Abstract
The use of raw sugarcane bagasse for cell immobilization by adsorption was evaluated in order to increase ethanol production. The aim of this work was to study the immobilization process of Saccharomyces cerevisiae ITV-01 RD on natural and pre-treated sugarcane bagasse. Pre-treatment with sulfuric acid was selected because of the efficiency and retention obtained (58% and 80 mg/g). The Guggenheim–Andersen–de Boer (GAB) model adjusted experimental data and demonstrated that the process involved monolayer and multilayer stages. No statistically different results were obtained between the use of solid:liquid ratios of 1:75 and 1:100, and immobilization was stable up to 20 days. In a packed bed reactor using glucose medium, at a dilution rate (D) of 0.11 h−1, 48 g/L ethanol was produced; meanwhile at 0.48 h−1, ethanol productivity was 12.1 g/Lh. Using sugarcane juice diluted to 100 g/L total sugars, an ethanol productivity of 5.03 g/Lh was attained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bioethanol produced from renewable biomass, such as sugar, starch, macroalgae (Rhizoclonium sp.), or lignocellulosic materials, is one of the alternative energy resources, which is both renewable and environmentally friendly [1,2,3]. During batch fermentation, many parameters can cause a decrease in the specific rate of yeast growth, and inhibition can be caused either by product or substrate concentration [1].
Ethanol production by immobilized yeast cells on conventional supports has been extensively investigated during the past few decades. Microbial cell immobilization showed certain technical and economic advantages over free cell systems. Cell immobilization for fermentation has been developed to eliminate inhibition caused by high substrate and product concentrations and to enhance ethanol production productivity and yield [4, 5]. Cell immobilization is a method of increasing cell mass concentration in the fermenter leading to greater process productivity and production cost minimization while offering advantages over free cell fermentation operations [6].
The use of natural structural materials like sorghum bagasse, sugarcane bagasse, and rice straw for cell immobilization was evaluated previously [6,7,8,9,10]. The advantages accruable from such biomaterials are reusability, freedom from toxicity problems, mechanical strength for necessary support, and opening of spaces within the matrix for growing cells [9]. Some pre-treatments, like diluted acid and delignification using alkali on raw material, have been employed as a strategy for the improvement of the immobilization process [6], so that the damage to lignocellulose residue integrity could increase the adsorption process. However, there are few studies that contribute to understanding the immobilization process. The most common isotherms applied in solid to liquid systems are the Langmuir theoretical isotherm equilibrium (the best known and most often used isotherm for the sorption of a solute from a liquid solution), the Freundlich, the Redlich–Peterson, and the Guggenheim–Andersen–de Boer (GAB) model [10]. The mechanism involved in the latter could be useful in order to understand yeast adsorption phenomena on lignocellulose residues such as sugarcane bagasse.
The use of the respiratory-deficient (RD) phenotype of S. cerevisiae has been recommended previously for bioethanol production [11,12,13]. In immobilized cell systems, oxygen availability is diminished because oxygen transfer rates are lower than in free cell systems. Thus, the RD phenotype could be useful, as damage to the respiratory chain causes less dependence on oxygen in RD strains than in respiratory-competent strains. Also, RD strains have a higher ethanol yield and productivity than respiratory-competent strains. The aim of this work was to study the immobilization process of S. cerevisiae ITV-01 RD on natural and pre-treated sugarcane bagasse.
2 Material and Methods
2.1 Microorganism
The osmotolerant low pH-resistant S. cerevisiae ITV-01 RD was obtained previously by ethidium bromide exposure with a deficiency on cytochrome c [13].
2.2 Sugarcane bagasse
Sugarcane bagasse was obtained from “El Modelo” factory in January 2012. Its composition was as follows: 39.3% cellulose, 30.5% hemicellulose, and 11.5% lignin. The sugarcane bagasse was washed in order to eliminate the remaining sucrose, and a solar dry was performed. Finally, it was sieved to obtain particle size from 3.36 to 6.36 mm (Retsch Mod. 30) and dried to constant weight at 70 °C.
2.3 Dilute acid and alkali pre-treatment
Dilute acid pre-treatment was carried out using 2% w/v sulfuric acid (H2SO4) with a 1:14 solid:liquid ratio, at 121 °C for 40 min. The alkali pre-treatment was performed using 2% w/v NaOH with a 1:6 solid:liquid ratio at 100 °C for 60 min [14]. Both pre-treatments were autoclaved at 121 ºC during 20 min. After, these were washed using water in order to eliminate sulfuric acid and NaOH, respectively. Finally, they were dried at sun until to have at less of 10% humidity.
2.4 Culture media
2.4.1 Conservation medium
S. cerevisiae ITV-01 RD was stored at 4 °C using a culture medium whose composition was as follows: glucose, yeast extract, and agar (20, 10. and 20 g/L, respectively).
2.4.2 Preculture and kinetic media
Preculture medium was glucose, yeast extract, KH2PO4, (NH4)2SO4, and MgSO4⋅7H2O (50, 1.0, 5.0, 2.0, 0.4 g/L, respectively). The kinetic medium was proposed previously by Ortiz-Muñiz et al. [15]: glucose, yeast extract, KH2PO4, (NH4)2SO4, and MgSO4⋅7H2O (150, 2.0, 8.0, 5.0, 1.0 g/L, respectively). The initial pH was adjusted to 3.0 using 85% v/v orthophosphoric acid. Preculture was performed in a 250-mL Erlenmeyer flask with 100-mL liquid medium stirred at 150 rpm. After inoculation, each Erlenmeyer flask was incubated at 30 °C for 24 h. Two precultures were prepared to obtain the inoculum [15].
2.5 Cell immobilization
The immobilization process was evaluated using 3 types of sugarcane bagasse: untreated, dilute acid, and alkali pre-treatments. The inoculum was prepared as follows: yeasts cells were incubated (New Brunswick Scientific classic series C24KC Refrigerated Incubator Shaker Edison NJ, USA) for 12 h at 30 °C, 150 rpm using preculture medium. Then, the yeast cells were centrifuged at 4500G for 20 min at 15 °C and suspended in 20-mL 9 g/L NaCl in order to adjust the inoculum size to the desired value. In 250-mL Erlenmeyer flasks, 1-g sugarcane bagasse was sterilized at 121 °C for 15 min, and inoculum was added under aseptic conditions; the samples were subsequently incubated at 30 °C, 80 rpm. Destructive sampling was carried out every 4 h for 24 h.
2.6 Solid to liquid ratio and immobilization stability
The solid to liquid ratio was evaluated using batch cultures in 250-mL flasks vessels using 1-g sugarcane bagasse with yeast cells immobilized under established conditions. The flasks were incubated at 150 rpm at 30 °C using an incubator (New Brunswick Scientific classic series C24KC Refrigerated Incubator Shaker Edison NJ, USA). Immobilization stability was evaluated by twenty sequential cultures using the best solid to liquid ratio found. The culture media was removed, and fresh culture media was added. All the experiments were performed in duplicate.
2.7 Effect of dilution rate and culture media
Fermentation in a packed bed reactor was carried out using a glass column with a double wall for temperature control. The column, packed with 83-g dry sugarcane bagasse, measured 4.6 cm inner diameter, 6.2 cm outer diameter, and 38 cm long; the total volume was 1.17 L. Immobilization was performed according to the results obtained, after which an isotonic sterile solution (9 g/L NaCl) was washed through for 10 h using a dilution rate of 0.2 h−1 in order to remove free cells from the system. The effect of dilution rate was evaluated at 0.11, 0.21, 0.32, and 0.48 h−1 using glucose media. At the better dilution rate, the culture medium effect was evaluated using sugarcane juice and molasses “B,” a co-product obtained from cane sugar production 15% richer in sugar content than the final molasses [16].
2.8 Analytical techniques
2.8.1 Biomass determination
Yeast growth was measured by direct count using a Thoma Chamber (Marienfeld Laboratory Glassware, Germany). Viability was ascertained by the methylene blue staining method proposed previously by Lange et al. [17]. In addition, a correlation cell count against cell dry weight was performed in order to adjust inoculum size to desired values.
2.8.2 Subtracts and products measure
The culture medium was centrifuged for 10 min at 10,000 rpm (Eppendorf Centrifuge 5424, Germany) and the supernatant stored at − 20 °C until its analysis. Sugars (sucrose, glucose, and fructose) and products (ethanol, acetic acid, and glycerol) were quantified by using a Waters Alliance HPLC (Model 2695, Water Corporation, Milford, MA) supplied with a refractive index detector (Waters 2414) and with a Shodex SH1011 column operating at 50 °C and 55 °C, respectively. The mobile phase of 0.05 mol/L H2SO4 was run at an elution flow rate of 0.60 mL/min. The concentration was obtained based on calibration with standards.
2.9 Immobilization parameters
The biomass adsorbed on sugarcane bagasse was calculated by dry weight using a Whatman paper No. 2 and a vacuum system (Oaklon Mod WP-15); it was dried at 70 °C. The control sample was a 9 g/L NaCl solution. Free cells were determined by direct cell count using a Thoma Chamber correlated to dry cell weight.
Cell retention on the bagasse (R) was calculated as the ratio of immobilized cell mass on the carrier to mass (g/g). The concentration of immobilized cells (Xi) was calculated as the ratio of immobilized cell mass on the carrier to medium volume (g/L). Immobilization efficiency (Yi) was calculated as Xi over XT (the concentration of total suspended plus immobilized cells, g/L) multiplied by 100 (%).
2.10 GAB modelling
The experimental data found at equilibrium were modelled using the Guggenheim–Anderson–de Boer (GAB) model, according to the following equation:
where \(\mathrm{X}\) is the amount of free cells at equilibrium; Xm specifies the monolayer sorption capability (g/L); α is the energetic constant, a parameter related to the difference in energy between molecules of the first and the other layer (cal/cal); k measures the chemical potential difference between the molecules of the second layer and the liquid phase (cal/cal); and C is the cells immobilized at equilibrium (g/L). The parameters involved in this model were calculated by non-linear regression.
2.11 Scanning electronic microscopy (SEM)
The bagasse carrier samples, dried at room temperature, were studied with an electron microscope. Subsequently, they were coated using gold–palladium and processed using a Jeol SEM, model JSM-5600lv (Jeol Ltd., Tokyo, Japan).
2.12 Lignocellulosic composition
The lignocellulosic composition was carried out in duplicate using the standard analytical procedure for biomass analysis provided by the National Renewable Energy Laboratory (NREL) [18], which consisted of a double acid hydrolysis. The first hydrolysis was carried out in a stainless steel electric thermostatic water bath with 72% H2SO4 for 60 min, 30 °C, stirring for 10 min. The second hydrolysis was carried out with 4% H2SO4 in an autoclave (AESA, CV-250, Mexico) at 121 °C, 1.1 atm for 60 min. The glucose, xylose, and arabinose contained in the supernatant were analyzed by HPLC (Waters 600 TSP Spectra System, Waters, Milford, MA) using a Shodex SH1011 8 Å-300 mm column (H203153, Japan), at 50 °C, with sulfuric acid 5 mM as mobile phase with a flow of 0.6 mL / min and a refractive index detector (Waters 2414, TSP Refracto Monitor V, Waters, Milford, MA, USA). Acid-soluble lignin was analyzed using a UV–Vis spectrophotometer (Agilent Cary 8454, Agilent Technologies). Insoluble lignin moisture was determined according to AOCS methodology (Ab 2–49) using a vacuum oven (Thermo Scientific Lab-Line, 3608-1CE), and finally ash determination was performed in a flask (Thermo Scientific Thermoline, model FB1310M-33) using AOCS methodology (Ba 5a-49).
3 Results and discussion
3.1 Characterization of raw and pre-treated materials
Regarding the composition of lignocellulosic material, sugarcane raw has 44.1% cellulose, 28.2% hemicellulose, and 18.3% lignin, while the sugarcane bagasse pre-treated by acid and alkaline hydrolysis presented 58.8–74.2% cellulose, 7.2–9.5% hemicellulose, and 28.7–10.6% lignin, respectively, as shown in Table 1.
3.2 Effect of pre-treatment
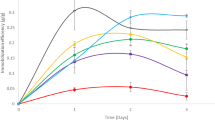
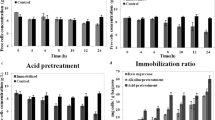
In order to evaluate cell retention of S. cerevisiae ITV-01 RD immobilization on untreated, acid, and alkali pre-treated sugarcane bagasse, sorption kinetics was performed using an inoculum size of 2.5 g/L dry cells. Although natural sugarcane bagasse showed the highest cell retention, it also showed the lowest efficiency. On the other hand, bagasse pre-treated with NaOH showed the highest efficiency and the lowest cell retention (Fig. 1). Pre-treatment with NaOH caused greater damage to sugarcane bagasse structure, and yeast cells cannot be strongly attached, only lying on the surface of the support, thus being easily removed during wash out. Desorption phenomenon can be observed in Fig. 1A; cell retention initially increased and subsequently decreased with time. These results also agree with those previously reported [6].
In Fig. 2, natural bagasse can be observed with a smooth surface where the sorption phenomenon becomes difficult, whereas pre-treated bagasse presents an irregular surface where the sorption phenomenon can occur. However, NaOH pre-treatment results in great damage when the sorption process occurs but with fewer cells than with the sulfuric acid pre-treatment. Cell retention and efficiency are important parameters in the cell immobilization process, and the best pre-treatment using sulfuric acid as a balance can be reached between both parameters. Thus, the sugarcane bagasse employed for the following experiments was that pre-treated with sulfuric acid.
Inoculum size (0.25, 0.5, 0.75, 1.0, 2.5, 5, 10, and 16 g/L dry cells) was evaluated using the sugarcane bagasse pre-treated with sulfuric acid. The results shown in Fig. 3 demonstrate that increasing inoculum size causes an increase in cell retention with maximum immobilization efficiency at 5 g/L. So, the brokerage inflection point, after which immobilization efficiency starts diminishing, could limit inoculum size for cell immobilization.
3.3 Cell immobilization isotherm
S. cerevisiae ITV-01 RD was immobilized on sugarcane bagasse previously pre-treated with sulfuric acid. The inoculum employed was from 0.25 to 16 g/L dry cells. Figure 4 shows the experimental data and the adjusted calculation of R2 = 0.85. We found that Xm = 1.99 g/L and smaller values caused monolayer sorption. Energetic constants α and k were 22.17 and 6.27, respectively. The assumptions of this isotherm include a uniform surface; furthermore, the absence of interactions between sorpted molecules makes it impossible to establish the physical meaning of these values because the system is more complex. However, it was previously established that the phenomenon occurs in two steps: first, the interaction between the support and cells, and second, where cell adhesion overlies the first cell layer [19].
3.4 Solid to liquid ratio
In order to evaluate the metabolic activity of yeast cells immobilized on sugarcane bagasse, the effect of the following solid:liquid ratios were evaluated: 1:25, 1:50, 1:75, and 1:100 g/L. Ethanol production results were statistically the same using either the 1:75 or 1:100 ratios. Table 2 showed that ethanol yield increased with an increasing solid to liquid ratio (from 0.39 to 0.47 g/g). A similar behavior was observed with ethanol productivity (2.0 to 2.4 g/Lh). In the immobilized system, ethanol productivity was greater than in the free cell system (1.7 g/Lh). However, ethanol production and ethanol yield (48–56.4 g/L and 0.39–0.47 g/g, respectively) were lower in the immobilized system compared with the free cell system (60.6 g/L and 0.49 g/g).
3.5 Immobilization stability
In order to evaluate cell immobilization stability, 20 repeated batches were carried out using a 1:75 solid:liquid ratio for 24 h. After that, the culture medium was removed, and fresh medium was added. The results are shown in Fig. 5. All the parameters monitored remained unchanged after 20 batches, indicating immobilization stability. In the first and twentieth batch, ethanol production was 63.6 and 62.5 g/L with a glucose consumption of 97.6 and 94.5% and productivity of 2.65 and 2.60 g/Lh, respectively. The ethanol yield remained unchanged at 0.42 g/g. These results are not in accordance with the data reported by Chandel et al. [20] who found that in the first batch, ethanol production was 22 g/L, whereas for the twelfth batch, only 10 g/L ethanol was produced. The immobilization of S. cerevisiae ITV-01 RD on sugarcane bagasse with acid pre-treatment is stable up to 20 days; this yeast continued to cover the support, so that the support could be used for more than 20 batches.
3.6 Effect of dilution rate
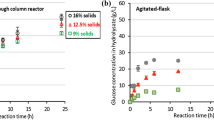
The stationary phase was reached when the glucose and ethanol concentrations in the outlet flow did not change for 3 residence times according to that previously established [21]. The greatest ethanol production (48 g/L) was obtained at D = 0.11 h−1, using a culture medium with 150 g/L glucose. Increased dilution rate diminished ethanol production. However, using greater dilution rates caused an increase in ethanol productivity from 5.1 to 12.1 g/Lh. In Fig. 6, the effect of dilution rate is shown. The specific growth rate of S. cerevisiae ITV-01 RD was 0.32 h−1, so dilution rates greater than the specific growth rate should be possible. At D = 0.48 h−1, 25 g/L ethanol production was obtained. In spite of continuous culture using free cells remaining unchanged, in these results, ethanol yield diminished from 0.5 g/g at D = 0.11 h−1 to 0.38 g/g at 0.48 h−1 (Table 3). However this decrease could be due to cell desorption caused by the increase in linear flow velocity. Process efficiency was 98% at D = 0.11 h−1, whereas at 0.48 h−1, this decreased to 65% This results agree with those previously reported using sorghum bagasse for immobilization [8].
Dilution rate 0.11 h−1 was selected because ethanol productivity was higher than that previously reported by Ortiz-Muñiz et al. [13] using S. cerevisiae ITV-01 RD free cells and because the ethanol concentration obtained was the greatest (48 g/L). Glucose was not completely consumed so the subsequent experiments were carried out using a culture medium with a total sugar content of g/L. No difference was found between using glucose at 100 or 150 g/L. Sugarcane juice and molasses “B” use was also evaluated under non-sterile conditions (Table 4). Ethanol production and productivity using sugarcane juice (47 g/L, 5.02 g/Lh) were better than with molasses “B” (41 g/L, 4.31 g/Lh). This could be due to the fact that sugarcane juice contains vitamins and other compounds that contribute to improved ethanol production, whereas in molasses “B,” these compounds could be eliminated because of the thermal process involved.
4 Conclusions
The use of pre-treatment with sulfuric acid increases cell retention due to the damage caused to sugarcane bagasse structure. The best inoculum size was 5 g/L; the best immobilization conditions (contact time 8 h, 30 °C) led to retention of 80 ± 4 mg/g with 58% efficiency. The data found adjust to the Guggenheim–Andersen–de Boer model (GAB Model) with a good correlation (R2 = 0.85), allowing us to state that for concentrations lower than 2 g/L inoculum, the cells are added in a monolayer, and the physical phenomenon which possibly prevails is adsorption; on the other hand, multilayer formation is a much more complex phenomenon. However, the micrographs show that high cell concentration promotes agglomerate formation in the cavernous parts of the support. The immobilization system was stable up to 20 days. In a packed bed column, a dilution rate of 0.11 h−1 was selected to obtain the highest efficiency (98%), 48 g/L of ethanol, and 5.1 g/Lh productivity. The use of non-sterile sugarcane juice for ethanol production in this system could be an option to improve process productivity. However, further studies should be carried out in order to increase ethanol production, maintaining the high productivity (12.1 g/Lh) obtained at the highest dilution rate evaluated (0.48 h−1), for instance, by increasing column length. Biotechnological ethanol production is improved by the use of culture systems in a packed bed column with S. cerevisiae ITV-01 RD immobilized on sugarcane bagasse hydrolyzed with sulfuric acid.
References
Nikolic S, Mojovic L, Rakin M, Pejin D (2009) Bioethanol production from corn meal by simultaneous enzymatic saccharification and fermentation with immobilized cells of Saccharomyces cerevisiae var. ellipsoideus. Fuel 88(9):1602–1607. https://doi.org/10.1016/j.fuel.2008.12.019
Mustafa G, Arshad M, Bano I, Abbas M (2020) Biotechnological applications of sugarcane bagasse and sugar beet molasses. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-01141-x
Khammee P, Ramaraj R, Whangchai N, Bhuyar P, Unpaprom Y (2020) The immobilization of yeast for fermentation of macroalgae Rhizoclonium sp. for efficient conversion into bioethanol. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00786-y
Yatmaz E, Germec M, Erkan SB, Turhan I (2020) Modeling of ethanol fermentation from carob extract–based medium by using Saccharomyces cerevisiae in the immobilized-cell stirred tank bioreactor. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-01154-6
Kourkoutas Y, Dimitropoulou S, Kanellaki M, Marchant R, Nigam P, Banat I, Koutinas A (2004) Immobilization technologies and support materials suitable in alcohol beverages production: a review. Food Microbiol 21(4):377–397. https://doi.org/10.1016/j.fm.2003.10.005
Santos DT, Sarrouh BF, Rivaldi JD, Converti A, Silva SS (2008) Use of sugarcane bagasse as biomaterial for cell immobilization for xylitol production. J Food Eng 86(4):542–548. https://doi.org/10.1016/j.jfoodeng.2007.11.004
Watanabe I, Miyata N, Ando A, Shiroma R, Tokuyasu RK, Nakamura T (2012) Ethanol production by repeated-batch simultaneous saccharification and fermentation (SSF) of alkali-treated rice straw using immobilized Saccharomyces cerevisiae cells. Bioresource Technol 123:695–698. https://doi.org/10.1016/j.biortech.2012.07.052
Yu J, Zhang X, Tan T (2007) An novel immobilization method of Saccharomyces cerevisiae to sorghum bagasse for ethanol production. J Biotechnol 129(3):415–420. https://doi.org/10.1016/j.jbiotec.2007.01.039
Iqbal M, Saeed A (2005) Novel method for cell immobilization and its application for production of organic acid. Lett Appl Microbiol 40(3):178–182. https://doi.org/10.1111/j.1472-765X.2004.01646.x
Ho YS (2004) Selection of optimum sorption isotherm. Carbon 42:2115–2116. https://doi.org/10.1016/j.carbon.2004.03.019
Oner ET, Oliver SG, Kirdar B (2005) Production of ethanol from starch by respiration-deficient recombinant Saccharomyces cerevisiae. Appl Environ Microb 71(10):6443–6445. https://doi.org/10.1128/AEM.71.10.6443-6445.2005
Ooi BG, Lankford KR (2009) Strategy for adapting wine yeasts for bioethanol production. Int J Mol Sci 10(1):385–394. https://doi.org/10.3390/ijms10010385
Ortiz-Muñiz B, Carvajal-Zarrabal O, Aguilar B, Aguilar-Uscanga MG (2012) Improvement in ethanol production using respiratory deficient phenotype of a wild type yeast Saccharomyces cerevisiae ITV-01. Renew Energ 37(1):197–201. https://doi.org/10.1016/j.renene.2011.06.019
Castañón-Rodríguez JF, Torrestiana-Sánchez B, Montero-Lagunes M, Portilla-Arias JA, Ramírez de León JA, Aguilar-Uscanga MG (2013) Using high pressure processing (HPP) to pretreat sugarcane bagasse. Carbohyd Polym 98:1018–1024. https://doi.org/10.1016/j.carbpol.2013.06.068
Ortiz-Muñiz B, Carvajal-Zarrabal O, Torrestiana-Sanchez B, Aguilar-Uscanga MG (2010) Kinetic study on ethanol production using Saccharomyces cerevisiae ITV-01 yeast isolated from sugar cane molasses. J Chem Technol Biotechnol 85(10):1361–1367. https://doi.org/10.1007/s00449-014-1183-8
Fernández-López CL, Torrestiana-Sanchez B, Salgado-Cervantes MA, Mendoza-García PG, Aguilar-Uscanga MG (2012) Use of sugarcane molasses “B” as an alternative for ethanol production with wild type yeast Saccharomyces cerevisiae ITV-01 at high sugar concentrations. Bioproc Biosyst Eng 35(4):605–614. https://doi.org/10.1007/s00449-011-0633-9
Lange H, Bavouzet J, Taillander P, Delorme C (1993) Systematic error and comparison of four methods for assessing the viability of Saccharomyces cerevisiae suspensions. Biotechnol Tech 7(1):223–228
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure 1617:1–16
Razmovki R, Pejin D (1996) Immobilization of Saccharomyces diastaticus on wood chips for ethanol production. Folia Microbiol 41(2):201–207
Chandel AK, Narasu ML, Chandrasekhar G, Manikyam A, Rao LV (2009) Use of Saccharum spontaneum (wild sugarcane) as biomaterial for cell immobilization and modulated ethanol production by thermotolerant Saccharomyces cerevisiae VS3. Bioresource Technol 100(8):2404–2410. https://doi.org/10.1016/j.biortech.2008.11.014
Cruz J, Domínguez J, Parajó J (2000) Xylitol production from barley bran hydrolysate by continuous fermentation with Debaryomyces hansenii. Biotechnol Lett 22:1895–1898. https://doi.org/10.1023/A:1005693709338
Acknowledgements
The authors acknowledged the economical support from the National Council of Science and Technology (CONACYT) and Tecnológico Nacional de México (TecNM) and the critical reading of Patricia Hayward Jones MSc. and Dulce María Barradas Dermitz MSc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Godoy-Salinas, A.J., Ortiz-Muñiz, B., Rodríguez, J.G. et al. Bioethanol production by S. cerevisiae ITV-01 RD immobilized on pre-treated sugarcane bagasse. Biomass Conv. Bioref. 13, 5973–5980 (2023). https://doi.org/10.1007/s13399-021-01602-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01602-x