Mohanty K (2018) Characterization of non-edible lignocellulosic biomass in terms of their candidacy towards alternative renewable fuels. Biomass Convers Biorefin 8(4):799–812. https://doi.org/10.1007/s13399-018-0332-8
Stella Mary G, Sugumaran P, Niveditha S, Ramalakshmi B, Ravichandran P, Seshadri S (2016) Production, characterization and evaluation of biochar from pod (Pisum sativum), leaf (Brassica oleracea) and peel (Citrus sinensis) wastes. Int J Recycl Org Waste Agric 5(1):43–53. https://doi.org/10.1007/s40093-016-0116-8
Omoniyi TE, Olorunnisola AO (2014) Experimental characterisation of bagasse biomass material for energy production. Int J Eng Technol 4(10):582–589
ASTM D7582–15 (2015) Standard test methods for proximate analysis of coal and coke by macro thermogravimetric analysis, ASTM International, West Conshohocken, PA, www.astm.org. Accessed 10 Mar 2021
Singh YD, Mahanta P, Bora U (2017) Comprehensive characterization of lignocellulosic biomass through proximate, ultimate and compositional analysis for bioenergy production. Renew Energy 103:490–500. https://doi.org/10.1016/j.renene.2016.11.039
Norm UNE 32019–84 (1999) Combustibles Minerales sólidos: Determinación del Contenido en Materias Volátiles; AENOR: Madrid, Spain
Basu P (2013) Biomass gasification, pyrolysis and torrefaction : practical design and theory. Academic Press, London, United Kingdom
Barbosa MM, Detmann E, Valadares Filho SC, Detmann KSC, Franco MO, Batista ED, Rocha GC (2017) Evaluation of methods for the quantification of ether extract contents in forage and cattle feces. An Acad Bras Ciênc 89(2):1295–1303. https://doi.org/10.1590/0001-3765201720160708
Ciolacu D, Ciolacu F, Popa IV (2011) Amorphous cellulose – structure and characterization. Cellulose Chem Technol 45(1–2):13–21. https://doi.org/10.1021/acs.jnatprod.6b00235
Karimi K, Taherzadeh MJ (2016) A critical review of analytical methods in pretreatment of lignocelluloses: composition, imaging, and crystallinity. Biores Technol 200(2016):1008–1018. https://doi.org/10.1016/j.biortech.2015.11.022
Ayeni AO, Adeeyo OA, Oresegun OM, Oladimeji TE (2015) Compositional analysis of lignocellulosic materials: evaluation of an economically viable method suitable for woody and non-woody biomass. Am J Eng Res 4(4):14–19
Mansor AM, Lim JS, Ani FN, Hashim H, Ho WS (2019) Characteristics of cellulose, hemicellulose and lignin of md2 pineapple biomass. Chem Eng Trans 72:79–84. https://doi.org/10.3303/CET1972014
Ogbebor OE, Ebhojiaye RS, Amiolemhen PE (2019) Characterization of dikanunt shell using scanning electron microscopy and X-ray diffractometry. Niger Res J Eng Environ Sci 4(2):828–833
Nádvorníková M, Banout J, Herák D, Verner V (2018) Evaluation of physical properties of rice used in traditional Kyrgyz Cuisine. Food Sci Nutr 6(6):1778–1787. https://doi.org/10.1002/fsn3.746
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Biores Technol 83:37–46
Eze-Ilochi NO, Oti WJ (2017) Characterization of selected Nigerian indigenous biomass wastes for their suitability in biofuel production. Int J Pharm Sci Invent 6(9):1–8
Sánchez-Orozco R, Balderas Hernández P, Roa Morales G, Ureña Núñez F, Orozco Villafuerte J, Lugo Lugo V, Flores Ramírez N, Barrera Díaz CE, Cajero Vázquez P (2014) Characterization of lignocellulosic fruit waste as an alternative feedstock for bioethanol production. BioRes 9(2):1873–1885
Li H, Mahmood N, Mac Z, Zhua M, Wanga J, Zhenga J, Yuanb Z, Weia Q, Xu C (2017) Preparation and characterization of bio-polyol and bio-based flexiblepolyurethane foams from fast pyrolysis of wheat straw. Ind Crops Prod 103:64–72. https://doi.org/10.1016/j.indcrop.2017.03.042
Tumuluru JS (2015) Comparison of chemical composition and energy property of torrefied switchgrass and corn stover. Front Energy Res 3:46. https://doi.org/10.3389/fenrg.2015.00046
Rambo MKD, Schmidt FL, Ferreira MMC (2015) Analysis of the lignocellulosic components of biomass residues for biorefinery opportunities. Talanta 144:696–703. https://doi.org/10.1016/j.talanta.2015.06.045
Nwokolo N, Mamphweli S, Meyer E, Tangwe S (2014) Electrical performance evaluation of Johansson biomass gasifier system coupled to a 150 KVA generator. Renew Energy 71:695–700. https://doi.org/10.1016/j.renene.2014.06.018
Pathak P, Mandavgane S, Kulkarni B (2017) Fruit peel waste: characterization and its potential uses. Curr Sci 113:444–454
Rather MA, Khan NS, Gupta R (2017) Hydrothermal carbonization of macrophyte Potamogeton lucens for solid biofuel production. Eng Sci Technol Int J 20(1):168–174. https://doi.org/10.1016/j.jestch.2016.08.015
Santos RM, Bispo DF, Granja HS, Sussuchi EM, Freitas Ramos ALD, LS, (2020) Pyrolysis of the Caupi bean pod (Vigna unguiculata): characterization of biomass and bio-oil. J Braz Chem Soc 31(6):1125–1136. https://doi.org/10.21577/0103-5053.20190277
Shah MA, Khan MNS, Kumar V (2018) Biomass residue characterization for their potential application as biofuels. J Therm Anal Calorim 134:2137–2145. https://doi.org/10.1007/s10973-018-7560-9
Pinto F, Gominho J, André RN, Gonçalves D, Miranda M, Varela F, Neves D, Santos J, Lourenço A, Pereira H (2017) Improvement of gasification performance of Eucalyptus globulus stumps with torrefaction and densification pre-treatments. Fuel 206:289–299. https://doi.org/10.1016/j.fuel.2017.06.008
Kozlov A (2019) The study of the kinetics of formation of gaseous products during thermochemical conversion of woody biomass. Energy Systems Research 2019. E3S web of Conferences 114, https://doi.org/10.1051/e3sconf/201911407008
Wu R, Beutler J, Baxter LL (2020) Non-catalytic ash effect on char reactivity. Appl Energy 260(2020):114358. https://doi.org/10.1016/j.apenergy.2019.114358
Davies A, Soheilian R, Zhuo C, Levendis YA (2014) Pyrolytic conversion of biomass residues to gaseous fuels for electricity generation. J Energy Resour Technol 136(2):021101. https://doi.org/10.1115/1.4025286
Zhou H, Meng A, Long Y, Li Q, Zhang Y (2014) Classification and comparison of municipal solid waste based on thermochemical characteristics. J Air Waste Manag Assoc 64(5):597–616. https://doi.org/10.1080/10962247.2013.873094
Danish M, Naqvi M, Farooq U, Naqvi S (2015) Characterization of South Asian agricultural residues for potential utilization in future ‘energy mix’. The 7th International Conference on Applied Energy – ICAE2015. Energy Procedia 75:2974–2980. https://doi.org/10.1016/j.egypro.2015.07.604
Zhou H, Long Y, Meng A, Li Q, Zhang Y (2013) The pyrolysis simulation of five biomass species by hemi-cellulose, cellulose and lignin based on thermogravimetric curves. Thermochim Acta 566:36–43. https://doi.org/10.1016/j.tca.2013.04.040
Rodriguez C, Gordillo G (2011) Adiabatic gasification and pyrolysis of coffee husk using air-steam for partial oxidation. J Combust. 2011:1–9. https://doi.org/10.1155/2011/303168
Wang Q, Sarkarp J (2018) Pyrolysis behaviors of waste coconut shell and husk biomasses. Int J Energy Prod Mgmt 3(1):34–43. https://doi.org/10.2495/eq-v3-n1-34-43
Abdeljaoued A, Querejeta N, Durán I, Álvarez-Gutiérrez N, Pevida C, Mohamed Chahbani H (2018) Preparation and evaluation of a coconut shell-based activated carbon for CO2/CH4 separation. Energies 11(7):1748. https://doi.org/10.3390/en11071748
Vallejos ME, Kruyeniski J, Area MC (2017) Second-generation bioethanol from industrial wood waste of South American species. Biofuel Res J 15(2017):654–667. https://doi.org/10.18331/BRJ2017.4.3.4
Bui NQ, Fongarland P, Rataboul F, Dartiguelongue C, Charon N, Vallee C, Essayem N (2018) Controlled pinewood fractionation with supercritical ethanol: a prerequisite toward pinewood conversion into chemicals and biofuels. C R Chim 21(6):555–562. https://doi.org/10.1016/j.crci.2018.03.008
Orémusová E, Tereňová L, Réh R (2014) Evaluation of the gross and net calorific value of the selected wood species. Adv Mater Res 1001:292–299. https://doi.org/10.4028/www.scientific.net/amr.1001.292
Ioelovich M, Morag E (2012) Study of enzymatic hydrolysis of pretreated biomass at increased solids loading. BioResources 7(4):4672–4682. https://doi.org/10.15376/biores.7.4.4672-4682
Gallego LJ, Escobar A, Peñuela M, Peña JD, Rios LA (2015) King Grass: a promising material for the production of second-generation butanol. Fuel 143:399–403. https://doi.org/10.1016/j.fuel.2014.11.077
Teng H, Li S, Lv Q (2011) Experimental study on agglomeration characteristics of king-grass combustion in fluidized bed. Renew Energy Resour 29:121–124 (in Chinese)
Mensah MB, Jumpah H, Boadi NO, Awudza JAM (2021) Assessment of quantities and composition of corn stover in Ghana and their conversion into bioethanol. Scientific African 12(2021):e00731. https://doi.org/10.1016/j.sciaf.2021.e00731
Santos LRO, Varanda LD, Hansted ALS, Da Róz A, Yamamoto H, Yamaji FM (2019) Different types of lignocellulosic materials for energy generation in the ceramic industry. Floresta Ambiente 26(Spec No 2):e20180440. https://doi.org/10.1590/2179-8087.044018
Rizal WA, Nisa’ K, Maryana R, J Prasetyo D, Pratiwi D, Jatmiko TH, Ariani D, Suwanto A (2020) Chemical composition of liquid smoke from coconut shell waste produced by SME in Rongkop Gunungkidul. IOP Conf Ser: Earth Environ Sci 462 012057. https://doi.org/10.1088/1755-1315/462/1/012057
Said M, John G, Mhilu C, Manyele S (2015) The study of kinetic properties and analytical pyrolysis of coconut shells. J Renew Energy. https://doi.org/10.1155/2015/30732
Milian-Luperón L, Hernández-Rodríguez M, Falcón-Hernández J, Otero-Calvis A (2020) Obtaining bioproducts by slow pyrolysis of coffee and cocoa husks as suitable candidates for being used as soil amendment and source of energy. https://doi.org/10.15446/rev.colomb.quim.v49n2.83231
Lubwama M, Yiga VA (2018) Characteristics of briquettes developed from rice and coffee husks for domestic cooking applications in Uganda. Renew Energy 118:43–55. https://doi.org/10.1016/j.renene.2017.11.003
Pach M, Zanzi R, Björnbom E (2002) “Torrefied biomass a substitute for wood and charcoal,” In: Proceedings of 6th Asia-Pacific International Symposium on Combustion and Energy Utilization, Kuala Lumpur
Ascough PL, Bird MI, Scott AC, Collinson ME, Cohen-Ofri I, Snape CE, Le Manquais K (2010) Charcoal reflectance measurements: implications for structural characterization and assessment of diagenetic alteration. J Archaeol Sci 37(7):1590–1599
Anukam AI, Goso BP, Okoh OO, Mamphweli SN (2017) Studies on characterization of corn cob for application in a gasification process for energy production. J Chem 2017, 6478389, 9. https://doi.org/10.1155/2017/6478389
Acknowledgements
U.S.E. is grateful for the PAMI fellowship at the African University of Science and Technology; Abuja. M.G.P. acknowledges support from the Ramon y Cajal Programme (RYC-2015-17516) of the Government of Spain, cofinanced by the European Social Fund.
Funding
This research was partially supported by the Ramon y Cajal Programme (grant number: RyC-2015–17516) of the Government of Spain, co-financed by the European Social Fund.
Author information
Authors and Affiliations
Contributions
USE: manuscript writing, conceptualisation, and data analysis. BNE: graph analysis and manuscript review and editing. MGP: characterisations and manuscript review and editing. END: characterisations. KF: manuscript review and editing. LEKA: manuscript review, editing, and general supervision. APO: manuscript review, editing, and general supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Ficus benjamina fruits biomass waste is morphologically porous
• It is eco-friendly with low sulphur and nitrogen contents
• The higher heating value is comparable to wood dust and switchgrass
• These fruits have potential for solid biofuel and other applications
Rights and permissions
About this article
Cite this article
Ezealigo, U.S., Ezealigo, B.N., Plaza, M.G. et al. Preliminary characterisation and valorisation of Ficus benjamina fruits for biofuel application. Biomass Conv. Bioref. 13, 12643–12654 (2023). https://doi.org/10.1007/s13399-021-02230-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02230-1