Abstract
For a more eco-friendly production of energy and chemicals (e.g. lactic acid), green biorefineries are implementing an environmentally conscious technique of using green biomass. To increase the amount of lactic acid in grass and rye silage, different ensiling treatments were conducted. Additionally, after separating the organic juice, the specific methane yield of the remaining solid residue of the ensiled material was determined. The amount of lactic acid was increased by 168.8 % (149.7 ± 20.9 g kg−1 dry matter (DM)) through applying homofermentative lactic acid bacteria together with carbonated lime to the raw material grass. For rye, while having a stable silage, the highest increase in lactic acid was achieved by chopping the raw material to a theoretical length of cut of 1 mm. As a result, an increase of 46.3 % (57.5 ± 0.6 g kg−1 DM) was attained. Taxonomic profiling by 16S amplicon sequencing revealed that the homofermentative species Lactobacillus plantarum was the most dominant species on both substrates with highest lactic acid production rate, though its growth on rye led to unstable silage conditions with butyric acid producing Clostridia. The specific methane yields of the corresponding solid residues were determined to be 335.7 ± 7.2 lN kg−1 organic dry matter (ODM) for grass and at 235.0 ± 2.6 lN kg−1 ODM for rye.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Climate change, the growing energy demand of the world’s population and the depletion of fossil fuels obligate the current industrial producers of goods and energy towards transforming production techniques towards sustainable and eco-friendly approaches. However, such approaches require reliable resources of raw materials, modernized production processes and with that, advanced approaches in research and development [1]. Biorefineries have the opportunity to contribute by substituting petroleum with renewable resources [2]. Green biorefineries apply green (grassland) biomass as raw material for the generation of bio-based products (e.g. poly lactic acid) and energy (e.g. biogas) [3]. By fractionating the biomass into a liquid and solid phase, the different pathways of utilization are initiated. The organic juice can be used to gain amino acids or platform chemicals such as lactic acid as feedstock for the chemical industry, while the remaining solid residue can be used for fibre products or biogas production [4]. To ensure a constant supply of raw material, in the northern hemisphere, it is necessary to ensile the biomass so as to accommodate the conversion by lactic acid bacteria of water-soluble carbohydrates into organic acids (lactic acid), to preserve nutrients and prevent detrimental microorganisms, mainly clostridia and coliform bacteria, from spoiling the crop [5–8]. The four stages of the ensiling process are well described in scientific literature [7–10]. Furthermore, the ensiling process defines the quality of the material that is used in the green biorefinery. Hence, an optimized ensiling procedure will directly affect the valuable ingredients in the silage and therefore increases the efficiency of the biorefining process. Although the ensiling process mainly depends on the epiphytic microflora and the chemical composition of the raw material (sugar content, water content, buffer capacity), different silage additives or ensiling treatments can be applied in order to influence certain characteristics of the silage [11]. Biological additives, such as homofermentative lactic acid bacteria which produce lactic acid as a main product or heterofermentative lactic acid bacteria, that produce lactic acid, acetic acid, ethanol and carbon dioxide with the disadvantage of creating higher dry matter (DM) losses, can be used to stimulate the fermentation process. High sugar contents, low water contents and low buffer capacity elevate the fermentability of the feedstock. Additional chemical additives can be utilized to inhibit or restrict undesirable fermentation or aerobic deterioration [7, 12]. The nominal chopping length also affects the amount of lactic acid in different raw materials [13]. By adding selected lactic acid bacteria in combination with carbonated lime (CaCO3), Haag et al. [14] demonstrated that the amount of lactic acid in maize silage was increased by 91.9 % up to 133.2 ± 3.7 g kg−1 dry matter. Larger amounts of lactic acid can be generated from the gradual decrease in pH value, furthermore preventing the artificially generated high density of lactobacilli from being inhibited. The same behaviour can be expected if grass or rye is treated with adequate ensiling techniques. While grass is the main feedstock for green biorefineries, rye is as well-established green crop which seems to be auspicious for high lactic acid contents, due to its promising sugar contents. In contrast to ensiling procedures which aim to preserve nutrients and the storability of forage [15], there is still a need within scientific literature to illustrate research on the increase of lactic acid content in grasses. Based on the study of Haag et al. [14], the aim of this research was to identify, while sustaining a stable silage, to which the yields of the lactic acid amount can be increased in grass and rye, by applying new ensiling techniques to create an additional source of income and an improvement to the value-added chain. Grass and rye consequently were treated with different silage additives and the build-up of platform chemicals (lactic acid, acetic acid, butyric acid, etc.) during the ensiling process was surveyed. In this context, also microbial composition of all conditions were analysed to reveal the key players responsible for high lactic acid production and silage stabilization. Additionally, the specific methane yield of the solid residue, after separating the organic juice, was determined in order to identify potential methane formation losses. The biogas potential and digestibility of remaining solid residues of grass and rye silage have not yet been described in literature.
2 Materials and methods
2.1 Raw materials
The raw material grass (permanent pasture mix, first cut) was collected from the University of Hohenheim (Agricultural Experiment Station: Location Kleinhohenheim, Germany). Harvesting of the grass was performed by an agricultural lawn mower (Disco 3100 F Profil, Claas, Germany). Additionally, the grass was wilted directly on the field to the desired dry matter content of over 350 g kg−1. The raw material rye (Secale cereale) was also obtained from the University of Hohenheim (Agricultural Experiment Station: Location Ihinger Hof, Germany) and harvested as a whole plant during ear emergence with a precision forage harvester (Jaguar Speedstar 870, Claas, Germany). Both raw substrates were reduced to an 8-mm theoretical length of cut.
2.2 Silage preparation
The ensiling process was performed in 1.5-l laboratory scale glass jars (Weck, Germany) at a constant temperature of 20 °C. Defined opening dates were set to 3, 14, 30 and 90 days to determine the course of ingredients over time. Silage additive application, compaction of raw material, the sealing of the jars and the weight calculation were performed according to Haag et al. [14] and standard sets by the German Agricultural Society [16]. Grass and rye were treated as shown in Table 1. Ho, is a homofermentative silage additive mixture consisting of Lactobacillus plantarum, Lactobacillus rhamnosus and Lactobacillus buchneri. L. buchneri is included to ensure aerobic stability for the silage. He is a heterofermentative silage additive containing L. buchneri. The amounts of the biological silage additives were applied according to the instructions of the producer (ISF GmbH, Germany). CaCO3 was used as a buffer substance to prevent a fast decrease in pH value [14, 17]. The adjustment of buffer amounts added to the respective raw substrate was based on expected amounts of produced organic acids and the corresponding dissociation equilibrium. Chopping to a 1-mm theoretical length of cut was performed by a cutting device (Thermomix, Vorwerk, Germany). Each treatment and opening date was performed in triplicates.
To separate the organic juice from the silages that were stored for 90 days, 400.0 g of each sample was pressed for 5 min at a working pressure of 200 bar, using a tincture press (HPH 2.5, HAPA Fertigungstechnik, Germany).
2.3 Analytical methods
All samples of raw material, silages and remaining residues were stored at −20 °C until chemical analyses and anaerobic digestion tests were conducted. Analyses of dry matter, organic dry matter (ODM) and the correction of lost volatile compounds during the drying process were fulfilled in accordance to DIN EN 12880, DIN EN 12879 [18, 19] and the study of Weissbach and Strubelt [20]. The formula of Weissbach [21] was used to determine DM losses along the ensiling process. The measurement of crude ash, crude protein, crude fat, crude fibre and total sugar was conducted according to the European regulations for feed analysis [22]. With reference to the Federation of German Agricultural Investigation and Research Institutes [23], the concentrations of neutral detergent fibre (NDF), acid detergent fibre (ADF) and acid detergent lignin (ADL) were analysed. Capillary gas chromatography (GC Type: CP 3800; VARIAN Medical Systems Inc., USA) and high performance liquid chromatography (HPLC, BISCHOFF Analysentechnik und –geräte GmbH, Germany) were performed, as described by Haag et al. [14], to determine volatile fatty acids, lactic acid, sugars and alcohols in the samples. The statistical analyses were performed using the statistical software R (R Core Team, 2012) to determine significant differences in lactic acid yields, acetic acid yields, pH values and DM losses between the different treatment variations. The significance test was based on Tukey’s studentized range test.
2.4 Taxonomic profiling
In order to determine the microbial composition on silages after 90 days, the separated organic juice, containing a representative picture of all organisms, was utilized for DNA extraction. For each treatment, the replicate with the medium lactic acid content was analysed; 300 μl were directly frozen as beads by dropping into liquid nitrogen. Disruption was accomplished by using a mixer (Mixer Mill MM 200, Retsch, Germany) with a shaking frequency of 30/s under cryogenic conditions. The resulting powder was resuspended in a 300-μl AL lysis buffer supplemented with 30 μl Proteinase K from Qiagen’s QIAamp DNA Mini Kit (Qiagen, Germany) and incubated for 10 min at 56 °C. The following steps were performed according to the manufacturer’s manual and eluted to an end of volume of 50 μl. DNA quantification was completed with the Qubit dsDNA HS Assay Kit (Thermo Fischer, USA).
Starting with 10 ng dsDNA, 16S amplicon libraries were generated according to Illumina’s 16S Metagenomics Sequencing Library Preparation protocol, except of using 1 U Phusion Hot Start Polymerase with HF buffer (Thermo Fischer) instead of KAPA HiFi HotStart ReadyMix (KAPA Biosystems, USA) in both rounds of PCR. The applied primer pair (S-D-Bact-0341-b-S-17 and S-D-Bact-0785-a-A-21) targets hypervariable regions V3 and V4 in a 464-bp amplicon [24]. Accordingly, each library was sequenced with an average depth of roughly 380,000 clusters with paired-ends and 2 × 250 cycles on Illumina HiSeq2500 in rapid mode. Sequences were quality-trimmed with a threshold phred score above Q28 and minimum read length of 50 bp using BBDuk from the BBMAP package 34.41. Subsequently, paired-end sequences were classified via Kraken [25] down to species level using a custom build database consisting of complete RefSeq (version 68) genomes for bacteria, archaea, virus and a set of 12 high quality fungal genomes. To reduce false-positive classification results introduced by contaminations, PCR or sequencing biases, subsequent analysis were only performed on species showing an abundance of at least 1 % of total classified reads in one of the investigated samples. The critical step of classifying such short reads uniquely to a certain taxon down to species level is overcome on the one hand by the specific architecture of the premade database containing each 31 bp k-mer in the sequence and the lowest common ancestor (LCA) in the taxonomy tree that contains that k-mer. On the other hand, Kraken classifies the reads by searching for the root-to-leaf path with the highest weight, which is the sum over all exact mapped k-mers in the path.
2.5 Batch anaerobic digestion test
The methane formation potential (norm conditions: 0 °C, 1,013 hPa) of the silages stored for 90 days and the corresponding solid residues, was determined by applying the patented Hohenheimer Biogas Yield Test (HBT) [26]. The anaerobic digestion test was conducted for 35 days under mesophilic conditions (37 ± 0.5 °C) in accordance to VDI Guideline 4630 [27]. For grass 1.3 g of fresh matter (FM) (0.4 g ODM), for rye 2.0 g FM (0.6 g ODM) of silage was used to run 1 batch reactor. Amounts of 1.1 g FM (0.4 g ODM) and 1.6 g FM (0.6 g ODM) of the solid residue were used, respectively. The mode of operation and calculation is described by Haag et al. [14]. The statistical analyses were performed using the statistical software R (R Core Team, 2012) to determine significant differences in the specific biogas yields and specific methane yields between the silage and the solid residue of the different variations. The significance test was based on a single factor ANOVA.
3 Results and discussion
3.1 Chemical composition of the raw materials
Table 2 displays the chemical analyses of the raw materials grass and rye. The DM contents of grass (374 g kg−1) and rye (353 g kg−1) were measured in an optimal range for the ensiling process [28]. The content of crude ash, crude protein and crude fat in the grass substrate was detected to be approximately twice as high in comparison to the rye substrate. With regard to the total sugar amount, both grass (180 g kg−1 DM) and rye (199 g kg−1 DM) showed high values, which is favourable for lactic acid production. Recalcitrant compounds for biogas and methane formation such as crude fibre, NDF and ADF were obtained in quantities slightly higher in rye than in grass. Furthermore, the ADL content, which is non-degradable in the biogas process, was observed 1.4 times higher in rye. The observed chemical composition of both grass and rye are comparable to values found in literature [29]. As these values are representing a chemical composition of the material which is desirable for further processing, the research has assumed that the cultivation of the described crops, grass and rye was performed in agreement with sound professional practice.
3.2 Silage characteristics
The concentrations of organic acids, alcohols, sugars, pH values and dry matter fermentation losses of grass after 90 days of storage are presented in Table 3. Treatment pH 1 (64.4cd ± 6.9 g kg−1 DM), pH 2 (63.2bcd ± 10.7 g kg−1 DM) and Ho (114.7e ± 5.8 g kg−1 DM) displayed an increase in the lactic acid content. However, the highest quantities of lactic acid in grass silages were observed for the combining treatments of Ho + pH 1 (142.5f ± 22.9 g kg−1 DM) and Ho + pH 2 (149.7f ± 20.9 g kg−1 DM). In comparison to the control treatment (55.7bc ± 1.7 g kg−1 DM), the lactic acid content was increased by 154.9 and 168.8 %. There were no additional DM losses during the ensiling process and acceptable pH values of 4.3 ± 0.3 and 4.3 ± 0.2 were reached. Weinberg et al. [30], showed lactic acid contents in ryegrass silage of up to 171.0 g kg−1 DM. The same behaviour was exhibited for maize silage by Haag et al. [14], whereby the lactic acid content was nearly doubled (133.2 ± 3.7 g kg−1 DM) by supplementing the same homofermentative lactic acid bacteria mix in combination with carbonated lime. Simultaneously, sugars (saccharose, glucose and fructose) were fermented more efficiently in the treatments Ho + pH 1 and Ho + pH 2 compared to the control, in spite of the treatments He + pH 1 and He + pH 2 exhibiting the best efficiency. However, for the treatments He (66.7e ± 1.4 g kg−1 DM), He + pH 1 (59.1df ± 3.3 g kg−1 DM) and He + pH 2 (68.1ef ± 6.7 g kg−1 DM), acetic acid was the main product, caused by the supplemented heterofermentative lactic acid bacteria. The pH values were measured in a desirable range (4.0 ± 0.5) for all treatments, beside pH 1 (4.8cde ± 0.3), pH 2 (4.9de ± 0.2) and He + pH 2 (5.3e ± 0.9), which is a strong indicator for having a stable silage [28].
The development of important ingredients in the control treatment assured that the ensiling process was realized without defect (Fig. 1). The chopped treatment, pH 1 and pH 2 exhibited an increase in lactic acid, as well an increase of acetic acid content. In treatments He, He + pH 1 and He + pH 2, the amount of acetic acid exceeds the amount of lactic acid between the third and last opening date, as well as an almost constant decrease in the lactic acid concentration. Such an observation may be caused by the limitation of hexose which enables heterofermentative lactic acid bacteria to metabolise lactic acid into acetic acid, anaerobically. Nevertheless, the limitation of hexose is unlikely, due to the high sugar contents in the raw material. In all treatments, the primary constituent of lactic acid was produced within the first 14 days. This remained consistent with the standard ensiling process [31]. Treatments Ho, Ho + pH 1 and Ho + pH 2 showed high increases in lactic acid and low production rates of acetic acid and ethanol. The development of lactic acid by treatment Ho + pH 2 indicates that its production might be increased to even higher values by elongating the storage time. This, and the fact that Ho + pH 2 creates highest lactic acid contents together with low DM losses and low pH values, proposes Ho + pH 2 as the most promising treatment for grass.
Similar to grass, rye revealed the highest lactic acid content for the treatment Ho + pH 2 (68.3g ± 2.3 g kg−1 DM) (Table 4). Nevertheless, the treatment was associated with the highest butyric acid content (10.1 ± 1.3 g kg−1 DM) which indicates poor silage quality. The same was obtained for the treatments pH 1, pH 2 and Ho + pH 1 with butyric acid contents of 4.9 ± 0.4 g kg−1 DM, 4.3 ± 0.3 g kg−1 DM and 5.3 ± 0.5 g kg−1 DM. The highest acetic acid contents were again detected in the treatments He + pH 1 (71.2e ± 5.5 g kg−1 DM) and He + pH 2 (73.8e ± 7.5 g kg−1 DM). Despite relatively high losses of DM (10.5d ± 0.4 %), the chopped treatment exhibited to be the most promising. This treatment presented an increase in lactic acid by 46.3 % up to 57.5e ± 0.6 g kg−1 DM, no detectable butyric acid and the lowest pH value of 3.7a ± 0.1. There was no other treatment that offered stable silage conditions with this increase in lactic acid.
The corresponding time resolved yields of lactic acid, acetic acid, butyric acid and ethanol during the ensiling process of rye are displayed in Fig. 2. The generation of ingredients in the control variation had implied that ensiling conditions were adequate. After day 30, lactic acid contents of treatment Ho + pH 1 and Ho + pH 2 had decreased while the butyric acid contents had increased. Such results had assumed a further decrease of lactic acid content with longer storage duration and reactivation of undesired microorganisms (e.g. clostridia, fungi). Furthermore, the increase of butyric acid in the treatments pH 1 and pH 2 was above the critical value of 3.0 g kg−1 DM [28], and will thus lead to deterioration. The reduction of lactic acid and the increased acetic acid synthesis in treatments He, He + pH 1 and He + pH 2 may be linked to the same reason as the grass silages. Ethanol contents were detected in fairly high quantities in the control, the chopped and all variations supplemented with homofermentative lactic acid bacteria or carbonated lime. Although this is not common in rye silage and would rather be expected in treatments with heterofermentative silage additives [11], it was not considered to be detrimental. Despite the fact that the ethanol content had indeed exceeded the lactic acid content in the chopped variant, this was also considered to have no effect on the silage quality of this treatment.
3.3 Microbial composition on silages
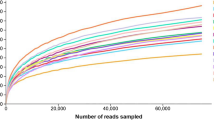
Figure 3a shows for both substrates the unique classification rates from family level down to species level without significant losses of read quantities (Supp. Table 1 A [32]). Through the combination of two different hypervariable regions inside the 16S rDNA and the exact match algorithm of Kraken, it was possible to uniquely assign roughly 96 % of all sequenced clusters to a certain species confirming the necessity of maximal possible read length.
Microbial composition on silages based on 16S amplicon sequencing. a Unique classification rate of 16S amplicon sequences with Kraken. b Venn diagram of detected species on grass and rye silage; a P. pentosaceus and b L. brevis, C. pasteurianum. Species population depending on treatment of grass (c) and rye (d) silage after 90 days
The overall microbial community, after correcting false positives, consisted of six and seven species in grass and rye silages, respectively, while five of them grew on both substrates (L. buchneri, L. plantarum, Lactobacillus sakei, Weissella koreensis and Leuconostoc mesenteroides, Fig. 3b). Each of these species is a member of the order Lactobacillales producing lactic acids, while L. plantarum is the only homofermentative producer.
On grass silage, L. plantarum’s population ratio relative to the other heterofermentative species is significantly increased to 80.0–90.0 % when treated with the homofermentative starter culture mix with L. plantarum, L. buchneri and L. rhamnosus (Ho, Ho + pH 1 and Ho + pH 2; Fig. 3c, Supp. Table 1 B), also explaining the higher production rates of lactic acid (Table 4). In all the other investigated conditions, the heterofermentative species L. buchneri remained the most dominant species. This was in correlation with the higher acetic acid concentrations. The exception, however, was the control silage, whereby a mixture of heterofermentative species was detected with L. mesenteroides to be the best adapted and proliferated organism. A significant buffer effect on species composition in grass could not be observed. However, for conditions that were not treated with bacteria starter cultures, a smaller proportion of Pediococcus pentosaceus was identified, which is also known to produce lactic acid [33] and whose growth obviously is inhibited by the starter culture composed of lactic acid bacteria.
Focussing on the rye silage, the treatments result in slightly different microbial compositions and dependencies. For example, Lactobacillus brevis is specifically growing on rye but not on grass silage, especially after chopping treatment. Also, L. plantarum’s growth was more dependent on the buffer treatment without heterofermentative bacteria starter culture and not exclusively on homofermentative culture additives (pH 1, pH 2, Ho + pH 1, Ho + pH 2 and Ho; Fig. 3d, Supp. Table 1 B). Accordingly, the population ratio of this species on rye had reached approximately 50.0 % under these conditions. However, despite the significant increase of lactic acid production, butyric acid was also produced (Table 4). This circumstance had indicated unstable silages, meaning that these treatments should not be applied in practice. With these treatments, a certain amount of Clostridium pasteurianum signatures were identified probably explaining the unstable conditions caused by the species ability to ferment glucose into butyric acid under certain conditions [34]. The most promising treatment on rye was to chop it, as this led to an increase in the lactic acid yield without the production of butyric acid. Interestingly, the mixed population of heterofermentative lactic acid bacteria represented 90.0 % of the community and only 10.0 % of L. plantarum without an increase in acetic acid yields. However, such an increase in acetic acid and a simultaneous decrease in lactic acid was observed when rye silage was exclusively treated with L. buchneri as starter culture presenting an almost 100.0 % pure monoculture in Fig. 3d (He). By trend, the buffer treatment in combination with L. buchneri starter culture (He + pH1 and He + pH 2) exhibited similar acid yield changes with the exception that lactic acid rates decreased to a lesser extent. Correspondingly, the research assumed that such results may be explained by the moderate growth of homofermentative L. plantarum.
In summary, the identical treatments presented different effects and results referring to the two tested substrates. Furthermore, identical treatments also seem to have different impact on lactic acid yield as well as on silage stability. This circumstance was also reflected on the level of microbial composition which might be the key to control these treatments more substrate-specifically. Furthermore, no signatures from L. rhamnosus were detected neither on grass nor on rye despite its inoculation within the homofermentative starter cultures had indicated that this species is not adequately adapted to the substrates and could be spared in the silage additives for these substrates.
3.4 Methane formation potential
Figure 4 illustrates the specific biogas yields (A) and methane yields (B) for silage and solid residue of all grass treatments. Specific methane yield of the control variation (375.8 ± 1.8 lN kg−1 ODM) is comparable to other investigations [35, 36] (Fig. 4b). The specific biogas yields of the grass silage treatments varied in a range from 647.3 ± 8.7 lN kg−1 ODM (Ho + pH 2) to 699.5 ± 6.2 lN kg−1 ODM (control), those of the corresponding solid residues from 574.1 ± 5.8 lN kg−1 ODM (chopped) to 669.1 ± 13.2 lN kg−1 ODM (pH 1) (Fig. 4a). Specific methane yields of the grass silage were obtained from minimum 348.5 ± 4.4 lN kg−1 ODM (Ho + pH 2) to maximum 380.0 ± 1.7 lN kg−1 ODM (He + pH 1), those of the corresponding solid residues from 289.2 ± 14.5 lN kg−1 ODM (He + pH 2) to 367.2 ± 3.3 lN kg−1 ODM (He + pH 1). Significant differences were detected between the specific biogas/specific methane yield of all silages and their corresponding solid residues, with the exception being the treatments Ho + pH 1 and Ho + pH 2. Organic acids, which are important for biogas production, had been lost during the separation process. This therefor led to a decrease in specific biogas and specific methane yields. Although the research did not have any assumptions to explain the phenomenon that the treatments Ho + pH 1 and Ho + pH 2 did not show significant differences, this incident was also observed for maize silage in the survey of Haag et al. [14]. In order to implement the concept of utilizing solid residue from these treatments in a green biorefinery, the research has considered it highly valuable that the specific methane yield remain sustained. Nevertheless, there is a loss of mass which has to be concerned in an economic consideration.
The same noteworthy occurrence, as in the treatments for grass, was observed for the specific biogas yield of rye treatments (Fig. 5a). Varying in a range from 475.3 ± 7.5 lN kg−1 ODM (Ho + pH 1, silage) to 580.9 ± 9.6 lN kg−1 ODM (He + pH 1, silage) and from 433.7 ± 9.1 lN kg−1 ODM (pH 2, solid residue) to 515.9 ± 9.5 lN kg−1 ODM (Ho + pH 2, solid residue), only the treatments Ho + pH 1 and Ho + pH 2 showed no significant differences in specific biogas yields between silage and solid residue. The specific methane yields varied from 257.3 ± 6.9 lN kg−1 ODM (Ho + pH 1, silage) to 319.1 ± 8.3 lN kg−1 ODM (He + pH 1, silage) as well as from 227.3 ± 6.7 lN kg−1 ODM (He + pH 2, solid residue) to 267.0 ± 6.9 lN kg−1 ODM (Ho + pH 1, solid residue) (Fig. 5b). In contrast to the specific biogas yield, the only treatment without a significant difference in specific methane yield between silage and solid residue was observed for Ho + pH 1. Furthermore, the difference between silage and solid residue in the treatment Ho + pH 2 was almost not statistically different. Once again, the treatments Ho + pH 1 and Ho + pH 2 presented unique properties for methane production. This might be caused by a change in structure of the fibres of the silage due to the treatment and hence improves anaerobic degradation. Chemical composition of the silages and solid residues should be addressed in further investigations. The control variation delivered a specific methane yield of 270.1 ± 1.1 lN kg−1 ODM for the silage, which is in line with expected methane yields for rye [36]. The chopped variation exhibited vast losses in specific methane yield from silage to solid residue (−60.6 lN kg−1 ODM). This in turn would reduce the potential of rye to be of high value of this study’s green biorefinery concept. In all anaerobic batch digestion tests, there was no inhibition detected, that had influenced the digestibility of the solid residues. This study is highly confident, that all the solid residues could be fermented in a full-scale agricultural biogas plant without any significant impact on the biogas process.
3.5 Production scheme
Calculating with an average harvest yield of 14.0 Mg ha−1 DM for grass and an overall lactic acid extraction efficiency of 76.5 % [37], the most promising treatment Ho + pH 2 could produce 1.6 Mg ha−1 of lactic acid. Considering that approximately 50.0 % of the harvested biomass (7.0 Mg ha−1 DM; 89.5 % ODM) can be used for further biogas production, after organic juice separation, a methane yield of 2,104,100.5 lN ha−1 is attainable. The increasing demand for lactic acid (e.g. biopolymer production) from the world market makes this source attractive for future concepts of biorefining. It is necessary to keep in mind that the purity of lactic acid and the costs to achieve high purity will have a huge influence on the economic feasibility of the procedure.
Assuming an average harvest yield of 11.0 Mg ha−1 DM for rye, the chopped variation would be able to deliver 0.5 Mg ha−1 of lactic acid. Approximately 50.0 % of the harvested biomass (5.5 Mg ha−1 DM; 96.6 % ODM) would remain for additional biogas production, which may assume a methane yield of 1,248,555.0 lN ha−1. The low amount of lactic acid that was available in rye, together with high deficits in specific methane yield due to organic juice separation, indicates that this plant would not be suitable for this concept of green biorefining.
4 Conclusions
The lactic acid content in grass silages can be increased by supplementing carbonated lime and homofermentative L. plantarum. Hence green biorefineries, using ensiled grasses with the aim to produce lactic acid and biogas, are able to be optimized by choosing an adequate ensiling technique and accordingly improve the value added chain. Due to its low amount of lactic acid contents and the inadequate specific methane yield, rye is no promising substrate for application in such a green biorefinery concept. Future research should focus on new ensiling methods of other raw substrates which hold promise for valuable platform chemicals. In addition, inoculation of the raw material with an overdose of the dominant lactic acid producing bacteria might be interesting for following studies. Furthermore, the transferring efficiency of carboxylic acids from the silage into the organic juice, the extraction efficiency out of the organic juice and the reintroduction of the processed organic juice into the biogas process would be of high interest for the economical evaluation of the entire concept.
References
Kamm B, Kamm M (2004) Principles of biorefineries. Appl Microbiol Biotechnol 64:137–145
Kamm B (2007) Production of platform chemicals and synthesis gas from biomass. Angew Chem - Int Ed 46:5056–5058
Kromus S, Wachter B, Koschuh W, Mandl M, Krotscheck C, Narodoslawsky M (2004) The Green Biorefinery Austria—Development of an integrated system for green biomass utilization. Chem Biochem Eng Q 18:7–12
Cherubini F, Jungmeier G, Wellisch M, Willke T, Skiadas I, van Ree R, de Jong E (2009) Toward a common classification approach for biorefinery systems, Biofuels. Bioproducts Biorefining 3:534–546
Weinberg ZG, Muck RE (1996) New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol Rev 19:53–68
Kandler O (1983) Carbohydrate metabolism in lactic acid bacteria, Antonie van Leeuwenhoek. Int J Gene Mole Microbiol 49:209–224
McDonald P, Henderson N, Heron S (1991) The Biochemistry of Silage. Marlow, Bucks
Bolsen KK, Ashbell G, Weinberg ZG (1996) Silage fermentation and silage additives—Review. Asian-Australasian J Animal Sci 9:483–493
Pitt RE, Muck RE, Leibensperger RY (1985) A quantitative model of the ensilage process in lactate silages. Grass Forage Sci 40:279–303
Lindgren S, Pettersson K, Kaspersson A, Jonsson A, Lingvall P (1985) Microbial dynamics during aerobic deterioration of silages. J Sci Food Agric 36:765–774
Herrmann C, Heiermann M, Idler C (2011) Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour Technol 102:5153–5161
Henderson N (1993) Silage additives. Anim Feed Sci Technol 45:35–56
Herrmann C, Heiermann M, Idler C, Prochnow A (2012) Particle size reduction during harvesting of crop feedstock for biogas production i: effects on ensiling process and methane yields. Bioenergy Res 5:926–936
Haag NL, Nägele H-J, Fritz T, Oechsner H (2015) Effects of ensiling treatments on lactic acid production and supplementary methane formation of maize and amaranth—an advanced green biorefining approach. Bioresour Technol 178:217–225
Mayne CS (1990) An evaluation of an inoculant of Lactobacillus plantarum as an additive for grass silage for dairy cattle. Anim Sci 51:1–13
German Agricultural Society, DLG (2000) DLG guideline for the testing of silage additives on DLG quality mark capability. DLG Verlag, Frankfurt am Main
Huenting K, Aymanns T, Pries M (2012) Fermentation potential of corn silage, XVI International Silage Conference 356–357
German Institute for Standardization (2001) DIN., DIN EN 12880: Characterization of sludges—determination of dry residue and water content; German version EN 12880:2000
German Institute for Standardization (2001) DIN., DIN EN 12879: Characterization of sludges—determination of the loss on ignition of dry mass; German version EN 12879:2000
Weissbach F, Strubelt C (2008) The correction of the dry matter content of grass silage as a substrate for biogas plants. Landtechnik 63:210–211
Weissbach F (1998) On the methodology of determining the fermentation losses in silage, Jahresbericht der FAL. 26–26
E. Commission Regulation (2009) Commission Regulation 2009/152/EC Laying down the methods of sampling and analysis for the official control of feed, Official Journal of the European Union. L 54/1
Federation of German Agricultural Investigation and Research Institutes, VDLUFA (2007) Method Book III—the chemical analysis for feedstuffs. VDLUFA Verlag, Darmstadt
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner F O (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41
Wood D E, Salzberg S L (2014) Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 15
Helffrich D, Morar M, Lemmer A, Oechsner H, Steingaß H (2005) Laboratory method for determining the quality and quantity of biogas produced from anaerobic decomposition of organic substances in a batch process
VDI-Society Energy and Environment, VDI (2006) VDI 4630: Fermentation of organic materials—characterization of the substrate, sampling, collection of material data, fermentation tests
Gerighausen H (2011) Praxishandbuch Futter- und Substratkonservierung. DLG Verlag, Frankfurt am Main
Bayerische Landesanstalt für Landwirtschaft, LFL (2015) Gruber Tabelle zur Fütterung der Milchkühe, Zuchtrinder, Schafe und Ziegen, LfL-Information. 37:1–94
Weinberg ZG, Ashbell G, Azrieli A, Brukental I (1993) Ensiling peas, ryegrass and wheat with additives of lactic acid bacteria (LAB) and cell wall degrading enzymes. Grass Forage Sci 48:70–78
Pahlow G, Muck RE, Driehuis F, Oude Elferink SJWH, Spoelstra SF (2003) Microbiology of ensiling. In: Al-Almoodi L, Barbarick KA, Volenec JJ, Dick WA (eds) Silage Science and Technology, American Society of Agronomy, Inc.; Crop Science Society of America, Inc. Soil Science Society of America, Inc., Madison, pp 31–93
Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman N J, Walker AW (2014) Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 12
Pritchard GG, Coolbear T (1993) The physiology and biochemistry of the proteolytic system in lactic acid bacteria. FEMS Microbiol Rev 12:179–206
Dabrock B, Bahl H, Gottschalk G (1992) Parameters affecting solvent production by Clostridium pasteurianum. Appl Environ Microbiol 58:1233–1239
Prochnow A, Heiermann M, Plöchl M, Linke B, Idler C, Amon T, Hobbs PJ (2009) Bioenergy from permanent grassland—a review: 1. Biogas. Bioresour Technol 100:4931–4944
Amon T, Amon B, Kryvoruchko V, Machmüller A, Hopfner-Sixt K, Bodiroza V, Hrbek R, Friedel J, Pötsch E, Wagentristl H, Schreiner M, Zollitsch W (2007) Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour Technol 98:3204–3212
Kromus S, Novalin S, Koschuh W, Hong Thang V, Krotscheck C (2003) Green biorefinery—separation of lactic acid from grass silage juice
Acknowledgments
The authors are grateful to Annette Buschmann (University of Hohenheim, State Institute for Agricultural Engineering and Bioenergy, Germany) for analysing numerous samples and her dedicated work in the laboratory. Furthermore, the authors thank Dietmar Ramhold and Thomas Fritz (ISF GmbH, Germany) for providing the silage additives. Finally, the authors thank Philip Stevens (Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB, Germany) for his support and helpful comments on Kraken.
Funding
This work was funded by the Federal Ministry of Education and Research (BMBF) within the scope of the project “GOBi - General Optimization of Biogas Processes; FKZ 03EK3525A.”
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLSX 18 kb)
Rights and permissions
About this article
Cite this article
Haag, N.L., Grumaz, C., Wiese, F. et al. Advanced green biorefining: effects of ensiling treatments on lactic acid production, microbial activity and supplementary methane formation of grass and rye. Biomass Conv. Bioref. 6, 197–208 (2016). https://doi.org/10.1007/s13399-015-0178-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-015-0178-2