Abstract
The purpose of this study was to bioprospect the volatile organic compounds (VOCs) of various Trichoderma harzianum strains to control black rot of postharvest snake fruit, an important fruit commodity in Southeast Asia, caused by the fungus Thielaviopsis paradoxa. Trough an indirect confrontation assay, T. harzianum InaCC F88 was found as the most suppressing strain among others. The strain inhibited T. paradoxa with growth relative to control (GRC) of 71.14%. A volatolomic analysis using Headspace GC–MS of this strain showed the most abundant VOC was isoamyl alcohol (36.06%), followed by 2-methyl-1-propanol (21.92%) and 2-cyclopentenone (10.72%). Isoamyl alcohol as the major compound inhibited T. paradoxa with GRC of 71.44, 28.88, and 2.86% after the addition of 10, 20, and 30 µL of the vapor of pure compound, respectively. Moreover, in a 1.5-L close-container assay, the addition of 300 µL isoamyl alcohol vapor was also able to reduce lesion tissue in the pre-infected fruit up to 29.15% after 7 days of storage in room temperature compared to 58.97% in the absence of the pure compound. In conclusion, T. harzianum InaCC F88 through its VOCs was potential to biocontrol black rot in snake fruit, thus extend its storage time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Snake fruit (Salacca sp.) is a tropical fruit that is commonly found in Southeast Asia. In Indonesia, this fruit is called “Salak” and is cultivated widely with a productivity of 35.33 ton/ha and a plantation area of 27,050 ha in 2019, thus playing a significant role in sustaining the economy of some areas, particularly in Sumatra, Java, and Bali Island [1]. Besides its unique flavor due to the presence of various methyl esters and sweet taste because of its high content of sugars [2], this fruit is also rich in antioxidants, polyphenols, vitamin C, as well as various organic acids [3]. Therefore, this fruit is one of the most popular fruits for daily consumption in Indonesia.
Postharvest loss (PHL) of snake fruit is relatively higher than other tropical fruits. This is mainly caused by the high intensity of decay by microbes. Generally, in developing countries, the PHL can reach 20–50% of the total production volume [4]. Due to its unique morphology, the tip of this fruit is very susceptible to pathogen infection. Black rot is a common disease found in the tip during storage in the market. The pathogen blackened or browned the infected fruit flesh. Further, the lesions became soft rot and produced an odious odor with sometimes an appearance of white mycelia on the rot part. This fruit rot contributes to large economic losses during storage and distribution. In addition, some of pathogenic microbes associated with postharvest rot produce mycotoxins which are dangerous to human health if the infected fruit is ingested [5]. Under these circumstances, the countermeasure action to prevent PHL due to postharvest disease is inevitable.
Chemical control is broadly considered the first strategy to control PHL due to microbial causes. Particularly, the application of synthetic chemical pesticides (SCPs) with their effective outcome is still a common practice. However, this method has been widely reviewed for some serious drawbacks. The foremost reason is the potential toxicity effect of SCPs on the surrounding environment and particularly on human health accompanied by their accumulative and persistent tendency [6]. Moreover, some cases of microbial pathogen resistance have been reported by the continuous sole application of SCPs [7]. As an alternative, chemical pesticides from vegetation sources have been investigated which is considerably more convenient [8]. However, this solution still requires a lot of improvements because of its ability to alter fruit aroma and the difficulty of standardizing the formula, hence influencing its effectiveness.
In the last decades, the utilization of volatile organic compounds (VOCs) derived from microbial sources, particularly fungi, has been a promising alternative to the biological control of PHL [9]. The microbial VOCs are considered effective and more environmentally friendly to reduce the use of SCPs. Trichoderma species are widely used in agricultural practice due to their various ability to improve plant growth and suppress phytopathogens. Among the species in the genus, T. harzianum is considered superior due of its ability to biosynthesize many secondary metabolites and potential enzymes. A previous study reported that T. harzianum emitted VOCs as secondary metabolites and signal molecules that mediated plant growth [10]. A more recent study showed that, besides stimulating the growth of the plants, the T. harzianum VOCs were able to combat phytopathogens as well [11]. In this study, the fungal VOCs were tested to inhibit the growth of Botrytis cinerea. However, it is unclear whether T. harzianum VOCs attacked the specific microbial target only. Hence, these metabolites might also target other microbial pathogens on various postharvest fruit disease. Therefore, this study aimed to bioprospect T. harzianum VOCs as a countermeasure for microbial pathogens causing the devastated black rot on Snake Fruit.
2 Materials and Methods
2.1 T. harzianum Strains and Maintaining Condition
Ten isolates of T. harzianum strain were obtained from the Indonesian Culture Collection (InaCC). The strains were isolated from two sources of samples, leaf litters (InaCC F86, InaCC F87, InaCC F89, InaCC F90, InaCC F91, InaCC F92, InaCC F116, and InaCC F144) and soils (InaCC F88 and InaCC F115). All strains were subcultured on PDA (Himedia, India) plates at 30 °C before use in the procedures.
2.2 Isolation of Pathogenic Fungi
The pathogenic fungi were isolated from rotten snake fruit (Salacca zalacca (Gaertner) Voss var. pondoh) collected from the Cibinong market, West Java Province, Indonesia. The fungal isolation was performed according to the surface sterilization method with some modifications [12]. The rotten S. zalacca was washed under tap water, dried with a sterile paper towel, and sterilized superficially by immersing in 70% ethanol for 1 min, after that with 1% sodium hypochlorite (NaOCl) for 2 min, and then thoroughly rinsed thrice with sterile distilled water. Afterward, the fruits were wrapped with a sterile paper towel and then dried for 3–4 h at ambient temperature to separate water from the surface. Rotten S. zalacca samples were prepared by cutting into 5-mm segments and subsequently placed on PDA plates. For each plate, four segments were placed individually. Cultures were then stored at 27 °C in the dark and were daily observed for 7–14 days. The actively growing fungal mycelia was transferred to new PDA plates and kept in a dark incubator at 27 °C. The purified fungal strains were then selected for working and backup cultures.
2.3 Identification of the Pathogenic Fungi
Pure pathogenic fungi were transferred and grown on PDA plates and then incubated at 27 °C for 5–7 days. Initial identification of fungi was based on morphological characteristics. Morphological identification was performed by observing both macroscopic and microscopic features. Observation of macroscopic characterizations included color, reverse color, texture, surface, colony shape, and exudate drop. Microscope slides were prepared from each selected strain using lactophenol as mounting medium. The observation of microscopic characterizations was done on a light microscope by investigating hyphae, pigmentation of the hyphae, clamp connection, septate, spores, conidia, and other reproductive structures.
Fungal strains were selected for their characterization using molecular approaches. Molecular identification was performed by DNA sequence analysis of the internal transcribed spacers (ITS1 and ITS2) of the rDNA region, which includes the 5.8S rRNA. Total fungal genomic DNA was isolated using the Nucleon PhytoPure, Plant and Fungal DNA Extraction Kit (GE Healthcare, USA) according to the manufacturer's instructions. DNA amplification of the ITS region of rDNA was performed by polymerase chain reaction (PCR). PCR amplification was performed in a 25 μl reaction mixture containing 10 μl distilled water, 12.5 μl GoTaq Green Master Mix (Promega, USA), 0.5 μl DMSO, 0.5 μl of each primer (10 pmol) and 1 μl (5–10 μl). ng) genomic DNA was taken as a sample. Approximately 550 nucleotides of ITS1 and ITS2 containing 5.8S rDNA were amplified using ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5(5′-GGAAGTAAAAGTCGTAACAAGG-3′) primer sets [13]. Amplification was done in a TaKaRa PCR Thermal Cycler P650 (TAKARA BIO Inc., Japan), programmed under the following conditions: initial denaturation at 95 °C for 3 min, 30 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min. The PCR products were purified and sequenced.
A preliminary construction of phylogenetic trees of selected strains was performed by editing raw sequence data using ChromasPro (http://www.technelysium.com.au/ChromasPro.html). Compiled sequences were retrieved from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nlh.gov/) using Muscle (http://www.ebi.ac.uk/Tools/msa/hardware). Phylogenetic analysis of the sequence data was performed according to the neighbor-joining (NJ) method [14] using the Molecular Evolutionary Genetics Analysis (MEGA) program version 7 [15]. The reliability of each branch was assessed by bootstrapping with 1000 resampling.
2.4 Indirect Confrontation Between T. harzianum and the Pathogenic Fungus
In vitro bioassays were performed using 9-cm Petri dishes containing approximately 15 mL of PDA medium. Two separate dishes were prepared. The first was inoculated into the center of a 6-mm hyphal disk containing a pathogenic fungal strain, and the other was inoculated with a T. harzianum strain. The lids were removed, the two plates were inverted, and the pathogenic fungi were placed on the T. harzianum plates. The two bases of the plates were sealed with a double layer of parafilm and kept at 30 °C. To avoid interference of antagonistic spores from plates inoculated with pathogenic fungi, the pathogens were on the upper plate. The time of assessment was determined 2 days post-confrontation and the diameter of pathogenic fungal mycelia was measured. The experiment was performed in triplicate for each T. harzianum strain. The percentage of growth relative to control (%GRC) was calculated as the percentage of the increasing in mycelium length after T. harzianum treatment (mm) and divided by the increasing in mycelium length of control (mm).
2.5 Untargeted Volatolomics of T. harzianum Strain by Headspace Gas Chromatography–Mass Spectrometry (GC–MS)
To analyze the volatile compounds within the selected T. harzianum strain, GC–MS was conducted according to the method by [16] with some modifications. The T. harzianum strain was inoculated in a 20-mL specific chromatography vial containing 1 mL of PDA and incubated at 30 ± 2 °C for 7 days. Headspace autosampler Shimadzu AOC 6000 (Shimadzu Corp, Japan) was performed to collect the VOCs by incubation for 1 min with agitation (250 rpm) at temperature of 30 °C. The sampler then inserted into the injection port of the gas chromatograph Shimadzu QP2020 (Shimadzu Corp, Japan), equipped with an SH-Rxi-5SilMS capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness). The temperature of oven was initially maintained at 40 °C for 3 min, then elevated to 230 °C then held for 5 min, at a rate of 7 °C min−1. The temperature of injector was 280 °C. The carrier gas was ultra-high purity helium with an initial column pressure of 61.8 kPa and a flow rate of 1.17 mL min−1. The ion source and quadrupole were set at 200 °C. Electron impact (EI) mass spectra were collected at an ionization voltage of 0.4 eV over the m/z range of 29–550. Volatiles and semivolatiles were identified based on computer searches ordered by the National Institute of Standards and Technology (NIST) 20 search chromatograms of mass spectral libraries.
2.6 Sealed Petri Dish Method of Pure Volatile Against Mycelial Growth of the Pathogenic Fungus
A sealed Petri dish method was developed and performed to determine the effect of the main volatile compounds involved in antifungal activity against pathogenic fungus. The compound isoamyl alcohol (3-methyl-1-butanol) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Isoamyl alcohol was dropped on a circular Whatman® (USA) paper filter (diameter around 6 mm) as much as 5–80 µL and placed on the lid of the plate. No isoamyl alcohol was used as the negative control. The tested plates were incubated at 30 ± 2 °C for 2 days, each treatment was administered three times. Mycelial colony expansion of pathogenic fungus was measured and %GRC was calculated.
2.7 In Vivo Assay of Volatile Against Postharvest Fruit Rot
In vivo experiments were conducted to investigate the effect of high VOCs on postharvest fruit rot of snake fruits according to the method by [16] with some modifications. Pathogenic fungus was fermented in PDB (Himedia, India) and grown under static conditions for 5 days at 30 ± 2 °C. Conidial suspension (5 μL) was applied to snake fruit for each experiment, consisting of three fruits (three replicates). Simultaneously, fungal inoculated snake fruits were incubated with isoamyl alcohol at 50–500 μL applied on a sterile tissue paper, without direct contact with the fruit, in a closed 16 × 16 × 7 cm plastic box for seven days in a dark incubator. Fungal inoculated snake fruits incubated without isoamyl alcohol served as controls. The fruit flesh only was examined to investigate the effect of the volatile compound on the infected fruit. The lesion part was marked by the soft and blackened/browned fruit flesh, while the uninfected part is rigid and yellowish. The percentage of the lesion part relative to whole fruit flesh (%LRW) was calculated as the percentage of fresh weight of lesion fruit (g) divided by the total fruit flesh (g).
2.8 Data Analysis
All data, including %GRC and %LRW, were subjected to analysis of variance (ANOVA). Statistically significant differences between treated samples and untreated controls were analyzed using Tukey's test with a threshold of P < 0.05. All graphics and statistical analyses were conducted using GraphPad Prism version 10.1.2 (San Diego, USA). The asterisk indicates the P-value of the comparison test, * (P < 0.05), **(P: 0.05–0.01), ***(P: 0.01–0.001), ****(P > 0.001). Without an asterisk sign, it is not significantly different (P > 0.05).
3 Results
3.1 Thielaviopsis sp. was the Fungal Pathogens Causing Tip Rot on Snake Fruit
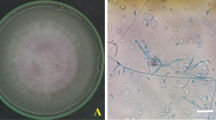
Six isolates of pathogenic fungi Thielaviopsis sp. (SPT DI-1, SPT DI-2, SPT DI-3, SPT DI-4, SPT DI-5, and SPT DI-6; see Appendix table) were isolated from rotten S. zalacca samples (Fig. 1A) collected from Cibinong market, West Java Province, Indonesia. Once isolated, the fungus based on its morphological characteristics was identified as Thielaviopsis sp. [17]. After 7 days of incubation on PDA with a temperature of 27 °C, gray or dark colonies occupied the entire 8 cm plate (Fig. 1B), conidiophores 100–200 × 6–10 µm, hyaline to light-brown, septated at the base, smooth. Conidia were sometimes cylindrical 4–12 × 2–3 µm, truncate at the ends, smooth, phialidic, hyaline, changing to light-brown or sometimes varying in shape, cylindrical oval or slightly ellipsoidal, 4–20 × 3–5 µm, smooth or in chains (Fig. 1C, D).
The rotten S. zalacca samples were presumably caused by pathogenic fungi Thielaviopsis sp. (A). The macroscopic and microscopic view of the representative Thielaviopsis sp. strains SPT DI-1 were isolated from snake fruit (B–D). The culture was grown in vitro on potato dextrose agar (PDA) media, 7 days incubation at T 27 °C. Neighbor-joining tree of three selected strains of pathogenic fungi Thielaviopsis sp. strain DI-1, DI-2, and DI-3 based on ITS rDNA sequence and Lasiodiplodia theobromae as outgroup (E). Only bootstrap values above 70 are shown
The molecular analysis was conducted on the three selected strains of pathogenic fungi Thielaviopsis sp. strain SPT DI-1, 2, and 3. The query length of ITS nucleotides was aligned online at NCBI (http://www.ncbi.nlm.nlh.gov/), consisting of 550–580 base pairs. The first–second closest taxa according to online BLAST alignment is shown in Appendix table. Fungal taxa Ceratocystis paradoxa is a fungus in the phylum Ascomycota with the asexual name Thielaviopsis paradoxa and is a common cause of black and stem end rot in its hosts. Phylogenetic analysis confirmed these molecular results. Based on the phylogenetic tree constructed by NJ analysis, the ITS sequences of the three representative strains of Thielaviopsis sp. SPT DI-1, 2, and 3 overlapped in the same clade as C. paradoxa strain BLH5-1 (KC415073). The pickup value for the C. paradoxa strain BCRC 34425 (GU358207) and the C. paradoxa strain CBA-HW01 (GU567771) was 98% (Fig. 1E). For further experiments, one isolate, Thielaviopsis sp. SPT DI-2 was selected as a representative for all isolates.
3.2 Volatile Compounds Produced by T. harzianum Strains Inhibit the Mycelial Growth of T. paradoxa
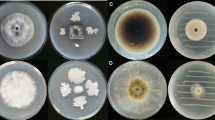
The sealed plate method was performed to test the effect of VOCs released by T. harzianum on the vegetative mycelial growth of T. paradoxa After two days of indirect confrontation in a dark incubator with a temperature of 30 °C, the colony diameter of T. paradoxa was affected by various degrees. In contact with T. harzianum InaCC F88, the mycelial growth of T. paradoxa was lower than other T. harzianum strains (Fig. 2). T. harzianum InaCC F88 was able to significantly suppress 71.14% (P < 0.0001, 95% CI of diff.) of T. paradoxa growth in comparison to the control plate. On the other hand, T. harzianum InaCC F86, F87, and F90 did not significantly inhibit the growth of T. paradoxa.
3.3 Identification of VOCs Emitted by T. harzianum InaCC F88
The results from indirect confrontation showed that T. harzianum InaCC F88 had a strong inhibition ability toward T. paradoxa. This was suggested that the VOCs emitted by this mold had antifungal activity. To identify and characterize the fungal VOCs, an untargeted approach of analysis was conducted by using GC–MS coupled with headspace to collect the emitted volatile compounds. A total of 9 compounds with similarity above 70% were identified using the NIST library (Table 1). The chromatogram of analysis is shown Appendix figure. As illustrated in Table 1, the identified VOCs of T. harzianum InaCC F88 were belong to various chemical groups, from alcohol (2-methyl-1-propanol and 3-methyl-1-butanol), ketone (2-cyclopentenone), ester (3-methyl-1-butyl ethanoate), amide (semicarbazide), carboxylic acid (ethyl hydrogen oxalate), alkene (2-methyl-1,3,5-hexatriene), enol (7-methyloctane-2,4-dione), and carboxylic ester (4-nitrophenyl hept-2-enoate). The most abundance VOC detected in this investigation was 3-methyl-1-butanol (the trivial name is isoamyl alcohol) at 3.932 min with 36.06% peak area, followed by 2-methyl-1-propanol at 2.324 min with 21.92% peak area, and 2-cyclopentenone at 2.200 with 10.72% peak area. The mass spectrum and molecular structure of isoamyl alcohol are shown in Appendix.
3.4 Effect of Pure VOC Against T. paradoxa
Isoamyl alcohol as the pure volatile compound with the most abundance percentage in the volatilome of T. harzianum InaCC F88 fermented in PDA, was subjected to an indirect confrontation with T. paradoxa in a 9-cm Petri dish containing PDA. The result showed that the antifungal activity was dependent on the concentration of isoamyl alcohol. Application of 40 µL and above of isoamyl alcohol to the plate assay inhibited the mycelial growth of T. paradoxa (Fig. 3). However, the application of 30 µL of the compound strongly inhibited the pathogen with the percentage of GRC reaching only 2.86%. The addition of 20, 10, and 5 µL of isoamyl alcohol resulted in 28.88, 71.44, and 96.28% of GRC, respectively, indicating the decreasing of inhibition ability. This result confirmed that isoamyl alcohol has antifungal properties against T. paradoxa.
3.5 Pure VOC of T. harzianum Strain Reduced Postharvest Rot in Snake Fruit
The previous result showed that isoamyl alcohol as the pure VOC of T. harzianum InaCC F88 has antifungal properties against T. paradoxa in PDA plate. Therefore, isoamyl alcohol was subjected to the pathogen in snake fruit rot during storage. To examine the effect of isoamyl alcohol on snake fruit during postharvest storage, the pathogen was inoculated to the tip of the fruit at a similar time to the exposure of the volatile compound. The result showed that post-incubation at room temperature and dark conditions, a blacked rotten tissue was observed in snake fruit with and without exposure to isoamyl alcohol. However, the percentage of this lesion part in fruit (%LRW) without isoamyl alcohol exposition was highest among other treatments and decreased gradually as the amount of isoamyl alcohol was increased (Fig. 4). The LRW value of fruit without the addition of isoamyl alcohol was 58.97%. The addition of 100 and 200 µL decreased the LRW to 47.65 and 35.17%, but not significantly different (P < 0.0001, 95% CI of diff). The application of 300 µL significantly decreased the LRW to 29.15%. The addition of isoamyl alcohol as much as 400 and 500 µL plummeted the LRW value to 11.82 and 3.83%, respectively.
4 Discussion
T. paradoxa is the main fungal pathogen causing tip rot disease in postharvest snake fruit [22]. Reported for the first time that T. paradoxa was observed to occur in rot snake fruit in Thailand. The pathogen caused blackened or browned discolor to the infected fruit flesh. The lesions became soft rot and produced a heinous odor with sometimes an appearance of white mycelia on the rot part. Besides snake fruit, T. paradoxa was also reported to infect other postharvest fruits in various countries. T. paradoxa was reported as the causal agent of black rot on pineapple in French Guinea [23]. In this country, T. paradoxa has been listed as a quarantine pathogen. In Turkey, this fungus was reported to cause main stalk rot on bananas [24]. T. paradoxa was not only reported to infect fruit but also other parts of various plants. An outbreak of trunk rot in coconut trees was caused by this fungus [25]. Similar to rotting in fruit, T. paradoxa also produced black or brown lesions in the coconut stem. In addition to the stem, T. paradoxa was able to infect the leaves of bottle palms as well and produce blackish brown lesions on the leaf tissue [26].
T. harzianum strains produced VOCs with anti-fungal properties. Various Trichoderma species have been previously reported to emit volatile compounds with biocontrol activity against phytopathogens as well as plant growth-promoting ability [10, 27]. However, this property was shown as strain-dependent. A similar study conducted by [28] showed that various T. harzianum strains have variability in inhibiting growth growth-altering morphology of some pathogenic wood rot fungal species through indirect confrontation bioassay. Another report showed that Trichoderma spp. differed in producing volatile compounds and potency as plant growth-promoting and biocontrol agents [29]. This intraspecies variation relies on genetic and epigenetic factors, which from an ecological and evolutionary standpoint is a strategy for keeping survivability under various unfavorable environmental conditions [30].
Isoamyl alcohol was found as the major volatile compound emitted by T. harzianum InaCC F88. isoamyl alcohol has been traced in volatilomes of various molds and yeasts as well. Isoamyl alcohol along with its ester, isoamyl acetate, was among the major VOCs produced by Candida maltose, isolated from cheese when it was grown on YPD agar [31]. Furthermore, in this study, isoamyl alcohol and isoamyl acetate inhibited the growth of 15 species of molds at 20 µL/dish and 160 µL/dish, respectively. Isoamyl alcohol was found as one of the major compounds emitted by Metarhizium brunneum, an entomopathogen mold, but the production depended on the strain, incubation period, and most notably nutrient condition [32]. Isoamyl alcohol was majorly produced in osmotic stress medium as well as low to intermediate C/N medium. Moreover, the study also showed the effectivity of isoamyl alcohol to control the growth of Pythium ultimum, a fungal phytopathogen that responsible for root rot disease of diverse crops, with the percentage of inhibition was above 80%. In addition to yeast and mold, isoamyl alcohol was also detected as a VOC produced by bacteria. In a peptone medium, isoamyl alcohol was detected produced by Bacillus spp. among other 50 VOCs [33].
Isoamyl alcohol is commonly found in food naturally and has been used widely as a food additive and fragrance. Isoamyl alcohol is a C5 alcohol, known also as a part of fusel alcohols, the by-products of alcoholic fermentation. Health assessment in humans reported that isoamyl alcohol is considered to be not genotoxic with NOAEL value of repeated dose toxicity reaching 1250 mg/kg/day [34]. However, isoamyl alcohol interacted antagonistically with some microbial cells. This specificity interaction with other kingdoms makes it a good candidate for the biocontrol of some microbial plant pathogens. A previous study has reported the mechanism of action of isoamyl alcohol toward its microbial cell target. Isoamyl alcohol was able to modify the permeability of fungal pathogens from the anthracnose infected plants, Colletotrichum gloeosporioides and C. acutatum, by increasing the level of peroxidation of membrane lipids [35]. This condition leads to an increase in ROS production and alteration of lipid layer composition, hence changing the permeability of the membrane, resulting its disintegration and finally cell death [36].
Due to its antifungal property against T. paradoxa, isoamyl alcohol has the potency to be developed as a biofumigant agent for postharvest snake fruit. However, for the effective application, proper formulation is required. The isoamyl alcohol as an active ingredient can be formulated as gas aerosol, in combination with edible films, or controlled-released system approach [9]. Moreover, several factors should be considered for the development of isoamyl alcohol-based biofumigants. The first thing is the compatibility, in terms of the interaction of the components of the formula (active ingredient and excipients) with the fruit being minimized to avoid the possible alteration of fruit quality post-application. Another factor is the safety consideration, concerning the proper concentration in formula and application dose to eschew the acute effect of the active compounds. The last thing is the economic consideration in the matter of cost production. The formula, including the preferable excipients, as well as the production process should be effective and efficient from the economical perspective.
5 Conclusion
Through the emission of fungal VOCs, T. harzianum InaCC F88 was able to reduce the mycelial growth of T. paradoxa, the fungal pathogen of black rot in snake fruit. Volatolomic analysis of InaCC F88 strain by Headspace GC–MS showed isoamyl alcohol as the most abundant compound. Moreover, the antifungal property of isoamyl alcohol has been proven through its ability to decrease the mycelial growth of T. paradoxa in plate tests and reduce the decay incidence of pre-infected snake fruit during storage. For future prospects, the bioformulation of isoamyl alcohol as a biofumigant for controlling postharvest fruit disease needs to be further developed.
Data Availability
Data will be made available on request.
Abbreviations
- GC–MS:
-
Gas chromatography–mass spectrometry
- GRC:
-
Growth relative to control
- InaCC:
-
Indonesian Culture Collection
- LRW:
-
Lesion part relative to whole fruit flesh
- PDA:
-
Potato dextrose agar
- PHL:
-
Postharvest losses
- SCPs:
-
Synthetic chemical pesticides
- VOCs:
-
Volatile organic compounds
References
Pusat Data dan Sistem Informasi Pertanian: Outlook Salak Komoditas Pertanian Subsektor Hortikultura. Pusat Data dan Sistem Informasi Pertanian Kementerian Pertanian, Jakarta (2020)
Supriyadi, A.; Suhardi, A.; Suzuki, M.; Yoshida, K.; Muto, T.; Fujita, A.; Watanabe, N.: Changes in the volatile compounds and in the chemical and physical properties of snake fruit (Salacca edulis Reinw) Cv. Pondoh during maturation. J. Agric. Food Chem. 50(26), 7627–7633 (2002)
Mazumdar, P.; Pratama, H.; Lau, S.E.; Teo, C.H.; Harikrishna, J.A.: Biology, phytochemical profile and prospects for snake fruit: an antioxidant-rich fruit of South East Asia. Trends Food Sci. Technol. 91, 147–158 (2019)
Dwiastuti, M.E.; Soesanto, L.; Aji, T.G.; Devy, N.F.; Hardiyanto: Biological control strategy for postharvest diseases of citrus, apples, grapes and strawberries fruits and application in Indonesia. Egypt. J. Biol. Pest Control 31, 1–12 (2021)
Bartholomew, H.P.; Bradshaw, M.; Jurick, W.M.; Fonseca, J.M.: The good, the bad, and the ugly: mycotoxin production during postharvest decay and their influence on tritrophic host–Pathogen–microbe interactions. Front. Microbiol. 12, 611881 (2021)
Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; Mohapatra, A.: Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: a comprehensive review. Front. Microbiol. 2022, 2833 (2022)
Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P.: The evolutionary origins of pesticide resistance. Biol. Rev. 94(1), 135–155 (2019)
Shahbaz, M.U.; Arshad, M.; Mukhtar, K.; Nabi, B.G.; Goksen, G.; Starowicz, M.; Nawaz, A.; Ahmad, I.; Walayat, N.; Manzoor, M.F.; Aadil, R.M.: Natural plant extracts: an update about novel spraying as an alternative of chemical pesticides to extend the postharvest shelf life of fruits and vegetables. Molecules 27(16), 5152 (2022)
Napitupulu, T.P.: Antagonistic fungal volatiles as potential biocontrol countermeasure for microbial postharvest fruit diseases. Egypt. J. Biol. Pest Control 33(1), 100 (2023)
Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W.: Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 3(1), 1–14 (2016)
Rubio, M.B.; Monti, M.M.; Gualtieri, L.; Ruocco, M.; Hermosa, R.; Monte, E.: Trichoderma harzianum volatile organic compounds regulated by the THCTF1 transcription factor are involved in antifungal activity and beneficial plant responses. J. Fungi 9(6), 654 (2023)
Ramadhani, I.; Rohadi, H.; Yuliani, Y.; Ilyas, M.: Study on endophytic fungi associated with Moringa oleifera Lam. collected from Lombok island West Nusa Tenggara. Ann. Bogor. 24(2), 66–73 (2020)
White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W.: Amplification and direct sequencing of fungal RNA genes for phylogenetics. In: Innis, M.A.; Gelfand, D.H.; Sninsky, J.J.; White, T.J. (Eds.) PCR Protocols, a Guide to Methods and Applications, pp. 315–322. Academic Press, San Diego (1990)
Saitou, N.; Nei, M.: The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987)
Kumar, S.; Stecher, G.; Tamura, K.: MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016)
Intana, W.; Kheawleng, S.; Sunpapao, A.: Trichoderma asperellum T76–14 released volatile organic compounds against postharvest fruit rot in muskmelons (Cucumis melo) caused by Fusarium incarnatum. J. Fungi 7(1), 46 (2021)
Ellis, M.B.: Dematiaceous Hyphomycetes. Commonw. Mycol. Inst. Kew 1, 1–608 (1971)
Siwek, A.; Stefańska, J.; Dzitko, K.; Ruszczak, A.: Antifungal effect of 4-arylthiosemicarbazides against Candida species. Search for molecular basis of antifungal activity of thiosemicarbazide derivatives. J. Mol. Model. 18, 4159–4170 (2012)
Zhou, Y.; Behrendt, J.; Sutherland, A.J.; Griffiths, G.: Synthetic molecular mimics of naturally occurring cyclopentenones exhibit antifungal activity towards pathogenic fungi. Microbiology 157(12), 3435–3445 (2011)
Micalizzi, E.W.; Smith, M.L.: Volatile organic compounds kill the white-nose syndrome fungus, Pseudogymnoascus destructans, in hibernaculum sediment. Can. J. Microbiol. 66(10), 593–599 (2020)
Ando, H.; Kurata, A.; Kishimoto, N.: Antimicrobial properties and mechanism of volatile isoamyl acetate, a main flavour component of Japanese sake (Ginjo-shu). J. Appl. Microbiol. 118(4), 873–880 (2015)
Soytong, K.; Jitkasemsuk, S.: First report of Thielaviopsis paradoxa causing fruit rot on Sala (Salacca edulis) in Thailand. Plant Dis. 85(2), 230–230 (2001)
Hubert, J.; Fourrier, C.; Laplace, D.; Ioos, R.: First report of pineapple black rot caused by Ceratocystis paradoxa on Ananas comosus in French Guiana. Plant Dis. 98(11), 1584–1584 (2014)
Demiray, S.T.; Akçalı, E.; Uysal, A.; Kurt, Ş: First report of Thielaviopsis paradoxa causing main stalk rot on banana in Turkey. Plant Dis. 104(10), 2733 (2020)
Warwick, D.; Passos, E.E.: Outbreak of stem bleeding in coconuts caused by Thielaviopsis paradoxa in Sergipe. Brazil. Trop. Plant Pathol. 34, 175–177 (2009)
Soytong, K.; Pongak, W.; Kasiolarn, H.: Biological control of Thielaviopsis bud rot of Hyophorbe lagenicaulis in the field. J. Agric. Technol. 1(2), 235–245 (2005)
Joo, J.H.; Hussein, K.A.: Biological control and plant growth promotion properties of volatile organic compound-producing antagonistic Trichoderma spp. Front. Plant Sci. 13, 897668 (2022)
Chan, M.E.; Tan, J.Y.; Lee, Y.Y.; Lee, D.; Fong, Y.K.; Mutwil, M.; Wong, J.Y.; Hong, Y.: Locally isolated Trichoderma harzianum species have broad spectrum biocontrol activities against the wood rot fungal species through both volatile inhibition and mycoparasitism. J. Fungi 9(6), 675 (2023)
Gualtieri, L.; Monti, M.M.; Mele, F.; Russo, A.; Pedata, P.A.; Ruocco, M.: Volatile organic compound (VOC) profiles of different Trichoderma species and their potential application. J. Fungi 8(10), 989 (2022)
Godhe, A.; Rynearson, T.: The role of intraspecific variation in the ecological and evolutionary success of diatoms in changing environments. Philos. Trans. Royal Soc. Lond. B Biol. Sci. 372(1728), 20160399 (2017)
Ando, H.; Hatanaka, K.; Ohata, I.; Yamashita-Kitaguchi, Y.; Kurata, A.; Kishimoto, N.: Antifungal activities of volatile substances generated by yeast isolated from Iranian commercial cheese. Food Control 26(2), 472–478 (2012)
Hummadi, E.H.; Cetin, Y.; Demirbek, M.; Kardar, N.M.; Khan, S.; Coates, C.J.; Eastwood, D.C.; Dudley, E.; Maffeis, T.; Loveridge, J.; Butt, T.M.: Antimicrobial volatiles of the insect pathogen Metarhizium brunneum. J. Fungi 8(4), 326 (2022)
Wright, S.J.L.; Linton, C.J.; Edwards, R.A.; Drury, E.: Isoamyl alcohol (3-methyl-1-butanol), a volatile anti-cyanobacterial and phytotoxic product of some Bacillus spp. Lett. Appl. Microbiol. 13(3), 130–132 (1991)
Api, A.M.; Belsito, D.; Botelho, D.; Browne, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Dagli, M.L.; Dekant, W.; Deodhar, C.; Fryer, A.D.: RIFM fragrance ingredient safety assessment, isoamyl alcohol CAS Registry Number 123-51-3. Food Chem. Toxicol. 110, S421–S430 (2017)
Dalilla, C.R.; Mauricio, B.F.; Simone, C.B.; Silvia, B.; Sergio, F.P.: Antimicrobial activity of volatile organic compounds and their effect on lipid peroxidation and electrolyte loss in Colletotrichum gloeosporioides and Colletotrichum acutatum mycelia. Afr. J. Microbiol. Res. 9(23), 1527–1535 (2015)
Vázquez, J.; Grillitsch, K.; Daum, G.; Mas, A.; Beltran, G.; Torija, M.J.: The role of the membrane lipid composition in the oxidative stress tolerance of different wine yeasts. Food Microbiol. 78, 143–154 (2019)
Funding
This work was financially supported by Research Joint Collaboration of Research Organization for Life Sciences and Environment, National Research and Innovation Agency (BRIN) Indonesia, fiscal year 2023 (Number 10/III.5/HK/2023).
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology, Validation, Data Curation, Writing - Original Draft, Visualization, Supervision, Project administration, and Funding acquisition were performed by Toga Pangihotan Napitupulu; Formal analysis and Investigation were conducted by Toga Pangihotan Napitupulu, Des Saputro Wibowo, and Muhammad Ilyas; Review and Editing were prepared by Toga Pangihotan Napitupulu and Muhammad Ilyas; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Appendix
Appendix
See Fig.
5 and Tables
2 and
3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Napitupulu, T.P., Wibowo, D.S. & Ilyas, M. Biocontrol of Thielaviopsis paradoxa Causing Black Rot on Postharvest Snake Fruit by Volatile Organic Compounds of Trichoderma harzianum. Arab J Sci Eng (2024). https://doi.org/10.1007/s13369-024-09539-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13369-024-09539-9