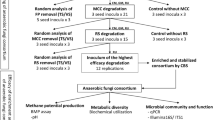
Abstract
Lignocellulosic biomass from agricultural waste seems promising feedstock for biofuel production; however, its degradation to fermentable sugars is challenging. Interestingly, fungi have shown substantial potential for the breakdown of lignocellulosic biomass and thus could be employed in lignocellulose-based biorefinery. Aiming at this, the current study was focused on screening the novel cellulolytic fungi from the surrounding environment. The preliminary molecular/morphological screening of 107 samples narrowed the experiment to 5 different fungi, designated: Aspergillus tubingensis AKF2, Aspergillus flavus AKF3, Pyricularia oryzae AKF4, Aspergillus nominus AKF5, and Aspergillus oryzae AKF6. The selected fungi were evaluated for their cellulolytic potential utilizing wheat straw, corn cob, and rice husk. The highest enzyme activity (CMCase: 68.2 U ml−1; FPase: 343.3 U ml−1; β-Gase: 86.3 U ml−1; xylanase: 234.6 U ml−1) was observed on the 8th day in the cultures of A. tubingensis AKF2 using wheat straw as compared to corn cob and rice husk. The total reducing sugars released after enzymatic breakdown were also high (437.05 mg g−1) in the wheat straw cultures of A. tubingensis AKF2 comprehending the higher cellulolytic activity of this fungus. To further investigate the compositional breakdown, scanning electron microscopy and FTIR were performed. Significant (LSD > 0.05) results were observed in the wheat straw cultures of A. tubingensis AKF2 with 48%, 36%, 39%, and 56% reductions in the cellulose, hemicellulose, lignin, and total mass contents, respectively. The superseding fungus (A. tubingensis AKF2) identified here could be further optimized for an eco-sustainable biorefinery and may have industrial applications for cellulase production.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lignocellulosic biomass is a remarkable resource for the extraction of bio-based fuels [1] and other value-added products such as organic acids, fructans, phenolics, mono-/oligomeric pentoses, hexoses, etc., [2]. Each year, staple crops leeave residues of ~ 5 billion metric tons worldwide [3] which could be potentially utilized for biofuel extraction without any ethical/environmental or arable land-use conflicts. Research is focused on producing eco-friendly and renewable bioenergy and making it a progressive alternative to fossil fuels due to their continuous depletion and environmental deterioratic effects [4, 5].
Lignocellulosic biomass is recalcitrant and primarily consists of cellulose, lignin, and hemicellulose polymers that intervened to form vegetal biomass [6]. Cellulose forms the internal core which is surrounded by lignin and hemicellulose [7]. Cellulosic polymers are formed by anhydrous gluconic rings (cellobiose) via β-1,4 glycosidic linkages. These cellulosic polymers are linked via hydrogen and Van der Waals bonding to form a highly complex crystalline structure. Lignocellulosic biomass cannot be used directly for biofuel production due to its highly polymerized structure that is inaccessible to the fermentable microorganisms ; thus, it must be broken down using any physical, chemical, or biological pretreatments to facilitate the structural breakdowns and to decrease the crystallinity [8]. The conversion of complex polymerized cellulosic sugars into simpler monomeric forms is done by the process known as saccharification [9] and achieved by the cooperative actions of various cellulolytic, hemicellulolytic, and ligninolytic enzymes produced by fungi and bacteria competing for the carbon cycle. Lignocellulosic biomass hydrolysis has been optimized by adopting various parameters such as acid-ionic pretreatment [10], combining dilute acid with high temperature/pressure [11], and mostly reporting mild-alkali pretreatments.
Enzymatic saccharification using fungi is a cost-efficient and sustainable approach. Cellulases are complex enzymes including endoglucanases (EG, EC 3.2.1.4) randomly cleaving the internal β-1,4-glycosidic bonds, exo-cellobiohydrolases (CBH, EC 3.2.1.91) releasing cellobiose from reducing/non-reducing ends, and β-glucosidases (BGL, EC 3.2.1.21) hydrolyzing the cellobiose to glucose monomers [12, 13]. Cellulases have several applications in the industry including paper, pulp, laundry processing, textile, food, bioconversion, and animal feed relying on lignocellulose substrates [14, 15]. The search for cellulase producers including white-rot/thermophilic fungi remained inevitable.
Filamentous fungal species such as Trichoderma reesei and Aspergillus niger have demonstrated the higher ability of lignocellulose hydrolysis in several studies [16,17,18]. This might be attributed to the ability of these fungi to colonize the soil near plants and subsequent penetrations into plant tissues through the development of their hyphae and enzyme’s release to hydrolyze the recalcitrant components [19]. The model strain of T. reesei RUT C30 yielding higher cellulases was screened after several rounds of induced mutations in the original T. reesei QM6 isolated during World War II [20]. Around 80–85% of the T. reesei core enzymes during saccharification constitute the exo-cellobiohydrolases (CBH, EC 3.2.1.91) cleaving the cellulose content [21]; thus, hemicellulose breakdown needs further improvements in the fungal secretome. The quest for the screening of new species is continued with renewed interests in the hydrolysis of the lignocellulosic biomass using minimum resources.
Aiming to identify some novel fungal species from the local biosphere degrading the accessible agricultural residues, we screened ~ 107 local fungal isolates from Punjab, Pakistan, for their cellulolytic potential. Limited data is available on the biodiversity of fungal species in Pakistan focusing the hydrolytic activities. Being an agricultural country, Pakistan has tremendous potential for the lignocellulosic biomass coming from the residues of major food crops like wheat, rice, and maize. This could offer a large reactor for rich microbial growth yielding higher cellulase activities. In the context of biorefinery perspectives, our work emphasized on local fungal strain selection with high biotechnological applicability.
We investigated 5 different fungal strains for their cellulolytic potential cultivated on different carbon sources (wheat straw, corn cob, rice husk). Further, structural modification in the biotreated lignocellulosic biomass was confirmed through compositional assays, scanning electron microscope (SEM) analysis, and Fourier transform infrared (FTIR) spectroscopy. The schematic flowchart of the experimental work is presented in Fig. 1.
2 Material and methods
2.1 Sample collection
Fungi were collected from various samples in the surroundings (approximate coordinates of one sampling point: 31.312533°N; 72.936067°E) including soil, wood residues, rotten fruits/vegetable waste, decaying leaves, etc. . The collected samples (~ 10 g each) were labeled, packed in plastic zipper bags, and were kept at 4 °C. Later, the samples were cultivated on Petri plates (100 × 15 mm) containing ~ 20 ml of potato dextrose agar (PDA; Merck®, Darmstadt, Germany) for 2–3 days at 28 °C for the primary fungi cultures by using the sprinkling/streak plate method [22]. After incubation, the primary fungi cultures were sub-cultured on PDA and Sabouraud dextrose agar (SDA; Merck®, Darmstadt, Germany) plates for 3–4 times to remove the cross-contamination and to screen the pure fungi species. The fungi Trichoderma reesei M182 was obtained from the culture collection lab of the Fermentation Technology Group, NIBGE, Faisalabad, Pakistan [18].
2.2 Molecular identification of the fungi isolates
Molecular identification of the isolated fungi was based on the PCR amplification of the conserved nucleotide sequence of the ribosomal internal transcribed spacer (ITS) of the 18S rRNA [23]. For this purpose, fungal genomic DNA was isolated and purified using the UltraClean Soil DNA-isolation Kit (Mo-Bio Laboratory, CA, USA). For ITS region amplification, primers [Forward: ITS-1 (5′-TCCGTAGGTGAACCTGAGG-3′); Reverse: ITS-4 (5′-TCCTCCGCTTATTGATATGC-3′)] were used using DREAM GREEN 2 × Master Mix (Fermentas®, Canada) with following PCR profile (initial denaturation @ 95 °C for 10 min; (denaturation @ 95 °C for 1 min; primer annealing @ 59 °C for 1 min; extension @ 72 °C for 1 min) × 35 cycles; and a final extension @ 72 °C for 10 min). The PCR products were resolved on 1% agarose gel and observed for product size. For molecular confirmation, DNA sequencing was performed (Macrogen®, Korea). Species were identified based on sequence homology through NCBI nucleotide BLAST analysis (http://www.ncbi.nlm.nih.gov/BLAST/). The obtained sequences were submitted to the NCBI database for accession numbers. Based on the sequencing results, phylogenetic analysis was performed for specie demarcation.
2.3 Primary screening for the cellulolytic potential of the isolates
At first, the cellulase production of the isolated fungi was analyzed by the carboxymethyl cellulose (CMC) agar plate method as described by Sazci et al. [24]. For this purpose, conidia harvested from 1-week-old fungi cultures (PDA) were resuspended in the center of the screening plates (CMC agar). After incubation of 3–5 days at 28 °C, then at 50 °C for the last 18 h, the plates were stained (30–60 min) with 1% Congo-red dye following washing with 1 M NaCl for 15–20 min. Based on the diameter (mm) of the cellulose hydrolysis zone around the colonies [25], potentially cellulolytic strains were screened.
2.4 Preparation of lignocellulosic biomass substrates
Lignocellulosic substrates comprising wheat straw, corn cob, and rice husk were collected from local agricultural fields (~ coordinates 31.314534°N, 72.936589°E) of Akhuwat-Faisalabad Institute of Research Science and Technology (FIRST), Faisalabad, Pakistan. After washing with tap water followed by sterile distilled water, the collected substrate samples were dried in a hot air oven (60 °C) and then processed into fine particles (~ 2 mm) using a hammer beater mill. The processed samples were packed in plastic zipper bags for further use.
2.5 Saccharification of lignocellulosic substrates
2.5.1 Fungal sample preparation
Based on prescreening cellulase activity (Congo red staining assays), five fungal strains (AKF2, AKF3, AKF4, AKF5, AKF6) were further selected for their hydrolytic potential. Pure cultures of the strains (AKF2, AKF3, AKF4, AKF5, AKF6) were preserved on PDA slants and were kept at 4 °C until further use. The spores of these selected strains were sub-cultured on sterile Petri plates containing fresh PDA (pH 5.0)and incubated upside down at 25 °C in the dark for 5 days. All chemicals and reagents used in the current study were of analytical grade.
2.5.2 Preparation of spore inoculum
To prepare the spore inoculum, 10 ml of sterile saline water was added to the 5-day-old fungi culture plates (PDA) and were scraped with a sterile spatula. The mixture was sterile filtered, and the spore count was calculated using a hemocytometer assisted with a light microscope. The number of spores per ml of inoculum was calculated using the following equation:
After calculating the number of spores, the spore suspensions were transferred to sterile 50-ml tubes and diluted with sterile saline water until a spore count of ~ 107 spores ml−1 was achieved.
2.5.3 Preparation of culture media and experimental design
For hydrolytic activity, a randomized complete block design (RCBD) experiment was plotted constituted of three blocks (lignocellulosic substrates), five treatments (the 5 selected fungi: AKF2, AKF3, AKF4, AKF5, AKF6), and 3 replicates for each treatment within a block. Following this RCBD, a total of 45 sterile flasks were prepared containing 1 g of lignocellulosic substrate and 100 ml of Mandel and Sternburg’s medium (yeast extract, 20 g/L; peptone, 0.25 g/L; Tween-80 1 ml/L; urea, 0.3 g/L and mineral composition (g/L): (NH4)2SO4, 1.4; K2HPO4, 2; CaCl2·2H2O, 0.3; MgSO4·7H2O, 0.3; FeSO4·7H2O, 0.05.; MnSO4·7H2O; 0.01; ZnSO4·7H2O, 0.01; CoCl2·6H2O; 0.02) [26]. The media containing flasks were autoclaved at 121 °C (15 psi) for 15–20 min.
2.6 Submerged fermentation and crude enzyme extraction
The culture flasks were inoculated with 1 ml of fungal spore (as mentioned above in Sect. 2.5.2), agitated at 120 rpm at 28 °C. At the 4th, 6th, 8th, and 10th days, 5 ml of aliquot sample was aseptically taken from each flask. The collected samples were centrifuged at 8000 rpm for 8 min at 4 °C to remove the undesirable particulate matter/spores. The scrap-free supernatant was used for further enzyme assays and to calculate the total proteins and reducing sugars.
2.7 Enzyme assays
All the experiments for enzyme assays were performed in triplicates. The absorbance readings were measured on a double-beam spectrophotometer (AE-S90-2D, Guangzhou, China). For reference concentration, a standard curve was plotted against the known concentrations of standard solutions. The enzyme activity was expressed as “U ml−1” where “U” represents the amount of enzyme required to liberate 1 µM glucose per minute. The enzyme activities were calculated according to the following equation:
where
- ∆A:
-
absorbance at spectrophotometer
- Std Factor:
-
1/slope
- Volume of QRM:
-
Volume of DNS
2.7.1 Cellulolytic enzyme assays
Filter paper assay (FPase) is a standard measure of total cellulase assay according to IUPAC recommendations and was used following the “Laboratory Analytical Methods” NREL, USA [27]. The assay mixture contained 0.5 ml of crude enzyme supernatant, 1 ml of sodium citrate buffer (pH 4.8), and Whatman #1 filter paper strips (6 × 1 cm), incubated at 50 °C for 60 min. After that, 3 ml of 3, 5-dinitrosalicylic acid (DNS) reagent was added to the reaction mixture, and the reaction was stopped at 100 °C and allowed for color development. Finally, the absorbance [optical density (OD)] was measured at 545 nm. Carboxymethyl cellulase assay (CMCase) was used for measuring the endo-β-1,4 glucanase following the method used by Ghose [28]. The assay mixture contained 0.5 ml of 1% (w/v) CMC solution as substrate (diluted with 50 mM citrate buffer (pH 4.8)) and 0.5 ml of crude enzyme. After an incubation of 30 min at 50 °C, 1 ml of DNS reagent was added to the reaction mixture, and the reaction was stopped at 100 °C and allowed for color development. Finally, the OD was measured at 545 nm. β-Glucosidase assay was performed by the method used by Herr [29]. The assay mixture contains 0.5 ml of 0.01 M pNPG (ρ-nitrophenyl β-d-glucopyranoside) in 0.05 M citrate buffer at pH 4.8 and 0.5 ml of crude enzyme extract. After incubation for 30 min at 50 °C, 4 ml of NaOH-Glycine buffer (50 mM and pH 10.6) was added to stop the reaction. Finally, the OD was measured at 420 nm. The standard activity of β-glucosidase is defined as the amount of enzyme required to liberate 1 µM of ρ-nitrophenyl under standard conditions. Xylanase activity was measured using 1% birchwood xylan as a substrate. The assay mixture contained 0.5 ml of 1% (w/v) birchwood xylan as substrate (diluted with 50 mM citrate buffer (pH 4.8)) and 0.5 ml of crude enzyme. After an incubation of 20 min at 50 °C, 1 ml of DNS reagent was added to the reaction mixture, and the reaction was stopped at 100 °C for 5 min and allowed for color development. Finally, the OD was measured at 545 nm.
2.7.2 Ligninolytic enzyme assays
Lignin peroxidase (LiP) and manganese peroxidase (MnP) activities were measured by following the methods described by Xu et al. [30]. For LiP activity, the assay mixture contained 4.34 ml of 0.2 M sodium tartrate buffer (pH 3.0), 0.6 ml of 10 mM veratryl alcohol, and 1 ml of diluted enzyme supernatant. The reaction was triggered by adding 60 µl of 2 mM hydrogen peroxide solution at 30 °C, and the absorbance was measured at 310 nm. The LiP activity was defined as the oxidation of veratryl alcohol to 1 mM veratraldehyde per min under standard conditions. For MnP activity, the assay mixture contained 0.1 ml of 40 mM MnSO4, 3.4 ml of 50 mM sodium tartrate solution (pH 5.0), and 0.4 ml of diluted enzyme supernatant. The reaction was triggered by adding 0.1 mM of 1.6 mM hydrogen peroxide solution at 30 °C, and the absorbance was measured at 310 nm. The MnP activity was defined as the enzyme required for the oxidation of Mn2+ to Mn3+ and that oxidized 1 mM MnSO4 per min under standard conditions.
2.8 Total protein and reducing sugar estimation
The total protein content was measured using the method devised by Lowery et al. [31] using bovine serum albumin (BSA) as a standard. At first, 0.5 ml of crude supernatant was mixed with 2.5 ml of alkaline copper reagent (ACR) and incubated at room temperature for 10 min. Then, 0.5 ml of freshly prepared Folin reagent was added, and the solution was processed for 30 min for color development. Finally, the OD was measured at 750 nm. The total reducing sugars released in enzyme supernatant were estimated using the DNS method [32]. For this purpose, 3 ml of DNS reagent was taken in a test tube (16 × 150 mm) and was mixed with 1 ml of crude enzyme supernatant. Finally, the reaction was stopped with boiling water (100 °C) incubation for 5 min and allowed for color development. The OD was measured at 550 nm. Absorbance was then translated into reducing sugar concentrations by the use of a glucose standard calibration curve.
2.9 Wheat straw biodegradation assays
2.9.1 Mass reduction and compositional analysis
The total mass of the wheat straw was measured before and after the biodegradation experiments. After the enzymatic hydrolysis, wheat straw was isolated from culture flasks and was dried in a hot air oven (60 °C) to evaporate the total moisture contents. The differences in values were recorded as total mass reduction and are presented as a mass reduction percentage (%). Similarly, compositional analysis of cellulose, hemicellulose, and lignin was done with sequential detergence using natural, acid, and 72% H2SO4 detergents following protocols described by Godin et al. [33]. The percentage reduction of total mass, cellulose, hemicellulose, and lignin was calculated using the following equation:
where
- M(B):
-
mass of the substrate before culture inoculation
- M(A):
-
obtained mass of the substrate after fermentation
2.9.2 SEM analysis of biotreated wheat straw
To investigate the structural modifications treated by fungal strains on wheat straw, SEM (JSM-6360LVPRIME, JEOL®, USA, fitted with Carl Zeiss Ultra Plus lens) analysis was performed. For this purpose, wheat straw was collected after saccharification and was dried in a hot air oven at 60 °C for 24 h. Untreated (control) and biotreated wheat straw samples were subjected to SEM analysis (device magnification scale (200 × –2.50 k ×), 10 kV accelerated voltage). SEM pictures were taken on a black carbon cord and gold-coated with a sputter coater from untreated and biotreated wheat straw.
2.9.3 FTIR analysis of wheat straw
To observe the functional group modifications in wheat straw due to fungal activities, FTIR analysis was performed using an instrument (IRAffinity-1, Shimadzu®, Japan, equipped with a 2-m gas cell and a DTGS KBr detector). Wheat straw obtained from post-cultural harvesting was brought into the FTIR analysis. For this purpose, 2 mg of wheat straw sample was grounded in an agate-mortar and blended with 200 mg of spectroscopic-grade KBr and pressed to create 13-mm (diameter) pellets. In the region of 4000–500 cm−1, the spectrums of untreated (control) and biotreated wheat straw were calculated.
2.10 Statistical analysis
The saccharification experiment was designed following the RCBD plot. The enzyme results are presented with bar graphs, in which each bar represents the mean ± standard error of the three replicates within a treatment. Statistical analyses were performed using Statistix software (Statistics 8.1, Analytical Software®, Tallahassee, FL 32,312, USA). Results were designated significant at P < 0.05* and designated with different letters (a/b/c/d/e) by comparing means using the LSD pair-wise comparison test. The bar values designated with same letters (a/b/c/d/e) are non-significant at P > 0.05 = ns.
3 Results and discussion
3.1 Fungal sample collection and potential species screening
Aiming to identify the potential fungi with higher biodegradation capability, nearly 107 different samples (leaf pieces, mixed fruit pieces, kitchen waste, wood residues, crop residues, etc.,) were collected for the in vitro cultivation of fungal species. From a fairly large number of cultures on PDA plates, we selected 20 different strains (AKF1–AKF20) based on their morphology and culturing conditions (Supplementary Fig. 1). For specie identification, the selected strains (AKF1–AKF20) were subjected to PCR fingerprinting for the amplification of the 18S ITS region. The PCR amplification resulted in the expected product size (500–700 bp) in all samples (PCR amplification of the 5 selected fungi, Supplementary Fig. 2A). To further identify the amplified PCR products, sequencing was performed for the ITS region analysis. The selected strains were assigned to the respective fungal specie based on the sequence similarity resulting from the BLASTN analysis. Based on > 98% of query coverage, the similarity index of each sample strain with the corresponding reference strain and the corresponding accession numbers assigned are presented in Table 1.
For the initial screening of cellulase-producers, the solid-state cultures (CMC-agar plates) of the selected strains (AKF1–AKF20) were subjected to Congo-red staining analysis. The cellulase activity was measured in context to the clearing halo (diameter) of hydrolysis produced by each sample (Table 1; Supplementary Fig. 2B). Among 20 different isolates, we selected 5 isolates (Aspergillus tubingensis AKF2, Aspergillus flavus AKF3, Pyricularia oryzae AKF4, Aspergillus nominus AKF5, Aspergillus oryzae AKF6) producing higher values of clearing halo diameter (Table 1) for further study. The preselection of fungal strains based on crude enzyme extracts using Congo-red staining is advantageous for the subsequent selection of higher cellulase producers [34]. The sequences of the selected fungi were submitted to NCBI and received the following accessions: Aspergillus tubingensis AKF2 (MZ356366), Aspergillus flavus AKF3 (MZ356370), Pyricularia oryzae AKF4 (MZ356384), Aspergillus nominus AKF5 (MZ356386), Aspergillus oryzae AKF6 (MZ356388).
3.2 Cellulolytic enzyme assays and determination of suitable carbon substrate
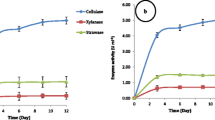
The cellulolytic activity (CMCase, FPase, β-GaSe, Xylanase) of the selected fungi (A. tubingensis AKF2, A. flavus AKF3, P. oryzae AKF4, A. nomius AKF5, and A. oryzae AKF6) was measured during submerged fermentation using wheat straw, corn cob, and rice husk as lignocellulose substrates. The enzyme assays were performed at the 4th, 6th, 8th, and 10th days of the fermentation time course. Previously, the fungal cellulolytic activities have been evaluated either on a single biomass type [16, 18, 35] or on different biomass types [30, 34]. Figure 2 A–D show the cellulolytic activities of CMCase, FPase, β-glucosidase, and xylanase of the sample fungi on wheat straw, corn con, and rice husk measured on the 10th day of fermentation. The results show that all fungi produced hydrolytic enzymes on different substrates; however, a significant variation (LSD; P < 0.05) was observed in the case of the substrate and fungal species (Fig. 2). Overall, A. tubingensis AKF2 showed higher cellulolytic activity (CMCase: 68.2 ± 0.37 U ml−1; FPase: 343.3 ± 5.63 U ml−1; β-Gase: 86.3 ± 1.37 U ml−1; xylanase: 234.6 ± 3.63 U ml−1) using wheat straw when compared to the rice husk and corn cob (Fig. 2). The higher cellulolytic activity of A. tubingensis AKF2 using wheat straw might be due to substrate saccharide contents constituting cellulose (36%), hemicellulose (26%), arabinoxylan, and starch [36]. Furthermore, Xu et al. [30] reported a significantly higher increase (20.1%) of mycelial growth of white-rot fungi (Inonotus obliquus) on wheat straw comparing rise and corn biomasses, correlating the effects of carbon substrates on fungal growth. The CMCase activity of A. tubingensis AKF2 in this work is comparable and higher than the productivities of other fungi reported previously [30, 37].
Enzymatic activities of the selected fungi (Aspergillus tubingensis AKF2, Aspergillus flavus AKF3, Pyricularia oryzae AKF4, Aspergillus nominus AKF5, and Aspergillus oryzae AKF6) cultivated with wheat straw, rice husk, and corn cob as lignocellulose substrates. The enzyme activity is detected on day 8th and expressed as “U ml−1” where “U” represents the amount of enzyme required to liberate 1 µM of glucose per minute. The bar values represent the average mean (Error bar = standard deviation) of 3 replicates. The bar values sharing the same letters are non-significant (LSD > 0.05)
Fermentation using corn cob also produced significant values of cellulolytic enzymes next to wheat straw (Fig. 2: A. tubingensis AKF2 (CMCase: 55.7 ± 0.31 U ml−1; FPase: 201.6 ± 3.65 U ml−1; β-Gase: 77.39 ± 1.31 U ml−1; xylanase: 201.6 ± 3.13 U ml−1)). Previously, Bakri et al. [38] reported the higher hydrolytic activity (174 ± 21.88 U ml−1) of Aspergillus strain FSS129 using corn cob as a carbon substrate comparing various biomasses. The other interesting results were obtained in case of A. oryzae AKF6 where a comparative less variation was observed in enzyme activity using wheat straw (CMCase: 31.1 ± 0.43 U ml−1; FPase: 177.1 ± 0.38 U ml−1; β-Gase: 63.5 ± 1.43 U ml−1; xylanase: 155.6 ± 1.01 U ml−1), corn cob (CMCase: 20.7 ± 0.12 U ml−1; FPase: 160.7 ± 1.79 U ml−1; β-Gase: 42.6 ± 1.12 U ml−1; xylanase: 162.3 ± 1.79 U ml−1) and rice husk (CMCase: 23.8 ± 0.37 U ml−1; FPase: 172 ± 0.74 U ml−1; β-Gase: 33.6 ± 1.37 U ml−1; xylanase: 178.3 ± 1.9 U ml−1) (Fig. 2). Aspergillus genus has been well known for the production of cellulolytic enzymes using wheat straw such as A. oryzae [39], A. flavus [40], and A. tubingensis [41].
The Pyricularia genus (P. oryzae AKF4) showed a poor cellulolytic response in the case of wheat straw and corn cob substrates (Fig. 2); however, by using rice husk, a significantly higher value (152.3 ± 0.18 U ml−1) was obtained in FPase (Fig. 2B). This might be due to the natural host of P. oryzae infecting rice crops [42]. Xylanase activity was high in cultures of wheat straw and corn cob in most of the samples as compared to the rice husk (Fig. 2D); however, in A. oryzae AKF6, significant higher activity was observed 178.3 ± 1.9 U ml−1. This follows the results of Xu et al. [30], where a five fold increase in xylanase activity was detected in rice straw culture of I. obliquus than the Trichoderma spp. used previously. In this study, the xylanase activity of A. tubingensis AKF2 cultures using wheat straw was 234.6 ± 3.63 U ml−1 on the 10th day of fermentation and nearly in line with the xylanase activity described previously [30]. The breakdown of lignocellulose substrates requires a blend of hydrolytic enzymes working synergistically [43]. The selection of suitable carbon substrate for the selected strains aimed to increase the saccharification process for a rapid bioconversion. The interesting results of this study suggest that the isolated A. tubingensis AKF2 has the potential for utilizing wheat straw as an effective carbon source with the highest cellulolytic activity among other isolated strains. The leading results of the enzyme assays were obtained with wheat straw cultures, and thus used as carbon substrate in further experiments.
3.3 Enzyme assays of A. tubingensis AKF2 and T. reesei M184
To proofread the higher cellulolytic activity of the best performing fungus, we repeated the enzymatic hydrolysis of wheat straw by using A. tubingensis AKF2 and T. reesei M182 (Fig. 3). The filamentous Trichoderma strains have been extensively used in industry for cellulolytic enzymes [44, 45], and here, T. reesei M182 was used as reference strain to compare the cellulolytic activity with A. tubingensis AKF2. The results show an interesting pattern of cellulolytic enzymes produced by both fungi (Fig. 3). The highest CMCase activity (125.3 ± 2.49 U ml−1) of T. reesei M184 was recorded at 144 h of cultivation time (Fig. 3A), whereas A. tubingensis AKF2 produced 132.6 ± 4.31 U ml−1 at the same cultivation time (Fig. 3A). These results are comparable to our previous study where we reported a higher CMCase activity (~ 195 U ml−1) of T. reesei M184 at 96 h of cultivation in SSF experiment [18]. Here, we reported the higher CMCase activities of both fungi at 144 h of cultivation, and this might be due to variations in culture conditions or SmF experiment. A very higher CMCase activity (750 U/gds) of A. tubingensis was also reported by utilizing copra meal in SSF [41]. Likewise, Khokhar et al. [46] reported the higher CMCase activity (257.5 U ml−1) of T. reesei at 144 h using wheat straw as a carbon source. The FPase activities of both fungi were recorded higher (A. tubingensis AKF2; 326.3 ± 6.32 U ml−1: T. reesei-M184; 350.3 ± 4.41 U ml−1) at 240 h (10th Day) of cultivation time (Fig. 3B). These results are in line with the findings of Khokhar et al. [46].
Enzymatic activities of the selected fungi (Aspergillus tubingensis AKF2 and Trichoderma reesei M168) cultivated with wheat straw as lignocellulose substrates. The enzyme activities were detected on 48, 96, 144, 168, and 240 h of cultivation time and expressed as “U ml−1” where “U” represents the amount of enzyme required to liberate 1 µM of glucose per minute. The values represent the average mean (Error bar = standard deviation) of 3 replicates. A CMCase activities of A. tubingensis AKF2, and T. reesei M168, B FPase activities of A. tubingensis AKF2, and T. reesei M168, and C Lip and MnP activity of A. tubingensis AKF2
The lignin-degrading ability of A. tubingensis AKF2 was analyzed through LiP and MnP enzyme activity. The selected fungi produced both ligninolytic enzymes with varying concentrations at different cultivation times (Fig. 3C). The highest LiP activity of A. tubingensis AKF2 was recorded as 146.36 ± 3.32 U ml−1 at 48 h of cultivation (Fig. 3C). White-rot fungi have been associated with ligninolytic activities with partial or full enzyme activities [47]. Xu et al. [30] reported a higher LiP activity (123.4 U ml−1) of Inonotus obliquus on day 4 utilizing wheat straw and proofread the higher LiP activity of A. tubingensis AKF2 produced here (Fig. 3C). The MnP activity reached the maximum (97.2 ± 2.86 U ml−1) at 96 h of cultivation and following the MnP activity of Phanerochaete chrysosporium at day 2 (48 h) with rice straw cultures [48]. Different Aspergillus sp. have been reported with ligninolytic potential utilizing wheat straw as a carbon source [49]. Trichoderma sp. is inefficient for lignin degradation, and here, the ligninolytic activity of T. reesei M168 was not detectable in the enzyme hydrolysate. Genetic bioengineering has been used to transform the T. reesei Rut C30 for the improved delignification of rice straw [50].
3.4 Determination of total proteins and total reducing sugars
The total proteins and reducing sugars were observed at 4th, 6th, 8th, and 10th day of enzymatic saccharification using supernatants of the enzyme extract from the wheat straw cultures (Fig. 4). The results show that the highest value (284.9 ± 3.89 µg ml−1) of protein was observed in the cultures of P. oryzae AKF4 at the 4th day while comparing to A. tubingensis AKF2 (254.65 ± 4.3 µg ml−1) obtained at the 8th day (Fig. 4A). The fungus P. oryzae AKF4 showed no significant results of enzyme activity using wheat straw comparing to other fungi (Fig. 2). This suggests that the extracellular proteins are not consistent with higher enzyme activity, and P. oryzae AKF4 excreted some other secondary metabolites at a higher dose at the start of enzymatic saccharification. The protein secretion was higher on the 4th day and then gradually decreases with time in most of the fungi culture (Fig. 4A). This relates to the effect of different lignocellulosic substrates, fungal growth phases, and culture conditions on the extracellular protein production by different fungi [51].
Total protein and reducing sugars released from cultures of fungi (Aspergillus tubingensis AKF2, Aspergillus flavus AKF3, Pyricularia oryzae AKF4, Aspergillus nominus AKF5, and Aspergillus oryzae AKF6) cultivated with wheat straw. The total protein is expressed as “µg/ml” and reducing sugars are expressed as “mg g−1” released under the given assay conditions. The bar values represent the average mean (Error bar = standard deviation) of 3 biological replicates. The bar values sharing the same letters are non-significant (LSD > 0.05)
The supernatants of crude enzyme extracts were evaluated for the total reducing sugars released during the saccharification of wheat straw cultures (Fig. 4B). A. tubingensis AKF2 and A. oryzae AKF6 showed higher values (437.05 ± 7.8 mg g−1 and 391.27 ± 7.13 mg g−1, respectively) of total reducing sugars compared to other strains (Fig. 4B). There was an increasing trend of reducing sugar release with saccharification time and reached the maximum on the 8th day and then decreased. This increased trend of sugars profile suggests the increased hydrolytic activity of fungal isolates with time and is inconsistent with many past studies [18, 52, 53]. Here, the reducing sugars (437.05 ± 7.8 mg g−1: Fig. 4B) released from wheat straw cultures of A. tubengenesis AKF2 cultures was higher than wheat straw treated with bacterial strains; Ochrobactrum oryzae BMP03, Bacillus sp. BMP01 (368 mg g−1) [53], rice straw hydrolyzed with fungal enzymes cocktail (315 mg g−1) [54], sweet sorghum bagasse treated with Coriolus versicolor (~ 150 mg g−1) [55], and wheat straw treated with co-cultures of T. reesei + Monascus purpureus (435 mg g−1) [18].
3.5 Biodegradation analysis
3.5.1 Compositional analysis of the hydrolyzed wheat straw
Fungi degrade the lignocellulosic biomass following different growth patterns, structural modifications, releasing enzyme cocktails, and having specificity for the carbon substrates. The fungal growth utilizing the substrate biomass could be used as a quantitative indicator for enzymatic breakdown [30, 56]. Some fungi utilize more biomass for their mycelial growth but release a low amount of sugars in media and are worthless. Fungi decomposing the lignocellulosic biomass in the shortest time with minimum removal of free sugars from media are desirable in biofuel research. Here, in this study, the compositional changes (reduction %) in the wheat straw cultures of the selected fungi (A. tubingensis AKF2, A. flavus AKF3, P. oryzae AKF4, A. nomius AKF5, A. oryzae AKF6) were evaluated as an indicator of fungi cellulolytic potential. The composition (%) of the wheat straw before fungi treatment was recorded as cellulose: 38.6 ± 1.2, hemicellulose: 25.2 ± 0.8, and lignin 17.2 ± 0.3, and correlates with past studies [18, 57].
The significant changes (LSD > 0.05) were observed in the cultures of A. tubingensis AKF2 with 48.1 ± 0.78, 36.1 ± 1.06, 39.6 ± 1.75, and 56.3 ± 1.21% reductions in cellulose, hemicellulose, lignin, and total mass, respectively (Table 2). Results of A. flavus AKF3 were indeterminate respective to the enzyme exhibit of this fungus (Fig. 2). The reduced digestibility in the cultures of A. flavus AKF3 might be due to the different nature of cellulolytic enzymes working on wheat straw and releasing fewer reducing sugars (Fig. 4B). The lowest reductions observed in the case of P. oryzae AKF4 cultures (Table 2) are inconsistent with lower enzymatic (Fig. 2), reducing sugar activity (Fig. 4B) of this fungus hydrolyzing wheat straw. The higher values of cellulose and hemicellulose reductions in the cultures of A. tubingensis AKF2, A. nomius AKF5, and A. oryzae AKF6 illustrate the fungal potentials for higher cellulolytic activity and subsequent release of reducing sugars. The higher amount of released sugars could be used for biofuel synthesis.
3.5.2 FTIR analysis
The FTIR analysis was used to investigate the modifications in functional groups (lignin, cellulose, and hemicellulose) of the biodegraded wheat straw and compared to the control (untreated). The results were analyzed through absorbance differences in FTIR spectra peaks (4000–500 cm−1) of the respective samples on the 8th day of hydrolysis (Fig. 5; Table 3). The results demonstrated a significant change in the absorbance values of the biotreated samples and suggest functional modifications due to fungal activities. The hydroxyl (–OH) linkage between cellulose and lignin at 3301 cm−1 indicated large peaks and represents the hydroxyl stretching on the surface of biodegraded wheat straw samples [18, 58]. Significant low absorbance peaks (3301–1511 cm−1) in samples of A. tubingensis AKF2 and A. nominus AKF5 represent the depolymerization of the lignin contents of the wheat straw (Fig. 5). Lignin and cellulose are covalently linked in wheat straw and to penetrate its expanding hyphae, fungi have to delignify it by producing a cocktail of hydrolyzing enzymes [59]. The lower peak intensity in the lignin functional groups at (1511–1730 cm−1) might be due to lignin digestibility through lignin-degrading enzymes (Table 3) and following the results of past studies using different fungi [18, 53, 60]. The A. tubingensis AKF2 strain showed the lowest absorbance peaks in FTIR spectra (Fig. 5; Table 3) and correlates with the best performing fungi (T. reesei and M. purpureus) reported earlier for wheat straw biodegradation [18]. The peaks around 1327–1422 cm−1 are characterized by C–H conformations in the cellulosic fibrils [60]. A significant decrease in absorbance at 1422 cm−1 in A. tubingensis AKF2 (0.92236) as compared to the control (0.9835) implies the structural breakdown in the cellulose/hemicellulose contents of the wheat straw (Table 3; Fig. 5). The hemicellulose bands at 1030–1034 cm−1 represent the C–O, C = O, C–C–O stretching and were significantly decreased after fungal hydrolysis (Table 3; Fig. 5), confirming the digestibility of polysaccharides in biomass [61]. Among all the five strains used in the current study, A. tubingensis AKF2 and A. nominus AKF5 performed excellently in wheat straw degradation evaluated through different assays.
3.5.3 SEM analysis
The morphology and micro-surface of wheat straw were analyzed through scanning electron microscope before/after fungal hydrolysis (Fig. 6). The nature of fungal hydrolytic enzymes, hyphal penetration, culture environment, and the substrate constituent ratio like cellulose, hemicellulose, and lignin are the major factors determining the biomass degradation [46, 62]. SEM image of untreated wheat straw (control) represents a regular and compact cellulosic structure with no pores on the upper surface (Fig. 6, control). SEM images of wheat straw after 8 days of fungal hydrolysis showed major structural modifications resulting in cellulosic breakdowns, irregular texture, and micro-surface openings. The prominent breakdown was observed in cases of A. tubingensis AKF2, A. nominus AKF, and A. oryzae AKF6 samples (Fig. 6). These fungi also produced a higher amount of cellulolytic enzymes in the current study (Fig. 2) and following higher biodegradation ability due to higher enzyme activities of different fungi reported earlier [18, 63, 64]. Wheat straw hydrolysis by A. tubingensis AKF2 resulted in a highly deformed, porous, and rough texture (Fig. 6). Fungal hyphae penetrate inside the compact structure to increase the surface area for further enzymatic breakdown and release of sugars. The fungi; A. flavus AKF3 and P. oryzae AKF4, produced minor structural modifications as compared to the results of the other fungi (Fig. 6). This relates to their lower hydrolytic activity detected in enzyme assays (Fig. 2) and substrate compositional assays (Table 2).
4 Conclusions
Environmental isolates of fungi have tremendous potential for plant biomass degradation. From 107 samples collected, we screened 20 different fungi based on their nucleotide sequence and growth morphology. Next, we further narrowed it to 5 fungi producing higher enzymes in a prescreening cellulase assay. The selected fungi were used for the biodelignification of 3 different lignocellulosic substrates (wheat straw, corn cob, and rice husk) to identify the hydrolytic potential of different fungi using different carbon sources. From enzymatic assays, fungi using wheat straw performed better as compared to other substrates. After that, wheat straw was also analyzed to observe structural, functional, and compositional modifications produced by the selected fungi. Conclusively, from all work, we found that Aspergillus tubingensis AKF2 produced higher cellulolytic enzymes, total reducing sugars release, more biomass reduction, and disruptions among all the selected strains. Further investigations for optimum saccharification conditions like temperature, media pH, substrate concentrations, inoculum size, and cultivation time could enhance the biodegradation potential of this fungus. The targeted genome editing to enhance the plant biomass-degrading enzymes could be implemented to increase the cellulolytic efficacy of these fungi. Recently, the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) system has revolutionized the fungal genetic engineering with site-specific reprogramming of fungal metabolite and/or enzyme production. For example, Liu et al. [65] used the CRISPR/Cas9 technology to engineer the thermophilic fungi, Myceliophthora thermophila, by targeting the cellulase production pathway. Specifically, multiplexed editing of cellulase-producing genes (cre-1, res-1, gh1-1, and alp-1) in M. thermophilia resulted in a 13-fold increase in cellulolytic activity compared to the wild strain. Similarly, CRISPR-mediated deletion of an amylolytic enzyme regulator (amyR) in M. thermophilia resulted in a threefold increase of lignocellulose activity in another study [66]. Thus, unmasking the cellulase potential of this fungus by combining different classical and/or genome engineering approaches may have broad applications in biofuel biorefinery. The fairly available lignocellulosic feedstock like wheat straw in Pakistan seems to be promising in this scenario for biofuel production by using the potential fungi isolates having higher cellulolytic performance.
References
Van Zessen E, Weismann M, Bakker R, Elbersen H, Reith J, Den Uil H (2003) Lignocellulosic ethanol, a second opinion. Report 2GAVE-03.11. NetherlandsAgency for Energy & Environment. Available from:, https://library.wur.nl/WebQuery/wurpubs/339809.
Rasmussen H, Tanner D, Sørensen H, Meyer AS (2017) New degradation compounds from lignocellulosic biomass pretreatment: routes for formation of potent oligophenolic enzyme inhibitors. Green Chem 19(2):464–473
Bar-On YM, Phillips R, Milo R (2018) The biomass distribution on Earth. Proc Natl Acad Sci U S A 115(25):6506–6511
Baykara SZ (2018) Hydrogen: a brief overview on its sources, production and environmental impact. Int J Hydrogen Energy 43(23):10605–10614
Hanif I, Raza SMF, Gago-de-Santos P, Abbas Q (2019) Fossil fuels, foreign direct investment, and economic growth have triggered CO2 emissions in emerging Asian economies: some empirical evidence. Energy 171:493–501
Patel SJ, Onkarappa R, Shobha KS (2007) Comparative study of ethanol production from microbial pretreated agricultural residues. J Appl Environ Manag 11(4):137–141
Chen H (2014) Chemical composition and structure of natural lignocellulose, Biotechnology of lignocellulose. Springer, pp 25–71
Baadhe RR, Potumarthi R, Mekala NK (2014) Influence of dilute acid and alkali pretreatment on reducing sugar production from corncobs by crude enzymatic method: a comparative study. Bioresour Technol 162:213–217
Salehi SA, Karimi K, Behzad T, Poornejad NJE (2012) Efficient conversion of rice straw to bioethanol using sodium carbonate pretreatment. Fuels 26(12):7354–7361
Goto M, Kamiya N (2016) Powerful peracetic acid–ionic liquid pretreatment process for the efficient chemical hydrolysis of lignocellulosic biomass. Bioresource Technol 214:487–495
Karimi K, Kheradmandinia S, Taherzadeh MJ (2006) Conversion of rice straw to sugars by dilute-acid hydrolysis. Biomass Bioenergy 30(3):247–253
Irshad MN, Anwar Z, But HI, Afroz A, Ikram N, Rashid UJB (2013) The industrial applicability of purified cellulase complex indigenously produced by Trichoderma viride through solid-state bio-processing of agro-industrial and municipal paper wastes. BioResources 8(1):145–157
Ahmed I, Zia MA, Iqbal HMNJC (2010) Bioprocessing of proximally analyzed wheat straw for enhanced cellulase production through process optimization with Trichoderma viride under SSF. Cellulose 2(W3):100
Ilyas U, Majeed A, Hussain K, Nawaz K, Ahmad S, Nadeem MJWASJ (2011) Solid state fermentation of Vignamungo for cellulase production by Aspergillus niger. World Applied Science Journal 12:1172–1178
Sharma A, Tewari R, Rana SS, Soni R, Soni SK (2016) Cellulases: classification, methods of determination and industrial applications. Appl Biochem Biotechnol 179(8):1346–1380
Kogo T, Yoshida Y, Koganei K, Matsumoto H, Watanabe T, Ogihara J, Kasumi T (2017) Production of rice straw hydrolysis enzymes by the fungi Trichoderma reesei and Humicola insolens using rice straw as a carbon source. Bioresour Technol 233:67–73
Arnthong J, Chuaseeharonnachai C, Boonyuen N, Tachaapaikun C, Chimchana D, Eurwilaichitr L, Champreda V, Chantasingh D (2018) Cooperative decomposition of rice straw by co-cultivation of cellulolytic fungI. J Sci 45:645–652
Fatma S, Saleem A, Tabassum R (2020) Wheat straw hydrolysis by using co-cultures of Trichoderma reesei and Monascus purpureus toward enhanced biodegradation of the lignocellulosic biomass in bioethanol biorefinery. Biomass Convers Biorefin 11(3):743–754
Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29(4):795–811
Mandels M, Reese ET (1957) Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J Bacteriol 73(2):269
Gritzali M, Brown RD Jr (1979) The cellulase system of Trichoderma: relationships between purified extracellular enzymes from induced or cellulose-grown cells. Adv Chem 181:237–260
Benny GL (2008) Methods used by Dr. RK Benjamin, and other mycologists, to isolate zygomycetes. Aliso J Syst Evol Bot 26(1):37–61
White TJ, Lee S-H, Taylor L, Shawe-Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Sazci A, Erenler K, Radford A (1986) Detection of cellulolytic fungi by using Congo red as an indicator: a comparative study with the dinitrosalicyclic acid reagent method. J Appl Bacteriol 61(6):559–562
Teather RM, Wood PJ (1982) Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43(4):777–780
Spano L, Medeiros J, Mandels MJUA, Natick, (1975) Enzymatic hydrolysis of cellulosic waste to glucose. Resource Recovery and Conservation 1(3):279–294
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure 1617(1):1–16
Ghose T (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268
Herr D (1979) Secretion of cellulase and β-glucosidase by Trichoderma viride ITCC-1433 in submerged culture on different substrates. Biotechnol Bioeng 21(8):1361–1371
Xu X, Xu Z, Shi S, Lin M (2017) Lignocellulose degradation patterns, structural changes, and enzyme secretion by Inonotus obliquus on straw biomass under submerged fermentation. Bioresour Technol 241:415–423
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Godin B, Agneessens R, Gerin PA, Delcarte JJT (2011) Composition of structural carbohydrates in biomass: precision of a liquid chromatography method using a neutral detergent extraction and a charged aerosol detector. Talanta 85(4):2014–2026
Anasontzis GE, Thuy NT, Hang DTM, Huong HT, Thanh DT, Hien DD, Thanh VN, Olsson L (2017) Rice straw hydrolysis using secretomes from novel fungal isolates from Vietnam. Biomass Bioenergy 99:11–20
Dias AA, Freitas GS, Marques GS, Sampaio A, Fraga IS, Rodrigues MA, Evtuguin DV, Bezerra RM (2010) Enzymatic saccharification of biologically pre-treated wheat straw with white-rot fungi. Bioresour Technol 101(15):6045–6050
McIntosh S, Vancov T (2011) Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenergy 35(7):3094–3103
Aggarwal NK, Goyal V, Saini A, Yadav A, Gupta R (2017) Enzymatic saccharification of pretreated rice straw by cellulases from Aspergillus niger BK01. 3 Biotech 7(3):158
Bakri Y, Masson M, Thonart P (2010) Isolation and identification of two new fungal strains for xylanase production. Appl Biochem Biotechnol 162(6):1626–1634
Hui L, Wan C, Hai-Tao D, Xue-Jiao C, Qi-Fa Z, Yu-Hua Z (2010) Direct microbial conversion of wheat straw into lipid by a cellulolytic fungus of Aspergillus oryzae A-4 in solid-state fermentation. Biores Technol 101(19):7556–7562
Gomathi D, Muthulakshmi C, Kumar DG, Ravikumar G, Kalaiselvi M, Uma C (2012) Submerged fermentation of wheat bran by Aspergillus flavus for production and characterization of carboxy methyl cellulase. Asian Pac J Trop Biomed 2(1):S67–S73
Prajapati BP, Kumar Suryawanshi R, Agrawal S, Ghosh M, Kango N (2018) Characterization of cellulase from Aspergillus tubingensis NKBP-55 for generation of fermentable sugars from agricultural residues. Bioresour Technol 250:733–740
Rais A, Shakeel M, Hafeez FY, Hassan MN (2016) Plant growth promoting rhizobacteria suppress blast disease caused by Pyricularia oryzae and increase grain yield of rice. Biocontrol 61(6):769–780
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4(1):7
Zhang F, Bunterngsook B, Li J-X, Zhao X-Q, Champreda V, Liu C-G, Bai F-W (2019) Regulation and production of lignocellulolytic enzymes from Trichoderma reesei for biofuels production. In Advances in bioenergy (Vol. 4, pp. 79-119). Elsevier
Seiboth, B., Ivanova, C. and Seidl-Seiboth, V., 2011. Trichoderma reesei: a fungal enzyme producer for cellulosic biofuels. Biofuel production-recent developments and prospects, pp.309-340
Khokhar Z-U, Syed Q, Nadeem M, Irfan M, Wu J, Samra Z, Gul I, Athar A (2014) Enhanced production of cellulase by Trichoderma reesei using wheat straw as a carbon source. World Appl Sci J 30(9):1095–1104
Manavalan T, Manavalan A, Heese K (2015) Characterization of lignocellulolytic enzymes from white-rot fungi. Curr Microbiol 70(4):485–498
Chang AJ, Fan J, Wen X (2012) Screening of fungi capable of highly selective degradation of lignin in rice straw. Int Biodeterior Biodegrad 72:26–30
Singh A, Bajar S, Bishnoi NR, Singh N (2010) Laccase production by Aspergillus heteromorphus using distillery spent wash and lignocellulosic biomass. J Hazard Mater 176(1–3):1079–1082
Gopalakrishnan RM, Manavalan T, Ramesh J, Thangavelu KP, Heese K (2020) Improvement of saccharification and delignification efficiency of Trichoderma reesei Rut-C30 by genetic bioengineering. Microorganisms 8(2):159
Vasina DV, Pavlov AR, Koroleva OV (2016) Extracellular proteins of Trametes hirsuta st. 072 induced by copper ions and a lignocellulose substrate. BMC Microbiol 16(1):106
Jiang Y, Duarte AV, van den Brink J, Wiebenga A, Zou G, Wang C, de Vries RP, Zhou Z, Benoit I (2016) Enhancing saccharification of wheat straw by mixing enzymes from genetically-modified Trichoderma reesei and Aspergillus niger. Biotechnol Lett 38(1):65–70
Tsegaye B, Balomajumder C, Roy P (2018) Biodegradation of wheat straw by Ochrobactrum oryzae BMP03 and Bacillus sp. BMP01 bacteria to enhance biofuel production by increasing total reducing sugars yield. Environ Sci Pollut Res Int 25(30):30585–30596
Dhiman SS, Haw J-R, Kalyani D, Kalia VC, Kang YC, Lee J-K (2015) Simultaneous pretreatment and saccharification: green technology for enhanced sugar yields from biomass using a fungal consortium. Bioresour Technol 179:50–57
Mishra V, Jana AK, Jana MM, Gupta A (2017) Enhancement in multiple lignolytic enzymes production for optimized lignin degradation and selectivity in fungal pretreatment of sweet sorghum bagasse. Bioresour Technol 236:49–59
Castoldi R, Bracht A, de Morais GR, Baesso ML, Correa RCG, Peralta RA, Moreira RDFPM, de Moraes MDLT, de Souza CGM, Peralta RM (2014) Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: study of degradation patterns and saccharification kinetics. Chem Eng J 258:240–246
Prasad S, Singh A, Joshi H (2007) Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour Conserv Recycl 50(1):1–39
Asghar U, Irfan M, Iram M, Huma Z, Nelofer R, Nadeem M, Syed Q (2015) Effect of alkaline pretreatment on delignification of wheat straw. Nat Prod Res 29(2):125–131
Talebnia F, Karakashev D, Angelidaki I (2010) Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol 101(13):4744–4753
Shahryari Z, Fazaelipoor MH, Setoodeh P, Nair RB, Taherzadeh MJ, Ghasemi Y (2018) Utilization of wheat straw for fungal phytase production. Int J Recycl Org Waste Agric 7(4):345–355
Zhang A-P, Liu C-F, Sun R-C, Xie J (2013) Extraction, purification, and characterization of lignin fractions from sugarcane bagasse. BioResources 8(2):1604–1614
Salvachúa D, Prieto A, López-Abelairas M, Lu-Chau T, Martínez ÁT, Martínez MJ (2011) Fungal pretreatment: an alternative in second-generation ethanol from wheat straw. Biores Technol 102(16):7500–7506
Kannaiyan R, Mahinpey N, Kostenko V, Martinuzzi RJ (2017) Enhanced delignification of wheat straw by the combined effect of hydrothermal and fungal treatments. Chem Eng Commun 204(7):803–812
Shah TA, Ullah R (2019) Pretreatment of wheat straw with ligninolytic fungi for increased biogas productivity. Int J Environ Sci Technol 16(11):7497–7508
Liu Q, Gao R, Li J, Lin L, Zhao J, Sun W, Tian C (2017) Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering. Biotechnol Biofuels 10(1):1
Xu G, Li J, Liu Q, Sun W, Jiang M, Tian C (2018) Transcriptional analysis of Myceliophthora thermophila on soluble starch and role of regulator AmyR on polysaccharide degradation. Bioresour Technol 265:558–562
Funding
This study was supported by the “Akhuwat Foundation,” a non-profit micro-finance organization working in Pakistan for the welfare of humanity. Akhuwat started working in 2001 to provide a small interest-free loan to poor families enabling them to become self-reliant. To fulfill the dream of a poverty-free society, Akhuwat started to provide “free education” to deserving students from all over Pakistan. Akhuwat-FIRST is one of the leading research institutes in Pakistan supported by “Akhuwat Foundation.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Fungi collected from the environment and prescreening through CMC-agar staining and ITS sequencing resulted in 5 different fungal isolates with cellulase activity.
• The selected fungi were evaluated for the enzymatic hydrolysis of wheat straw, rice straw, and corn cob through lignocellulolytic enzyme assays.
• Compositional and structural modifications of biodegraded wheat straw proofread the higher cellulolytic activity of fungi.
• The potential fungus “Aspergillus tubingensis AKF2” identified in this study have a great capacity for lignocellulose biodegradation and could be promising for biofuel extraction.
Supplementary Information
Below is the link to the electronic supplementary material.
13399_2021_1932_MOESM1_ESM.jpg
Supplementary file1 Morphological characterization of the 20 different fungi (AKF1-AKF20) taken after 5 days of culture on PDA Petri plates. (JPG 161 KB)
13399_2021_1932_MOESM2_ESM.jpg
Supplementary file2 A: Molecular confirmation of 5 fungi (AKF2, AKF3, AKF4, AKF5, AKF6) for the ITS band (500–700 bp). B: CMC-agar plates with clearing halo (diameter) of hydrolysis. (JPG 51 KB)
Rights and permissions
About this article
Cite this article
Awais, M., Fatma, S., Naveed, A. et al. Enhanced biodegradation of organic waste treated by environmental fungal isolates with higher cellulolytic potential. Biomass Conv. Bioref. (2021). https://doi.org/10.1007/s13399-021-01932-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-021-01932-w