Abstract
Cellulose is a promising alternative material as a sorbent for the removal of pollutants. The availability of hydroxyl groups on cellulose allows for the application of various modification reactions for the development of novel sorbents with different functional groups. In this work, a cellulose sorbent modified with N-methyl-glucamine was prepared and tested for the removal of boron. A batch adsorption process was used to further explore the boron sorption kinetics, isotherms, thermodynamics, mechanism, and reuse of the prepared sorbent. It was found that the optimum sorbent dose for boron removal was 0.2 g/25 mL. Moreover, the initial pH of the solution was found to affect the removal rate and was found to be ≥ 4. The sorption of boron reached equilibrium within 60 min. The maximum sorption capacity was calculated to be 4.7 mg B/g sorbent. The sorption process was found to be exothermic and the negative value of ∆S in the range of 30–60 °C is related to a decrease in randomness at the solid/solution interface during the sorption of boron on the sorbent. The sorption/regeneration experiments have shown that the removal rate of the sorbent remains the same over 5 cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Although boron (B) is one of the essential nutrients for humans and plants, it has a toxic effect on plants when present in excessive concentrations [1]. It is normally present in marine and groundwater but its concentration is proportional to the surrounding geology. However, anthropogenic factors also increase its concentration in water [2]. Boric acid is used in a variety of industries including optoelectronics, semiconductors, ceramics and borosilicate glass, B-containing fertilizers, herbicides, and insecticides [3]. The B concentration in such wastes changed from micrograms per liter to milligrams per liter. The concentration of boron in wastewater from hydraulic fracturing was 123 mg/L [4], while in wastewater from liquid crystal displays, it was 820 mg/L [5]. The World Health Organization (WHO) has set 2.4 mg/L as a guideline value for drinking water [6].
The various water treatment techniques such as boron-selective resins, coagulation, membrane processes, and sorption processes used to remove boron and the advantages/limitations of these methods have been studied by various groups [2, 7, 8].
The sorption process is one of the most efficient methods for removing boron at low concentrations. For this purpose, chelating resins, activated carbon, fly ash, and natural materials can be used [9]. The use of low cost, environmentally friendly, and abundant sorbents for water treatment has gained friction. The low cost, biodegradable, renewable, and modifiable properties make cellulose one of the materials with great potential for water treatment [10].
In recent years, various functional groups have been attached to cellulose and then used for contaminant removal. Parlak and Arar [11] used sodium periodate (NaIO4) and sodium metabisulfite (Na2S2O5) successively for the preparation of sulfonated cellulose and then applied it for the removal of copper (Cu2+). The capacity of the sorbent produced was reported to be 8.2 mg Cu2+/g. The authors also pointed out that the sorption of Cu2+ is a fast kinetic and exothermic process [11].
Özdemir et al. [12] applied a two-pot oxidation process for the preparation of diacetate-containing cellulose and tested it for the removal of beryllium (Be2+). Oxidation of cellulose was achieved by sequential oxidation with NaIO4 and sodium chlorite (NaClO2). The sorption of Be2+ reached equilibrium within 3 min. The capacity of the sorbent was reported to be 4.54 mg/g. The author also reported that 0.1 M HCl or H2SO4 solutions can be used for the regeneration of the sorbent loaded with Be [12].
Anirudhan et al. [13] prepared quaternary ammonium containing cellulose for the removal of chromium (Cr6+). The removal of Cr6+ reached equilibrium in 1 h. The capacity of the sorbent was 123.60 mg/g, and the Cr-loaded sorbent was regenerated with 0.1 M NaOH solution [13]. However, to our present knowledge, there is no study on the preparation of N-methyl-glucamine containing cellulose by graft polymerization technique.
Graft polymerization is an attractive method that allows the surface of materials to be functionalized without greatly affecting the properties of the bulk [14]. Graft polymerization, which is used for a variety of applications, has several advantages, such as ease of use, ability to impart different functionalities, tunable surface properties, compatibility and stability, relatively low cost, fast modification rates, and adjustable grafting percentages [14,15,16]. In our case, the grafting method allowed us to produce a sorbent with good stability and significantly improved the reusability of the sorbent.
In this work, an N-methyl-glucamine containing sorbent for the removal of boron from an aqueous solution was prepared and investigated. The batch sorption parameters for boron removal were also studied. In addition, the sorption kinetics and sorption capacity of the sorbent as well as the thermodynamic parameters were calculated. In the final phase of the research, the optimum regenerant for the regeneration of the B-loaded sorbent was found.
2 Experimental
2.1 Chemicals and materials
Glycidyl methacrylate (GMA, 97%, Sigma-Aldrich) and N-methyl-d-glucamine (NMG, 99%, Acros) were used to prepare boron-selective cellulose. The crude cellulose samples were obtained from Denkim Kimya A.Ş. (Denizli, Turkey) and used without pretreatment. The aqueous solution of boric acid was prepared by appropriate dissolution of boric acid (H3BO3, Merck) in deionized water.
2.2 Preparation of glycidyl methacrylate-N-methyl-d-glucamine monomer
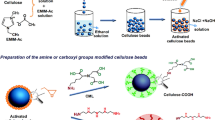
The glycidyl methacrylate-N-methyl-d-glucamine (GMA-NMG) monomer was prepared as described in [17]. Briefly, 150 mL of 0.83 M NMG solution was prepared and added to the reaction cell. Then, 20.4 mL of GMA solution (97%, d = 1.042 g/mL) was slowly added to the NMG solution. The N2 gas was passed through the solution for 15 min. The reaction cell was placed in a silicone oil bath at 70 °C for 5 h with vigorous stirring. At the beginning of the process, the mixture was turbid and at the end of the reaction, a single clear phase was observed. The reaction between GMA and NMG is shown in Fig. 1a.
The product was rinsed with diethyl ether (3 times at 50 mL) to remove unreacted GMA. A separatory funnel was used to separate the organic phase from the aqueous phase. The GMA-NMG monomer was in the aqueous phase.
2.3 Preparation of GMA-NMG-tethered cellulose
Fifteen grams of crude cellulose and 150 mL of GMA-NMG were added to the reaction cell, and then, N2 gas was passed through this mixture. The initiator (0.3366 g K2S2O8) was added to the mixture and the reaction cell was placed in an oil bath at 70 °C for 24 h under N2 atmosphere to allow the reaction of the hydroxyl groups of cellulose with ethylene groups. The reaction of cellulose with GMA-NMG is shown in Fig. 1b.
2.4 Characterization of the prepared sorbent
Infrared spectra of crude and GMA-NMG containing cellulose were determined using an infrared spectrometer (PerkinElmer, model One-B). The elemental composition of the crude and prepared sorbent was determined using an elemental analyzer (Leco, CHNS-932).
The determination of boron in the samples was measured spectrometrically as described in [18].
The removal efficiency (r) and capacity (q) of the sorbent were calculated according to Eqs. 1 and 2, respectively.
3 Results and discussion
3.1 Characterization of the sorbent
The infrared spectrum of raw and modified cellulose is shown in Fig. 2. The peaks at 3263 cm−1 and 2924 cm−1 are attributed to the O–H and -CH3/-CH2 stretching vibrations, respectively. After modification of cellulose, a decrease in this transmittance was observed. The band at 1713 cm−1 belongs to the C = O bending vibration. Glycidyl methacrylate has C = O groups and the binding of the new C = O groups decreased the transmittance. A new peak appeared at 1557 cm−1 belonging to amine groups, indicating that GMA-NMG functional groups were successfully attached to cellulose. The peaks at 1028 cm−1 can be attributed to C-O stretching [11, 12, 19,20,21].
The elemental composition (carbon C, hydrogen H, nitrogen N, and oxygen O) of crude cellulose was found to be 41.89% C, 6.21% H, and 51.89% O, respectively. The nitrogen (N) was not observed in crude cellulose. In the case of GMA-NMG, the sorbent composition was found to be 44.08% C, 5.31% H, 48.62% O, and 1.99% N. After the introduction of GMA-NMG groups into cellulose, nitrogen was found in the elemental composition, supporting the results of FTIR and further proving the successful grafting of GMA-NMG onto cellulose. It was also observed that the mass fraction of oxygen decreased after modification, which can be attributed to a lower oxygen mass ratio in the GMA-NMG group.
3.2 Effect of sorbent dose on boron removal
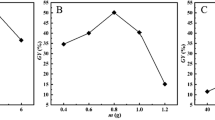
To find the optimal sorbent dose for boron removal, a series of experiments were performed. 25 mL of a boron-containing solution was contacted with different amounts of sorbent (0.1–0.3 g), where the boron concentration in the solution was 5 mg-B/L and the pH of the solution was 6. The variation of boron removal as a function of sorbent dose is shown in Fig. 3.
From Fig. 3, it can be seen that the increase in sorbent dose resulted in an increase in the percentage of boron removal. It is known that the number of available functional groups is linearly proportional to the sorbent dose. When the sorbent dose was increased, the number of functional groups increased and so did the removal rate. Figure 3 also shows that the maximum removal rate was 69% for 0.2 g and further addition of sorbent did not change the removal percentage. Therefore, a sorbent dose of 0.2 g was determined to be the optimum dose and was used in further experiments. Experiments were also conducted with plain cellulose, and as can be seen in Fig. 3, the removal of boron was not observed. These results show that grafting of cellulose improves the removal rate.
3.3 Effect of initial solution pH on boron removal
In this series of experiments, 0.2 g of GMA-NMG-containing cellulose was contacted with 25 mL of boron-containing solution (5 mg-B/L) with different initial pH values of the solution. The results are shown in Fig. 4.
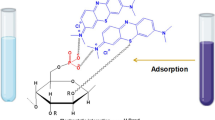
When the pH of the solution was adjusted to 2, 64% of the boron was removed from the solution. Increasing the pH of the solution to 4 increased the boron removal to 69% and further increasing the pH to 10 did not change the boron removal rate. The removal of boron by a sorbent containing NMG is a two-step process [22]. First, boric acid dissociates into its anionic form, borate, and hydronium ions (Eq. 3), and in another step, the borate anion forms a chelate with the cis-diol of N-methylglucamine groups, as shown in Fig. 5.
The resulting H3O+ ions are sorbed by the ternary amine groups of the NMG [23]. The higher complexability of the N-methyl-d-glucamine group would be an electrostatic attraction between the borate anion and the quaternary ammonium group (after protonation of the ternary amine group of NMG) [24]. When the pH of the solution decreases, the equilibrium shown in Eq. 3 shifts to the left side, then the molecular form of boric acid occurs and thus the removal of boron decreases.
3.4 Sorption kinetics
To find the optimum contact time for boron sorption, 8.0 g of sorbent was contacted with 1.0 L of a B-containing solution (5 mg-B/L, pH 6). The solution was mixed, and 10-mL samples were taken at specific time points. The changes in boron removal versus time are shown in Fig. 6.
As can be seen from Fig. 6, boron sorption reached a plateau after 60 min. The kinetic data are applied to pseudo-first-order (Eq. 4) and pseudo-second-order (Eq. 5) kinetic models [25, 26].
The calculated kinetic data are summarized in Table 1. The regression coefficients (R2) of the pseudo-second-order model were larger than those of the pseudo-first-order model, indicating that the pseudo-second-order kinetic model was more appropriate for the kinetic data obtained.
3.5 Sorption isotherms
0.2 g of sorbent was contacted with 25 mL of a boron-containing solution. The boron concentration in the solution varied from 25 to 400 mg-B/L (at pH 6). The changes in the capacity of the sorbent compared to the initial boron concentration are shown in Fig. 7. As can be seen in Fig. 7, the sorbent capacity increased with increasing boron concentration, and finally, the sorbent capacity reached its maximum value.
The experimental sorption results are applied to the Langmuir and Freundlich isotherm models. The linear form of the Langmuir model is shown in Eq. 6 and that of the Freundlich model is shown in Eq. 7 [27].
The isotherm parameters were calculated and are shown in Table 2. The obtained sorption data showed a better agreement with Langmuir’s isotherm model. This result shows that B sorbed on the prepared sorbent as a monolayer.
The capacity of the sorbent is compared with other sorbents reported in the literature, and the results are summarized in Table 3. The sorbent capacity varied from 0.16 to 199 mg/g. This difference could be due to the type of sorbent, its functional group, modification steps, and composition of the sorbent. For example, a similar work was carried out by Inukai et al. where the authors first modified cellulose with GMA and then further modified the epoxide groups of the grafted GMA with NMG [28]. The authors reported the adsorption capacity of their sorbent as 11.89 mg/g, which is higher than our sorbent. However, their sorbent was tested for only three reuse cycles, and after the second cycle, the recovery percentage decreased to 98%, while in our case, the recovery percentage was almost 100% even after 5 cycles (see Sect. 3.7).
Kamcev et al. have prepared a sorbent containing two NMG functional groups at different locations of the sorbent, which increase the sorption capacity [34]. The use of different starting materials that can bind more than one functional group can increase the removal capacity.
3.6 Thermodynamic studies
Thermodynamic studies were performed by varying the solution temperatures (30, 40, 50, and 60 °C). 0.2 g of the sorbent was mixed with 25 mL of boron-containing solution (5 mg-B/L, pH 6).
The changes in standard free energy (ΔG°), entropy (ΔS°), and enthalpy (ΔH°) were estimated using the following Eqs. 8–10 [42].
The calculated enthalpy change (ΔH°) was found as − 25.87 kJ/mol. The negative values indicate that the sorption phenomena are exothermic. The entropy change was (ΔS°) − 0.097 kJ/mol K, which means the loss of vibrational and rotational freedom of the groups of OH in GMA-NMG was involved in complexation with the boron atom [43]. The calculated values of standard free energy change (ΔG°) were positive for all temperature changes, implying that the extent of sorption is limited [44]. This could be due to the complexation of boron especially with cis-diol groups.
3.7 Regeneration and reuse of the sorbent
The regeneration process for the exhausted sorbent is the same as explained elsewhere [11]. The regeneration efficiency (RE, %) was calculated according to Eq. 11. The results are presented in Table 4. The results show that the sorbent can be regenerated with 0.5 M HCl or 0.5 M H2SO4 with efficiency more than 99%
The desorption and reuse tests of the prepared sorbent were carried out. In this part of the experiments, the 0.2 g sorbent was contacted with 25 mL of a boron-containing solution (5 mgB/L) for 1 h; then, the sorbent was removed from the solution and washed with deionized water. Regeneration of the sorbent was performed with 0.5 M 25 mL H2SO4 solution. The sorbent was kept in contact with the H2SO4 solution for 1 h. Then, it was removed from the acidic solution and washed with NaOH solution (pH 12) to remove the excess of H2SO4 from the sorbent and convert the quaternary groups to the free base form. After washing with NaOH solution, the sorbent was washed with deionized water to remove excess alkali from the sorbent and then used for further sorption tests. The sorption regeneration performance of the sorbent is shown in Fig. 8. As can be seen from the figure, the performance of the sorbent did not change after 5 sorption cycles, which can be considered a promising advantage from the environmental and economic point of view.
4 Conclusions
In summary, cellulose containing N-methyl-glucamine was prepared by a two-step process. In the first step, the glycidyl methacrylate-N-methyl glucamine monomer was synthesized, and in the second step, the monomer was attached to the cellulose. FTIR and elemental analysis confirmed the successful preparation of the sorbent. The results showed that optimal boron removal can be achieved at pH ≥ 4. The sorption reached equilibrium in 60 min. The maximum sorption capacity was found to be 4.7 mg B/g sorbent. Thermodynamic studies showed that the sorption of boron is exothermic and followed by a decrease in the randomness of the system. More than 99% of boron was desorbed during the regeneration process, and the sorbent can be used for at least five adsorption–desorption cycles.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- b :
-
Langmuir constant (L/mg)
- C 0 :
-
Initial boron concentration in the solution (mg/L)
- C e :
-
Boron concentration in the solution at equilibrium (mg/L)
- k 1 :
-
Pseudo-first-order rate constant (min−1)
- k 2 :
-
Pseudo-second-order rate constant (g/mg min)
- K F :
-
Freundlich adsorption constant
- m :
-
Mass of the sorbent used in the experiment (g)
- n :
-
Freundlich adsorption constant
- Q 0 :
-
Maximum sorption capacity for Langmuir model (mg/g)
- q e :
-
Amount of B sorbed onto sorbent at equilibrium (mg/g)
- q t :
-
Amount of B sorbed onto sorbent at any time (mg/g)
- r :
-
Removal efficiency (%)
- R :
-
Universal gas constant (8.314 J/mol-K)
- RE:
-
Regeneration efficiency (%)
- T :
-
Absolute temperature (K)
- t :
-
Time (min)
- V :
-
Volume of solution (L)
- ΔG°:
-
Standard free energy change (kJ/mol)
- ΔH°:
-
Standard enthalpy change (kJ/mol)
- ΔS°:
-
Standard entropy change (kJ/mol K)
References
Chen M, Dollar O, Shafer-Peltier K et al (2020) Boron removal by electrocoagulation: removal mechanism, adsorption models and factors influencing removal. Water Res 170:115362. https://doi.org/10.1016/j.watres.2019.115362
Lin J-Y, Mahasti NNN, Huang Y-H (2021) Recent advances in adsorption and coagulation for boron removal from wastewater: a comprehensive review. J Hazard Mater 407:124401. https://doi.org/10.1016/j.jhazmat.2020.124401
Hasenmueller EA, Criss RE (2013) Multiple sources of boron in urban surface waters and groundwaters. Sci Total Environ 447:235–247. https://doi.org/10.1016/j.scitotenv.2013.01.001
Sari MA, Chellam S (2015) Mechanisms of boron removal from hydraulic fracturing wastewater by aluminum electrocoagulation. J Colloid Interface Sci 458:103–111. https://doi.org/10.1016/j.jcis.2015.07.035
Mohapatra D, Chaudhury GR, Park KH (2008) Solvent extraction approach to recover boron from wastewater generated by the LCD manufacturing industry: part 1. Mining, Metall Explor 25:175–180. https://doi.org/10.1007/BF03403405
Guidelines for drinking-water quality WHO (2003) Boron in drinking-water background. Heal criteria other Support Inf 2:1–17
Wolska J, Bryjak M (2013) Methods for boron removal from aqueous solutions — a review. Desalination 310:18–24. https://doi.org/10.1016/j.desal.2012.08.003
Güler E, Kaya C, Kabay N, Arda M (2015) Boron removal from seawater: state-of-the-art review. Desalination 356:85–93. https://doi.org/10.1016/j.desal.2014.10.009
Guan Z, Lv J, Bai P, Guo X (2016) Boron removal from aqueous solutions by adsorption — a review. Desalination 383:29–37. https://doi.org/10.1016/j.desal.2015.12.026
Qin F, Fang Z, Zhou J et al (2019) Efficient removal of Cu2+ in water by carboxymethylated cellulose nanofibrils: performance and mechanism. Biomacromol 20:4466–4475. https://doi.org/10.1021/acs.biomac.9b01198
Parlak E, Arar Ö (2018) Removal of copper (Cu2+ ) from water by sulfonated cellulose. J Dispers Sci Technol 39:1403–1408. https://doi.org/10.1080/01932691.2017.1405818
Özdemir VT, Tuğaç HM, Arar Ö (2020) Two-pot oxidative preparation of dicarboxylic acid containing cellulose for the removal of Beryllium (Be2+) from aqueous solution. Curr Anal Chem 16:. https://doi.org/10.2174/1573411016999200719232310
Anirudhan TS, Nima J, Divya PL (2013) Adsorption of chromium(VI) from aqueous solutions by glycidylmethacrylate-grafted-densified cellulose with quaternary ammonium groups. Appl Surf Sci 279:441–449. https://doi.org/10.1016/j.apsusc.2013.04.134
Bhattacharya A (2004) Grafting: a versatile means to modify polymers: techniques, factors and applications. Prog Polym Sci 29:767–814. https://doi.org/10.1016/j.progpolymsci.2004.05.002
Gowda DV, Koshy T, Godugu K, Srivastava A (2016) Polymer grafting-an overview. Am J Pharmatech Res 6:1–13
Wang S, Wang Z, Li J et al (2020) Surface-grafting polymers: from chemistry to organic electronics. Mater Chem Front 4:692–714. https://doi.org/10.1039/C9QM00450E
Arar O, Kabay N, Sánchez J et al (2014) Removal of arsenic from water by combination of electro-oxidation and polymer enhanced ultrafiltration. Environ Prog Sustain Energy 33:918–924. https://doi.org/10.1002/ep.11876
Holdich RG, Cumming IW, Perni S (2006) Boron mass transfer during seeded microfiltration. Chem Eng Res Des 84:60–68. https://doi.org/10.1205/cherd.05015
Abdul Razak S, Mahadi AH, Abdullah R et al (2020) Biohydrogen production from photodecomposition of various cellulosic biomass wastes using metal-TiO2 catalysts. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01164-4
Arar Ö (2020) Co-precipitative preparation of a sulfonated cellulose-magnetite hybrid sorbent for the removal of Cu2+ Ions. Anal Sci 36:81–86. https://doi.org/10.2116/analsci.19SAP01
Puri S, Sharma S, Kumari A et al (2020) Extraction of lignocellulosic constituents from cow dung: preparation and characterisation of nanocellulose. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01119-9
Sarıçiçek EN, Tuğaç MM, Özdemir VT et al (2021) Removal of boron by boron selective resin-filled electrodeionization. Environ Technol Innov 23:101742. https://doi.org/10.1016/j.eti.2021.101742
Kabay N, Sarp S, Yuksel M et al (2007) Removal of boron from seawater by selective ion exchange resins. React Funct Polym 67:1643–1650. https://doi.org/10.1016/j.reactfunctpolym.2007.07.033
Yoshimura K, Miyazaki Y, Ota F et al (1998) Complexation of boric acid with the N-methyl-D-glucamine group in solution and in crosslinked polymer. J Chem Soc Faraday Trans 94:683–689. https://doi.org/10.1039/a707790d
Ho Y, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Ho Y, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124. https://doi.org/10.1016/S1385-8947(98)00076-X
Alyüz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J Hazard Mater 167:482–488. https://doi.org/10.1016/j.jhazmat.2009.01.006
Inukai Y, Tanaka Y, Matsuda T et al (2004) Removal of boron(III) by N-methylglucamine-type cellulose derivatives with higher adsorption rate. Anal Chim Acta 511:261–265. https://doi.org/10.1016/j.aca.2004.01.054
Kavak D (2009) Removal of boron from aqueous solutions by batch adsorption on calcined alunite using experimental design. J Hazard Mater 163:308–314. https://doi.org/10.1016/j.jhazmat.2008.06.093
Masindi V, Gitari MW, Tutu H, Debeer M (2016) Removal of boron from aqueous solution using magnesite and bentonite clay composite. Desalin Water Treat 57:8754–8764. https://doi.org/10.1080/19443994.2015.1025849
Ruiz M, Tobalina C, Demey-Cedeño H et al (2013) Sorption of boron on calcium alginate gel beads. React Funct Polym 73:653–657. https://doi.org/10.1016/j.reactfunctpolym.2013.01.014
Cengeloglu Y, Tor A, Arslan G et al (2007) Removal of boron from aqueous solution by using neutralized red mud. J Hazard Mater 142:412–417. https://doi.org/10.1016/j.jhazmat.2006.08.037
Ferreira OP, de Moraes SG, Durán N et al (2006) Evaluation of boron removal from water by hydrotalcite-like compounds. Chemosphere 62:80–88. https://doi.org/10.1016/j.chemosphere.2005.04.009
Kamcev J, Taylor MK, Shin D et al (2019) Functionalized porous aromatic frameworks as high-performance adsorbents for the rapid removal of boric acid from water. Adv Mater 31:1808027. https://doi.org/10.1002/adma.201808027
Recepoğlu YK, Kabay N, Yılmaz-İpek İ et al (2017) Deboronation of geothermal water using N-methyl-D-glucamine based chelating resins and a novel fiber adsorbent: batch and column studies. J Chem Technol Biotechnol 92:1540–1547. https://doi.org/10.1002/jctb.5234
Liu S, Xu M, Yu T et al (2017) Radiation synthesis and performance of novel cellulose-based microsphere adsorbents for efficient removal of boron (III). Carbohydr Polym 174:273–281. https://doi.org/10.1016/j.carbpol.2017.06.012
Wang J, Wang T, Li L et al (2014) Functionalization of polyacrylonitrile nanofiber using ATRP method for boric acid removal from aqueous solution. J Water Process Eng 3:98–104. https://doi.org/10.1016/j.jwpe.2014.05.015
Polowczyk I, Ulatowska J, Koźlecki T et al (2013) Studies on removal of boron from aqueous solution by fly ash agglomerates. Desalination 310:93–101. https://doi.org/10.1016/j.desal.2012.09.033
Wahib SA, Da’na DA, Ashfaq MY, Al-Ghouti MA (2021) Functionalized cellulose nanocrystals as a novel adsorption material for removal of boron from water. Case Stud Chem Environ Eng 4:100121. https://doi.org/10.1016/j.cscee.2021.100121
Ting T-M, Hoshina H, Seko N, Tamada M (2013) Removal of boron by boron-selective adsorbent prepared using radiation induced grafting technique. Desalin Water Treat 51:2602–2608. https://doi.org/10.1080/19443994.2012.749054
Hong M, Li D, Wang B et al (2021) Cellulose-derived polyols as high-capacity adsorbents for rapid boron and organic pollutants removal from water. J Hazard Mater 419:126503. https://doi.org/10.1016/j.jhazmat.2021.126503
Babu DK, Ravindhranath K, Mekala S (2021) Simple effective new bio-adsorbents for the removal of highly toxic nitrite ions from wastewater. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01677-6
Dawber JG, Matusin DH (1982) Potentiometric and polarimetric studies of the reaction of boric acid and tetrahydroxyborate ion with polyhydroxy compounds. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases 78:2521. https://doi.org/10.1039/f19827802521
Shahwan T (2021) Critical insights into the limitations and interpretations of the determination of ∆Go, ∆Ho, and ∆So of sorption of aqueous pollutants on different sorbents. Colloid Interface Sci Commun 41:100369. https://doi.org/10.1016/j.colcom.2021.100369
Acknowledgements
The authors thank Denkim Kimya A.Ş. for proving the cellulose samples. The authors also thank Dr. Emre Seyyal for his valuable comments on this paper and proofreading the manuscript.
Funding
This study is supported by Ege University Scientific Research Projects Coordination Unit (Project Number: FLP-2020–22167).
Ege Üniversitesi,FLP-2020–22167,Özgür Arar
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yetgin, A.G., Dündar, O.A., Çakmakçı, E. et al. Removal of boron from aqueous solution by modified cellulose. Biomass Conv. Bioref. 13, 13081–13090 (2023). https://doi.org/10.1007/s13399-021-02133-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02133-1