Abstract
A detailed understanding of chemical composition and thermal degradation behavior is very important for a biomass before processing it into a pyrolysis or gasification unit for energy production. In the present work, the physico- and thermo-chemical characterization of four different types of walnut shells (PSW, TSW, MSW and HSW) is carried out to evaluate their application as furnace oil. The thermal degradation behavior during the thermal decomposition of different walnut shells (WS) samples is studied using thermogravimetric analysis at three different heating rates (5, 10 and 15 °C min−1). It is observed that the complete moisture removal is below 152 °C, and the degradation of lignocellulosic biomass is occurred between ≈ 250 to ≈ 400 °C in the oxidizing atmosphere. For all different WS samples, the heating values are observed in the range of 13.8–18.4 MJ kg−1, which is comparable to the wood waste and lignite coal. The cellulose, hemicellulose, lignin and extractives in walnut shell are found to vary from 32.3 to 34.5, 21 to 27, 39 to 43 and 1.4 to 1.7%, respectively. The functional characterization of different WS is carried out using FTIR, and the most prominent FTIR band peak has been found at wave numbers of 3400, 2931, 1420 and 1050 cm−1, which is due to the stretching vibrations of –OH, CH–, aromatic C=C, and aliphatic ether and alcohol groups, respectively. Scanning electron microscopy analysis indicated the rough texture and heterogeneous structures of biomass. Further, the X-ray diffraction analysis showed the crystalline structure, which is due to the presence of cellulose. Therefore, it can be concluded that the walnut shell is a potential candidate for energy generation through thermo-chemical conversion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass contributes approximately 14% of the world energy supply and is categorized as the fourth energy source in many of the developing countries [1, 2]. In recent years, there is much emphasis on alternative energy resources for energy production due to continuous depletion of fossil fuels, rising prices of crude oil, increasing demand for energy and environmental problems. Biodegradability, environmental friendliness and sustainability are the important features which have made the biomass as primary candidate for production of bio-energy. The biomass source includes wood, woody crops, wood and agricultural wastes, herbaceous species, bagasse, industrial residues, waste paper, municipal solid waste, sawdust, waste from food processing, biosolids, grass, algae, animal wastes, aquatic plants, etc. Animal waste and crop residues are the significant biomass resource for electricity generation, especially in many developing countries [3].
Walnut shell is one of the agricultural biomass waste materials obtained from the walnut dry fruit. It consists of 60% kernel (the oily material) and 40% shell (the hard covering of the walnut) [4, 5]. The kernel, which comes out when cracked, is mainly used as dry fruit, medicine preparation, cosmetics and several other applications, whereas the shell remains mostly unused. In India, ≈ 250 thousand metric tonnes of walnut is produced in the year 2014–2015, which resulted in 100 thousand metric tonnes of walnut shells. The shell has no utilization except direct use for combustion in domestic application or burned in open environment. From this point of view, it may be considered as a potential source of biofuels and chemical feedstock.
Physico- and thermo-chemical and compositional analyses are important characterization for any biomass before considering it as alternative fuel in both domestic and industrial applications. The physico-chemical properties significantly influence the biomass conversion, and the properties of interest are moisture content, high heating value (HHV), fixed carbon (FC), volatile matters (VM), ash/residue, alkali metal and cellulose/lignin ratio [6]. In a biomass, the major components are cellulose, hemicellulose and lignin with extractives as minor components [7, 8]. These properties not only determine the conversion process, but in general, also influence the cost evaluation of the conversion technology. Another important tool to predict thermal behavior of a biomass during pyrolysis is thermogravimetric (TG) analysis, where the mass loss of the feedstock is determined against temperature under the control of heating rate and the atmospheric conditions (air/inert) [9,10,11,12,13,14,15,16,17,18,19]. Nanda et al. [20] carried out fast pyrolysis of pine wood, wheat straw and timothy grass at 450 °C to produce bio-chars. The biomass samples were characterized using Raman spectroscopy and high-pressure liquid chromatography (HPLC) to study the cellulose, hemicellulose and lignin contents. The XRD characterization was carried out to investigate the crystalline structure of different feedstocks. Shadangi and Mohanty [21] reported the thermal degradation behavior of four different biomass seeds (Mahua, Karanja, Niger and Linseed) during pyrolysis at a heating rate of 5, 10 and 15 °C min−1 under inert atmosphere. From TG/DTG and DSC analyses, the composition of hemicelluloses, cellulose and lignin in different biomass seeds was reported. The functional groups in different biomass seeds were characterized using FTIR.
For walnut shells, Yuan and Liu [22] reported the thermal decomposition behavior at different heating rates (5, 10, 20, 30 and 40 K min−1) under inert atmosphere at a flow rate of 20 mL min−1. The aim was to produce bio-char, which can be used as fuel or as adsorbent. The activation energy values obtained were varied from 120 to 150 kJ mol−1. Acikahn [13] and Uzun and Yaman [23] studied the thermal degradation behavior of walnut shells of Turkey region, using thermogravimetric analyzer, at different heating rates (2, 10 and 15 °C min−1, and 5 and 15 °C min−1, respectively), under inert atmosphere. Three distinct zones were reported, using thermogravimetric curve, which was mainly attributed to removal of water, decomposition of hemicelluloses and cellulose, and decomposition of lignin. The activation energy values, predicted using kinetics studies, were found to be varying from 45.6 to 78.4 [13] and 180 to 192 kJ mol−1 [23]. Findorak et al. [24] compared the thermal characteristics of walnut shells of Slovak region with the sawdust, at three different heating rates (5, 10 and 15 °C min−1) under air atmosphere. It was shown that the mass loss characteristics of walnut shell were similar as of sawdust under air atmosphere; however, the thermal degradation of WS was higher than sawdust.
It was observed that the physico-and thermal-characterization is limited to one particular type of walnut shell, and it is mostly limited to the TGA/DTG analysis. However, the physico- and thermo-chemical analysis, functional groups, morphology and crystalline structures of different types of walnut shells have not been discussed in the literature. Therefore, the present work is focussed on the physico- and thermo-chemical characterization of four different walnut shells obtained from paper-shelled walnut (PSW), thin-shelled walnut (TSW), medium-shelled walnut (TSW) and hard-shelled walnut (HSW) commonly available as agriculture waste product in Jammu & Kashmir, India. Further, compositional analysis, in terms of cellulose, hemicelluloses, lignin and extractives, was found by using analytical method. The TG analysis was performed at different heating rates. Furthermore, walnut shells were also characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectrometry.
Materials and methods
Walnut shells of different types (PSW, TSW, MSW and HSW) were collected from the local walnut industry (Srinagar, Jammu & Kashmir, India) and sun-dried for 3 days at an average temperature of 25 °C under 47% humidity. It was then crushed by high-speed ball mill (Fritsch Pulverisette, Germany). The ground material was passed through standard screens 10 ASTM in order to obtain particle size of 2 mm. Fine-powdered samples of different walnut shells were packed in air-tight containers and stored in desiccators for further characterizations.
Compositional analysis for cellulose, hemicellulose, lignin and extractives was performed according to the analytical method reported by Li et al. [25]. The proximate analysis of the walnut shells was carried out as per ASTM standard procedures: E871-82 (2013), D1102-84 (2013) and E872-82 (2013) for moisture, ash and VM contents, respectively [26,27,28]. Moisture contents were determined at 103 ± 1 °C temperature for 2 h, or till constant mass was obtained in an oven. Then the sample was kept at a temperature of 580 °C for 30 min and 950 °C for 7 min to obtain ash and volatile matters (VM), respectively. Fixed carbon (FC) was determined by subtracting the summation of the percentages of moisture, ash and VM from 100. All calculations for ash, VM and FC were on the basis of same moisture-free reference. The elemental mass percentage of carbon, hydrogen, oxygen, nitrogen, sulfur and ash in different types of walnut shells was performed using a CHNS elemental analyzer (Euro EA3000, Euro vector, Italy) as per ASTM procedure D5373-08 [29]. Higher heating value of the biomass residues was determined by bomb calorimeter (CC01/M3, Toshniwal, India) using ASTM procedure D2015-85 [30].
TG analysis was carried out under oxidizing atmosphere using a Diamond TG/DTA, Perkin Elmer, USA, thermogravimetric analyzer. The instrument was applied for measuring and recording the changes in sample mass with variation in temperature during thermal degradation. The thermal degradation experiments were performed non-isothermally at three different heating rates (5, 10 and 15 °C min−1) over temperatures ranging from 30 to 1200 °C. The carrier gas used was oxygen, at a constant flow rate of 200 mL min−1. Samples were placed on open platinum sample pans during the TG analysis. The morphology of the biomass residue was determined by using SEM (JEOL JSM-6390 LV, USA). Images were taken at 15 kV with 10,000 × magnification. The XRD was performed on the biomass residue using the diffractometer (Bruker D8-Advance, USA). One gram of the sample was taken for powder diffraction using X-ray source with 2.2 kW Cu anode (40 kV, 40 mA) under angular range 2θ (5°–120°). Further, different walnut shells were characterized using FTIR in order to get information about the functional groups. The Avatar 370, Thermo Nicolet, USA, FTIR instrument was used for the purpose with the sample powder diluted in 1% potassium bromide (KBr). The FTIR spectra in the range of 500–4000 cm−1 were gathered with a resolution of 4 cm−1 and an accumulation of 64 scans. The ATR was corrected mathematically for a generated spectrum.
Results and discussion
Proximate and ultimate analysis
The proximate and ultimate analysis and the corresponding VM/FC, H/C, O/C and HHV data are shown in Tables 1 and 2. The proximate analysis was reported on dry-ash-free (daf) basis (Table 1). For the purpose of comparison, the earlier work reported on walnut shells and other common biomasses such as wood, wood bark and wheat straw have also been included.
For a biomass residue to be considered as fuel, moisture content is an important component. The higher moisture content lowers the heating value, which in turn affects the behavior of biomass during pyrolysis. The physical properties and the quality of the pyrolysis products are also affected [8]. The results showed that moisture contents in the present walnut shells were in the range of 8–9.26% and were comparable with the data of Uzen and Yaman [23] and Lee et al. [31] for walnut shells. The moisture contents observed in walnut shells, in the present work, were higher as compared to the value (2.5%) reported by Acikalin [13], whereas it is much smaller than the values 20 and 16% reported by Mckendry [6] for wood and WS*, respectively. For biofuel application, the moisture contents in biomass residue should be < 10% [18]. The higher moisture content present in biomass residue results in higher heat requirement to remove moisture from biomass. Therefore, the walnut shells are suitable for pyrolysis as the moisture content is very less.
Volatile matters (VM) and fixed carbon (FC) contents are significant in order to measure the ignition and then gasification/oxidation characteristics of biomass, depending on the type of its utilization as an energy source [6]. For different walnut shells used in the present work, the VM contents fall in the range from 73.9 to 79.8% and are comparable with the results reported in the literature (Table 1). The biomass with higher VM is more reactive and can be easily volatilized and also produce less char [32]; therefore, walnut shells are suitable for the pyrolysis and hence production of biofuel. Fixed carbon contents of walnut shells are almost varied from 20 to 26% which is comparable with the values reported by Mckendry [6] for WS*. When compared with values reported by other researchers, it was higher.
Higher ash contents in the biomass reduce the energy content of the fuel proportionately. Also during thermo-chemical conversion process, the chemical composition of the ash can create significant operational problems, such as combustion processes due to formation of slag from ash at elevated temperatures. In the present work, for different walnut shells considered, the ash contents were found in the range of 2–5.84%, which is lower than bituminous coal (9 mass%), lignite (6 mass%) and barley straw (6 mass%) [6]. Ash contents in different walnut shells are also comparable with the ash contents in WB [31] and WS* [6]. However, they are not comparable with [13, 23], who reported 0.33 and 0.64%, respectively, for WS. The level of ash contents in the samples was well within the range and very much suitable for combustion and pyrolysis conversion processes.
The elemental analysis of the samples showed that the carbon, hydrogen, nitrogen, sulfur and oxygen contents were comparable for PSW, TSW, MSW and HSW. The CHNS values for different walnut shells are also in good agreement with the results available in the literature for different biomass residues [33]. From the O/C and H/C ratios of walnut shells, it can be seen that the ratios overlap with good-quality lignite coal. HHVs for walnut shells were found in the range varying from 13.8 to 18.45 MJ kg−1 and fall in the range of biomass having large heating values [34] and good-quality lignite coals [35]. The values were comparable with the results reported by Acikalin [13] for WS, Mckendry [6] for WS* and Gaur et al. [33] for other biomasses.
Compositional analysis
The proportions of cellulose, hemicellulose, lignin and extractives are significant in biomass from the standpoint of conversion processes. The decomposition of the first three components depends on the temperature, heating rate and different contaminants present in the biomass [34] and affects the pyrolysis behavior of the biomass [18]. The degradation temperatures for cellulose, hemicelluloses and lignin were 220–315, 315–400 and 500–900 °C, respectively [21]. The higher percentage of cellulose and hemicellulose in the biomass enhances the rate of thermal degradation during pyrolysis, due to their lower degradation temperatures. The lignocellulosic composition obtained in the present work for different types of walnut shells was similar with the results available in the literature [3, 23, 36] for different biomass. Lignocellulosic components and extractives in the present study were almost comparable with each other. However, when compared with the values reported in the literature, extractives and lignin are on the lower side, whereas hemicellulose is comparable and cellulose is on the higher side. The presence of extractives in biomass less than 10% does not affect the thermal conversion process and can be ignored [35]. In the present study, the extractives were < 2% and support the biomass suitability for pyrolysis (Table 3).
Thermogravimetric analysis (TGA)
Thermoanalysis techniques like TGA/DTA are widely accepted to describe the thermal behavior of biomass [13, 18, 21]. Thermogravimetric (TG) curves of walnut shells in an inert atmosphere at three different heating rates (5, 10 and 15 °C min−1) are shown in Fig. 1i–iv. The mass-loss range can be categorized into four zones. In the first zone, ≈ 9–11% mass loss is recorded for the temperature range between 38 to 116 °C at three heating rates for all types of walnut shells. In this part, maximum mass loss occurred in TSW (11%), as shown in the Fig. 1ii, and lowest in PSW (9%) as reflected in Fig. 1i at a heating rate of 5 °C min−1. This loss is due to the removal of the moisture and the light volatile components present in the biomass. The second zone starts at 116 °C and finishes at 229 °C where minimum mass loss was varied from ≈ 2–5% at all heating rates. In third zone, i.e., from 229 to 375 °C, where maximum mass loss occurred from 72 to 84% at 5 °C min−1, 77 to 85% at 10 and 15 °C min−1 in all different walnut shell samples, due to the presence of cellulose and hemicelluloses, which undergo oxidation/devolatization reactions. This zone is also referred to as the active pyrolysis zone [13, 18, 37].
Thermal decomposition behaviors may be explained by the individual components of walnut shells, where cellulose, hemicellulose and lignin are the main components. From the extensive literature carried out, it was observed that different biomasses show different thermogravimetric behavior of individual biomass components. It has also been observed that the decomposition of cellulose, hemicellulose and lignin was completed at temperature intervals of 310–400, 210–325 and 160–900 °C, respectively [38,39,40,41]. Therefore, it can be concluded that in the active pyrolysis zone the minor and major reactions may be attributed to the decomposition of cellulose and hemicellulose. The final zone, i.e., fourth zone, starts at ≈ 400 °C and finishes at ≈ 1200 °C, where the percentage mass loss was very low, which may be due to very slow degradation of biomass. This zone is known as passive zone, and it occurs due to degradation of lignin in a long temperature range [18, 42]. Similar reports have also been given by other investigators with respect to the thermal degradation behavior of biomass in this zone [42,43,44]. In the present study, it was observed that the thermal degradation in the oxidizing atmosphere above 400 °C was almost negligible.
SEM and XRD analysis
The scanning electron microscopy (SEM) was carried out to investigate the surface morphology of walnut shells and is shown in Fig. 2. It is clearly visible that the structures are agglomerated with rough texture and heterogeneous structure without any defects like cracks within the structures. The PSW and MSW sample showed planner sheet-like structure, while TSW and HSW showed rocky structure.
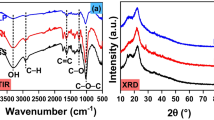
The XRD patterns of walnut shells are shown in Fig. 3 which shows an amorphous character similar to those of wood, coal and petroleum coke. The peaks were in the range of 10°–80° on the base line of the diffractograms. The peaks at 15.5° (d-space ~ 5.631 Å), 21.5° (d-space ~ 4.06 Å), 26° (d-space ~ 3.36 Å) and 34° (d-space ~ 2.58 Å) were assigned to cellulose and hemicellulose, respectively [36]. The broad peaks can be seen at 15.5° and 21.5° for all samples, which are the indicator of crystalline region of biomass due to the presence of cellulose [20, 36].
FTIR analysis
FTIR is a powerful characterization tool that has direct information about the functional groups. The FTIR has been used to predict the presence of hemicelluloses, cellulose and lignin in the biomass residue, such as non-edible oil seeds, like mahua, karanja, niger and linseed [21], empty fruit bunch briquettes [45], date palm [46], and Tectona grandis and Sorghum bicolour stalk [47]. Figure 4 shows FTIR spectra of different types of walnut shells in the range of 400–4000 cm−1 with a resolution of 4 cm−1. The most prominent band peaks were obtained at the wave numbers of 3407, 3400, 3398 and 3404 cm−1 for PSW, TSW, MSW and HSW, respectively, which indicated the –OH stretching vibration due to the presence of lignin and carbohydrates [40]. The second prominent peaks were obtained at ≈ 2931 cm−1, which represent the CH stretching vibrations methyl/methylene or methane functionalities in lignin. The bands at 1738–1739 cm−1 were due to the C=O esters vibrations in all walnut shells, which indicated the presence of acetyl group in the hemicelluloses fraction. Peak at 1509 cm−1 indicated aromatic C=C ring stretching vibration in lignin. The medium-intensity band between 1430 and 1420 cm−1 may be assigned to the aromatic C=C ring stretching vibration in lignin [48]. The band between 1060 and 1030 cm−1 confirmed the presence of aliphatic ether C–O– and alcohol C–O stretching due to presence of cellulose, hemicellulose and lignin. Table 4 shows the different bands referring to stretching of different groups.
Conclusions
In the present work, the detailed analysis of physico- and thermo-chemical characteristics along with SEM, XRD and FTIR analysis of different walnut shells (TSW, PSW, MSW and HSW) was carried out. The thermogravimetric analysis of different walnut shell samples was carried out at three different heating rates, i.e., 5, 10 and 15 °C min−1. The mass loss during thermal degradation was categorized into four different zones for all different WS samples: (1) in the first zone, for temperature varying from 30 to 116 °C, there was complete moisture removal; (2) second zone was attributed to the oxidation/devolatization of cellulose and hemicellulose for temperature ranging from 116 to 326 °C; (3) the third zone has different temperature ranges for different walnut shells and attributed to the thermal degradation of cellulose; and (4) the final zone started at ≈ 400 °C and finished at ≈ 1200 °C temperature, where degradation of lignin was taking place very slowly over a large temperature range. Cellulose and hemicellulose decomposed in a narrower temperature range (≈ 230–400 °C), depending on heating rate, whereas lignin decomposed in a wider range of temperature, thus showing apparent thermal stability during pyrolysis. Low moisture and ash content and high volatile matter and fixed carbon contents along with thermal decomposition behavior suggested that the different WS may act as a potential source for production of bio-oil through pyrolysis. The surface morphology of walnut shells showed heterogeneous structure, while XRD confirmed crystalline region of biomass due to the presence of cellulose. FTIR analysis of biomass showed that the biomass composition was dominated by oxygenated compounds. Thus, it is evident from the characterization that walnut shells are good potential source for energy production through thermo-chemical conversion processes.
References
Hall DO, Rosillo-Calle F, de Groot P. Biomass energy lessons from case studies in developing countries. Energy Policy. 1992;20:62–73.
McGowan F. Controlling the greenhouse effect: the role of renewables. Energy Policy. 1991;49:111–8.
Demirbas A. Combustion characteristics of different biomass fuels. Prog Energy Comb Sci. 2004;30:219–30.
Global agricultural Information network (GIAN). Report number IN5116; 2016.
Malhotra SP. World edible nuts economy. 2008; ISBN 13-978-81-8069-561-2.
Rather MA, Khan NS, Gupta R. Production of solid biofuel from macrophyte Potamogeton lucens. Eng Sci Technol. 2017;20:168–74.
McKendry P. Energy production from biomass (part 1): overview of biomass. Biores Technol. 2002;83:37–46.
Antal, MJ Jr. Biomass pyrolysis: a review of the literature part 2—lignocellulose pyrolysis. In: Boer KW, Duffle JA, editors. Advances in solar energy, vol 2. American Solar Energy Society, New York, 1983, p. 175–255.
Ceulcuoglu E, Ue Nay E, Karaosmanoglu F. Thermogravimetric analysis of the rapeseed cake. Energy Sour. 2001;23:889–95.
Yao F, Wu Q, Lei Y, Guo W, Xu Y. Thermal decomposition kinetics of natural fibers: activation energy with dynamic thermogravimetric analysis. Polym Degrad Stab. 2008;93:90–8.
Jiang G, Nowakowski DJ, Bridgwater AV. A systematic study of the kinetics of lignin pyrolysis. Thermochim Acta. 2010;498:61–6.
Al-Harahsheh M, Al-Ayed O, Robinson J, Kingman S, Al-Harahsheh A, Tarawneh K. Effect of demineralization and heating rate on the pyrolysis kinetics of Jordanian oil shales. Fuel Process Technol. 2011;92:1805–11.
Acikalin K. Thermogravimetric analysis of Walnut shells as pyrolysis feedstock. J Therm Anal Calorim. 2011;105:145–50.
Sasmal S, Goud VV, Mohanty K. Determination of salutary parameters to facilitate bio-energy production from three uncommon biomasses using thermogravimetric analysis. J Therm Anal Calorim. 2013;111:1649–55.
Antal MJ, Varhegyi G. Cellulose pyrolysis kinetics, the current state of knowledge. Ind Eng Chem Res. 1995;43:703–17.
Liou TH, Chang FW, Lo JJ. Pyrolysis kinetics of acid-leached rice husk. Ind Eng Chem Res. 1997;36:568–73.
Zhu X, Chen Z, Xiao B, et al. Co-pyrolysis behaviors and kinetics of sewage sludge and pine sawdust blends under non-isothermal conditions. J Therm Anal Calorim. 2014;119:2269–79.
Verma AK, Mondal P. Physicochemical characterization and kinetic study of pine needle for pyrolysis process. J Therm Anal Calorim. 2016;124:487–97.
Ghaffar SH, Fan M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenerg. 2013;57(57):264–79.
Nanda S, Mohanty P, Pant KK, Naik S, Kozinski JA, Dalai AK. Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenergy Res. 2013;6:663–77.
Shadangi KP, Mohanty K. Kinetic study and thermal analysis of the pyrolysis of non-edible oil seed powders by thermogravimetric and differential scanning calorimetric analysis. Renew Energy. 2014;63:337–44.
Yuan HR, Liu RH. Study on pyrolysis kinetics of walnut shells. J Therm Anal Calor. 2007;3:983–6.
Uzun BB, Yaman E. Thermogravimetric pyrolysis of walnut shell an assessment of kinetic modeling. In: International conference on “industrial waste and waste water treatment valorization” held in Athens, Greece, 21st–23rd May 2015.
Findorak R, Frohlichova M, Findorakova J, Findorakova L. Thermal degradation and kinetic study of sawdusts and walnut shells via thermal analysis. J Therm Anal Calorim. 2016;125:689–94.
Li S, Xu S, Liu S, Yang C, Lu Q. Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process Technol. 2004;85:1201–11.
ASTM. Standard test method for moisture analysis of particulate wood fuels, ASTM E871-82. Pennsylvania: ASTM International; 2013.
ASTM. Standard test method for ash in wood, ASTM D1102-84. Pennsylvania: ASTM International; 2013.
ASTM. Standard test method for volatile matter in the analysis of particulate wood fuels, ASTM E872-82. Pennsylvania: ASTM International; 2013.
ASTM. Standard test methods for instrumental determination of carbon, hydrogen and nitrogen in laboratory samples of coal, ASTM D5373-08. Pennsylvania: ASTM International; 2008.
ASTM, Standard test method for gross calorific value of coal and coke by the adiabatic bomb calorimeter, ASTM D2015-85.
Lee Y, Park J, Ryu C, Gang KS, Yang W, Park YK, Jung J, Hyun S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500° C. Biores Technol. 2013;148:196–201.
Graboski M, Bain R. Biomass gasification: principles and technology. In: Reed TB, editor. Noyes data corporation, New Jersey, 1981. p. 154–82.
Gaur S, Reed TB. An Atlas of thermal data for biomass and other fuels. National renewable energy laboratory USA. 1998; DE-AC36-83CH10093.
Jahirul MI, Rasul MG, Chowdhury AA, Ashwat N. Biofuels production through biomass pyrolysis—a technological review. Energies. 2012;5:4952–5001.
Ranzi E, Cuoci A, Faravelli T, Frassoldati A, Migliavacca G, Pierucci S, Sommariva S. Chemical kinetics of biomass pyrolysis. Energy Fuels. 2008;22:4292–300.
Vassilev SV, Baxter D, Andersen LK, Vassileva CG, Morgan TJ. An overview of the organic and inorganic phase composition of biomass. Fuel. 2012;94:1–33.
Jeguirim M, Trouve G. Pyrolysis characteristics and kinetics of Arundo donax using thermogravimetric analysis. Biores Technol. 2009;100:4026–31.
Bisht AS, Singh S, Kumar SR. Use of pine needle in energy generation application. Int J Res Appl Sci Eng Technol. 2014;2:59–63.
Cai JM, Bi LS. Kinetic analysis of wheat straw pyrolysis using isoconversional methods. J Therm Anal Calorim. 2009;98:325–30.
Chutia RS, Kataki R, Bhaskar T. Thermogravimetric and decomposition kinetic studies of Mesua ferrea L. deoiled cake. Biores Technol. 2013;139:66–72.
Mishra G, Bhaskar T. Non isothermal model free kinetics for pyrolysis of rice straw. Biores Technol. 2014;169:614–21.
Ackalın K. Pyrolytic characteristics and kinetics of pistachio shell by thermogravimetric analysis. J Therm Anal Calorim. 2012;109:227–35.
Lopez-Velazquez MA, Santes V, Balmaseda J, Torres-Garcia E. Pyrolysis of orange waste: a thermo-kinetic study. J Anal Appl Pyrolysis. 2013;99:170–7.
Lapuerta M, Hernández A, Rodríguez J. Kinetics of devolatilisation of forestry wastes from thermogravimetric analysis. Biomass Bioenergy. 2004;27:385–91.
Nyakuma BB, Johari A, Ahmad A, Amran T, Abdullah T. Thermogravimetric analysis of the fuel properties of empty fruit bunch briquettes. J Teknol. 2014;3:79–82.
Sait HH, Hussain A, Salema AA, Ani FN. Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Biores Technol. 2012;118:382–9.
Balogun AO, Lasode OA, McDonald AG. Thermo-physical, chemical and structural modifications in torrefied biomass residues. Waste Biomass Valor 2018;9:131–8.
Faix O. Classification of Lignins from different botanical origins by FT-IR spectroscopy. Holzforschung. 1991;45:21–7.
Acknowledgements
The authors would like to acknowledge to Sophisticated Test & Instrumentation Centre (SAIF), Cochin University of Science and Technology, Cochin, Kerala, India, for analyzing various biomass. We greatly appreciate the financial support provided by Minister of Human Resources Department (MHRD), Govt. of India. The authors also thankful to the Department of Chemical Engineering, Indian Institute of Technology Roorkee, and Department of Chemical Engineering Department, National Institute of Technology, Srinagar, Jammu & Kashmir, India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shah, M.A., Khan, M.N.S. & Kumar, V. Biomass residue characterization for their potential application as biofuels. J Therm Anal Calorim 134, 2137–2145 (2018). https://doi.org/10.1007/s10973-018-7560-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7560-9