Abstract
Drilling fluids are crucial for the safe and effective extraction of hydrocarbons from deep petroleum reserves. Lubricating, suspending, and conveying drilled cuttings to the surface are some of the functions of drilling fluids. Their performance depends on lubricity, fluid loss (FL) control, and rheology. Nanoparticles (NPs) have emerged in the oil and gas industry as an efficient fluid additive to modify and stabilize the properties of drilling fluids. NPs are thermally, chemically, and physically stable in drilling fluids; however, field data show they are inefficient at reducing drill string wear. Graphene nanoplatelets (GNPs) are now a useful drilling fluid agent because of their small particles with high specific surface area, good dispersion, high thermal and electrical stability, and their ability to lower stress and wear on the drill string. Thus, this study examined GNPs in drilling fluids, including surface modification methods and their effects on rheology, FL management, and lubricity. The unique properties of GNPs that make them a potential game-changer in drilling fluid are highlighted. The techniques used to modify GNP surfaces to improve drilling fluid compatibility, stability, and dispersion were also addressed. The broad study of laboratory GNP-modified drilling fluids is the central focus of this review. A scrutiny of the mechanisms by which GNPs influence the rheological behavior of drilling fluids and their impact on drilling efficiency and wellbore stability was also highlighted. Beyond laboratory tests, GNP’s real-world applications and commercialization possibilities were examined, taking economic and environmental considerations into account. Comparative examination of methods and results helps optimize GNP-enhanced drilling fluids. Small concentrations of GNPs (0.1–2.5 g) increased the base fluid lubricity and rheology. They also reduced the FL by 40–89%. Due to hydroxyl groups on clay surfaces, GNP has a strong affinity for organophilic clays. The challenges and limitations of GNP-modified drilling fluids were highlighted, along with future research directions. Finally, using GNPs as a fluid modification agent may improve drilling fluid lubricity, FL control, and rheology. These attributes will improve oil and gas drilling safety and efficiency. This review consolidates information and lays the groundwork for this growing field's study and innovation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The most crucial factor in drilling operations is the drilling fluid, and the effectiveness of the drilling fluid has a direct bearing on any drilling operation’s success [1]. Oil-base mud (OBM) is very effective in drilling high-temperature zones, shale formation, salt gypsum formation, and over-pressured formation. This is due to its rheological properties, good lubricating characteristics, low formation damage potential, excellent wellbore stability at high temperatures, and effectiveness against corrosion [2, 3]. However, higher toxicity, additional treatment cost before disposal, strict government regulation for onshore and offshore disposal of waste, kick detection problem, difficult lost circulation remediation and reduced effectiveness of some logging tools has limited the applicability of OBM. Water-based mud (WBM) has been commonly used in lieu of OBM because it is relatively inexpensive and easy to treat. Nevertheless, WBM has its limitations, which are the low rate of penetration, clay hydration, shale problems, and unstable wellbore [4]. Polymers have been used to improve the properties of WBM owing to their viscoelastic properties, rapid formation of gels at low shear rate and high shear stress which are desirable during drilling operations. But the increase in thermal gradient with depth of a well causes degradation of the polymer macromolecules resulting in loss of the vital properties of polymers [5, 6].
Over the past years, nanotechnology has become more prevalent and is being used in more areas of the oil and gas industry [7, 8]. Laboratory studies and field application have demonstrated that nanoparticles (NPs) can inhibit shale hydration, enhance the filtration and rheological properties of drilling mud and still retain their properties at high temperature and pressure [9, 10]. Graphene nanoplatelets (GNPs) are of interest due to their high specific surface area, tiny size, plate-like shape, superior high aspect ratio, thermal, electrical, mechanical, and chemical stability. They are viewed as the next-generation nanomaterial for the oil and gas industry [11]. Besides, GNPs low cost over graphene and modified graphene has made it one of the most sought-after nanomaterials [11]. For instance, Ridha et al. [12] evaluated the effectiveness of GNPs as a filtration material in WBM. They reported that GNPs showed excellent filtration properties at high temperatures compared to nanosilica. In similar vein, Arain et al. [13] formulated a novel OBM enhanced with GNPs and investigated its performance at high temperatures. GNPs improved the plastic viscosity (PV) and yield point (YP) of the OBM by 11% and 42%, respectively.
Furthermore, GNPs have the potential to improve oil well cement durability by modifying the microstructure of the hydration product within the cement, thereby increasing the mechanical strength of oil well cement. For example, Alkhamis and Imqam [14] utilized GNPs to reduce gas leakage and improve wellbore integrity by improving the compressive and tensile strengths by 10% and 30%, respectively. Also, GNPs can abate the impact of mud residues on the bonding strength of oil well cement. Tabatabaei et al. [15] modified the surface of GNPs to limit the deteriorating impact of OBM on oil-well cement bonding. The experimental results indicate that the GNPs inhibited the impact of mud residues at the interface and increased the bonding strength.
GNPs have been used as additives in drilling fluid [11,12,13], as well as in oil well cement [14, 15] and in the field [9]. All of these studies showed that GNPs worked very well to improve processes. In recent studies, researchers have synthesized NPs that are safe for the environment using the green approach [11]. An attempt was made by Srivastava et al. [16] to synthesize manganese oxide NPs from plant extract, while Aftab et al. [17], synthesized Tween 80 and zinc oxide nanocomposite. It was shown that these NPs improved thermal stability, improved rheology, and reduced fluid loss (FL) in both studies. There are numerous additives used in the drilling industry that contribute to the performance of drilling fluids. The materials have, however, been found to pose a threat to the environment and the workforce operating on the site. Drilling fluid additives are currently being investigated on the basis of two criteria: first, offering the properties required of drilling fluids; and second, being environmentally friendly, biodegradable, and sustainable.
Furthermore, engineers are in search of drilling fluids that are not only physically, chemically, and thermally stable but also have all the drilling fluid characteristics that will avoid blocked pipe accidents, protect the formation, reduce bit or drill string wear, and make the drilling operation easier. The reason for such demands is the increasing complexity of drilling operations over time. Engineers are currently exploring and extracting hydrocarbons from ultra-deep strata where temperatures and pressures are very high. Aside from stabilizing drilling fluids, NPs are ecologically friendly due to the small amount of their tiny particles employed in the drilling fluids. In theory, NPs are compatible with WBM and contribute greatly to mud stability. Furthermore, dispersion forces, including electrostatic attraction and Van der Waals forces, are effective in WBM. These forces greatly aid in the dispersion of NPs in the fluid system, allowing them to fulfil their intended goal [15]. As a result, a thorough examination of GNP synthesis, modification, and use in oil well drilling and completion operations may provide additional information on NPs for process improvement in the oil and gas industry. It will also supplement current knowledge and address the knowledge gaps on GNP-based drilling fluid.
Herein, a comprehensive review of the modification of GNPs during oil well drilling and completion is presented. Likewise, the feedstock for the synthesis of GNPs was discussed for chemists, pharmacists, material, chemical, mechanical, and petroleum engineers from academia and industry to have a critical stance on how various parameters influence the synthesis and modification of GNPs. Thus, the methods of synthesizing GNPs were discussed. Subsequently, GNP surface modification methods were examined. Likewise, the application of GNPs in oil well drilling and completion was reviewed. Finally, the challenges encountered have opened new opportunities for research and are highlighted in detail.
The rest of this review is structured as follows: The subsequent section focused on GNP synthesis methods like mechanical exfoliation, oxidative exfoliation-reduction, liquid-phase exfoliation (LPE), chemical vapor deposition (CVD), and feedstock for these synthesis methods. Surface modification methods such as elemental doping, covalent, and noncovalent surface modification were discussed thereafter. The next section depicts the factors influencing surface modification. Application of GNPs in oil well drilling and completion was presented, and a review of the important results and conclusions from recent studies was evaluated afterward. Finally, the challenges, projections, and forthcoming trend of GNP modification and application were highlighted. The overview of this study is illustrated in Fig. 1.
2 Graphene Nanoplatelets (GNPs)
Graphene is widely regarded as the revolutionary material of the twenty-first century because of its unique properties. This is a single graphite monolayer, the thickness of which is one atom (0.34 nm), but its lateral size could be several orders of magnitude greater. The material has few graphite layers, varying in thickness from 0.7 to 100 nm [18]. GNPs are stacks of graphene layers in a two-dimensional form and so are called two-dimensional nanosheets [19]. In addition to having excellent electrical, mechanical, and thermal conductivity due to their atomic structure (Fig. 2), GNPs exhibit high aspect ratios [20]. Carbon nanotubes (CNTs), carbon nanofibers (CNFs), graphene, and other advanced materials, such as active carbon (carbon black), will become increasingly important as the effort to overcome the major continues. Several synthesis techniques have been used to produce ideal graphene sheets that are highly ordered, have outstanding surface areas (2630 m2 g−1), exhibit high Young's modulus (1 TPa), have high thermal conductivity (5000 Wm K−1), are chemically durable and have high electron mobility (2.5 105 cm2 V−1 s−1) [21]. Despite its low absorption ratio of 2.3% of white light, intrinsic GNP is considered a semimetal or zero gap semiconductor because of its peculiar electronic properties [22]. Experimental measurements have shown a remarkable increase in electron mobility at room temperature, exceeding 15,000 cm2 V−1 s−1. This resistivity is lower than that of silver, which is considered the lowest resistible substance at room temperature [23].
Graphene nanoplatelets structure [40]
Biodegradation, flexible electronic devices, spintronics, photonics, optoelectronics, sensors, energy storage and conversion, and biomedical have all been studied with graphene for various applications [24, 25]. Graphene has robust electrical properties along with high optical transparency and flexibility that make it a great candidate for touch screens, liquid crystal displays (LCDs), photovoltaic cells, and organic light-emitting diodes (OLEDs) [23]. In addition to its potential as a nanomaterial, graphene has potential applications in the fields of nanoelectronics, molecular separation, composite additives, catalysis, nanosensors, and transport [26, 27]. The oil and gas industry is still developing graphene's presence despite its extensive use in many fields.
It is possible to fabricate nanostructures by using both top–down and bottom–up approaches [21]. Bottom-up synthesis refers to piling atoms on top of one another to synthesize nanostructures. Stacking crystal planes generates nanostructures by stacking them on top of one another [23,24,25]. By combining the building blocks with the substrate, a bottom-up method is able to form complex nanostructures. By using top-down synthesis, the bulk material is carved or cut into nano-sized particles with appropriate properties [28]. Bottom-up approaches are more advantageous than top–down approaches because they are likely to produce nanostructures with fewer defects, more consistent chemical alignment, and improved short-range and long-range organization. In a wide variety of products such as electronics, displays, paints, batteries, micro-machined silicon sensors and catalysts, NPs are expected to provide the performance improvements that they are already known for [29, 30].
2.1 GNPs Synthesis
A graphene atom is arranged in a two-dimensional honeycomb lattice and is one of the allotropes of elemental carbon. As a new material, it offers a number of intriguing properties that may have potential for future applications and composite industries [31, 32]. Through different routes, graphite has been converted into graphene-like structures, GNPs (quasi 2D), and wrinkled graphene (2D). Graphene with more than ten layers is known as GNPs or multi-layer graphene. Graphite, on the other hand, has more than 100 layers. A number of methods have been used to produce GNPs from crystalline graphite, including acid intercalation [33], intercalation-exfoliation [34] and LPE [35]. It was demonstrated that single and multiple layered graphite can be formed by the thermal decomposition of carbon on platinum substrates, marking the start of the monolayer GNPs synthesis [36]. It was found that these sheets failed to have consistent properties across different crystal planes of platinum, and there was no identification of beneficial applications for this product at that time, so it was not extensively studied. GNP are advantageous as they are less expensive and easier to produce than CNTs or single layer graphene [37]. In composite synthesis, uniform dispersion and effective use of secondary phases are two of the most challenging problems. There is no comparison between single layer graphene and CNTs when it comes to dispersibility of GNPs. CNTs are prone to tangles during dispersion and single-layer graphene curls during dispersion. The high surface area inherent in GNPs geometry makes it particularly promising for creating strong interfacial bonds with the matrix [38]. Graphite mesas were mechanically exfoliated to isolate GNPs as the first steps in the isolation process [39]. GNPs can be produced using this method at small scales, but large-scale production is not possible. There have been several reported synthetic methods for GNPs in the literature to overcome this shortcoming [18, 40,41,42].
2.2 GNPs Synthesis Methods
As a result of GNPs promising properties, nanocomposite materials research has increased significantly [43]. Although there are 2D materials being developed, large-scale manufacturing systems are still lacking [21]. It is difficult for the system to operate because of low production rates and high costs of graphene [44,45,46]. The cost of graphene needs to be competitive with those of current materials in order to successfully be applied in industry. Developing synthesis processes that are affordable, highly reliable, scalable, and produce high yields and quality is a major challenge. The accessibility, process ability, large-scale production, and low cost of GNPs have led to their use as substituting materials [47, 48] to address these issues. Cataldi et al. [19] refer to GNPs as 2-D nanosheets composed of stacked graphene layers. Sengupta et al. [49] indicated that GNPs are a good replacement for nanostructured fillers in material science. In most cases, LPE is used to obtain the material. Aside from microwave radiation exposure, shear exfoliation, ball-milling, and wet-jet milling are also methods of producing acid-intercalated graphite [20, 50]. Due to their atomic structure, GNPs are excellent conductors of electrical, mechanical, and thermal energy [20].
There are two main approaches to the synthesis of GNPs: top-down (destruction) and bottom-up (construction) [51, 52] as illustrated in Fig. 3. Top–down methods such as mechanical exfoliation [21], arc discharge [53], oxidative exfoliation-reduction [54, 55], LPE [56, 57] and CNT unzipping [53, 58] are typically effective in isolating and de-laminating graphite layers to produce GNPs with a single, bi- and few layers. The method destroys larger precursors, such as graphite and other carbon-based precursors, to produce GNPs that is nanoscale. Bottom-up methods utilize carbon sources other than graphite to synthesize GNPs and its derivatives. GNPs are constructed from precursors of atomic size using these methods. In addition to CVD and epitaxial growth [59, 60], other methods of bottom-up synthesis include the template route [61] and total organic synthesis [62].
2.2.1 Mechanical Exfoliation
It is possible to categorize mechanical exfoliation by directional routes, such as normal force vectors and shear force vectors as seen in Fig. 4. Among the recent studies on the normal force synthesis route is the peeling of graphite using advanced machinery of ultra-sharp single crystal diamond wedges [63]. A valuable advantage of this method is that it eliminates manual operation, as well as reducing labor costs and time. GNPs of 1.13–1.41 nm were produced using a three-roll mill machine, which has been a technique used in the rubber industry [64, 65]. Cleavage and exfoliation are mechanical or chemical techniques to separate GNPs sheets by breaking the bonds between them. Viculis et al. [46] made an attempt to form dispersion of carbon sheets by intercalating potassium metal between graphite sheets and exfoliating them with ethanol. After sonication, the exfoliated nanocarbon sheets formed nanoscrolls. According to transmission electron microscopy (TEM) analysis, each sheet contained 40 ± 15 layers. While these carbon nanosheets were much thicker than GNPs, the method demonstrated that graphene layers can also be separated from graphite in practice [66]. Lapshin [67] observed box-shaped graphene nanostructure using mechanical cleavage of highly oriented pyrolytic graphite (HOPG). A result having a quadrangular cross section was observed, the nanostructure consists of parallel hollow channels arranged in layers along its surface, and an approximate 1-nm-thick channel wall/facets are present. Through mechanical cleavage of graphite, Jayasena and Subbiah [63] synthesize a few layer GNPs using an ultra-sharp single crystal diamond wedge. This process is capable of synthesizing GNPs layers with small areas in few microns, according to the obtained layers.
Despite its rapid development and characterization, pristine graphene cannot be produced by mechanical exfoliation in enough quantities for most applications that require larger quantities of the material. As a result, alternative methods of producing graphene are required to ensure its wide distribution at large scales. The production of graphene has been facilitated by several alternative technologies in recent years. It has been shown that CVD on metallic catalysts can be used for patterned growth of graphene, which is particularly useful for applications that require a thin, highly conductive graphene material containing several layers [68,69,70]. Graphite can be directly exfoliated to produce graphene with low defect ratios [18, 71,72,73,74]. Though they are beneficial in bulk applications where graphene is a component within a mixture, they are constrained by huge size and thickness distributions.
2.2.2 Oxidative Exfoliation-Reduction
In addition to Brodie’s route, Staudenmaier’s route, Hofmann’s route, and Hummers’ route, there are four main routes for synthesizing GNPs. As shown in Fig. 5, these methods exhibit reaction pathways that occur at temperatures lower than 100 °C [54, 75]. Maintaining a relatively low synthesis temperature can result in low production costs. Nevertheless, toxic gases are generated by these methods, including nitrogen dioxide (NO2) and dinitrogen tetroxide (N2O4) [76, 77]. Therefore, environmental costs and process safety must be considered during process scaling. Since graphite intercalation compounds are incorporated into the graphite during oxidation, the distance between layers increases from 0.335 to 0.625 nm, or greater [78]. A high degree of hydrophilicity of GNPs, owing to its oxygen-containing functional groups, allows it to dissolve in a broad range of solutions, including water, ethylene glycol, N-methyl-2-pyrrolidone (NMP), or tetrahydrofuran (THF) [79, 80].
One of the most effective processes for the low-cost, mass manufacturing of graphene has been thought to involve oxidative exfoliation of natural graphite by acid treatment and subsequent chemical reduction. However, the harmful strong acids (such as H2SO4 and HNO3), oxidants (such as KMnO4, K2S2O8, and H2O2), as well as the carcinogenic reductant hydrazine (other reductants have also been utilized), are used in significant quantities during the process. Additionally, due to the severe oxidation conditions utilized, graphene sheets generated through oxidation–reduction are often tiny in size and contain many flaws. Furthermore, the reduction process results in very hydrophobic graphene sheets that have a propensity to clump irreversibly, which significantly impedes their manufacture, storage, and processing. The unusual structure of graphene is generally known to be the source of its exceptional characteristics. As a result, keeping the qualities of graphene depends on protecting its structure. It is vitally necessary to develop new methods for producing graphene in big quantities with good qualities.
2.2.3 Liquid Phase Exfoliation (LPE)
The surface energy, surface tension, Hildebrand solubility and Hansen solubility parameters determine which solvent is most appropriate for LPE since it exfoliates graphite by overcoming van der Waals forces [52, 65]. The LPE synthesis method has been used for GNPs production since 2008, and is one of the widely used methods to synthesis the material [74]. The LPE synthesis process involves three main steps: dispersion of graphite in a suitable solvent, exfoliation, and purification [81] as shown in Fig. 6. The LPE of graphene has been studied with more than 40 types of solvents by [82]. Not only can GNPs products be produced in a very small volume of solvent with the information obtained, but it is also feasible to use it to make high concentrations of GNPs products. The process can also be scaled up by selecting solvents that are less hazardous, less expensive, and more stable to disperse GNPs.
Liquid-phase exfoliation of graphene nanosheets [267]
The novel method Hernandez et al. developed involves dispersion and exfoliation of pure graphite in N-methyl-pyrrolidone after weighing the pros and cons of the LPE process [74]. Further processing could increase monolayer yield to 12 wt% from 1 wt%. Solvent-graphene interactions countered the energy required to exfoliate graphite into graphene by having similar surface energies as graphene, as the solvent was similar in surface energy to graphene. Large-scale graphene production can be achieved through this liquid-based exfoliation of graphite in organic solvents. According to Lotya et al. [83], graphite powder was dispersed in sodium dodecyl benzene sulfate (SDBS) and then exfoliated using sonication to yield single- or few-layered graphene [83]. In addition to Si and Samulski [84], Tung et al. [85], Tung et al. [86], Nethravathi and Rajamathi [87], many other attempts were made to produce graphene either from graphite or graphite oxide powder.
A recent modification has allowed the large-scale production of graphene through LPE, which is a process that has shown good promise for synthesizing graphene on a large scale. As a result of oxidation and reduction processes, the structure of the LPE graphene has lots of defects, which results in very poor structural properties. For this process to reach industrial scale production, future improvements must focus on controlling layers and reducing impurities.
2.2.4 Chemical Vapor Deposition (CVD)
Thermal CVD is a relatively new method of synthesizing graphene. CVD-synthesized planar few layer graphene (PFLG) was first reported in 2006 [88]. This work synthesized graphene on Ni foils using camphor, an eco-friendly, low-cost, natural precursor. An argon-gas gas carrier was used to pyrolyze camphor at 700–850 °C, after it had first been evaporated at 180 °C. In CVD, hydrocarbon gases like methane (CH4), acetylene (C2H2), ethylene (C2H4), and hexane (C6H14) plus biomass materials are decomposed at elevated temperatures (650–1000 °C) to form graphene sheets on metallic catalysts such as copper (Cu) and nickel (Ni) films [89, 90]. It dissociates into free carbon atoms and hydrogen (H2) atoms when it reaches the hot surface of the metal catalyst. When carbon solubility is reached on the metal surface, the atom will diffuse through the surface and the bulk of the catalyst, forming GNPs sheet [91, 92]. In addition to having a large surface area and low defects, CVD is able to produce high quality GNPs with a dense structure and low defects [90].
As a result of thermal CVD, Yu et al. [93] reported the formation of three to four layers of graphene on poly-crystalline Ni foils (here of a thickness of 500 µm). For the synthesis process, the following mixture of CH4, H2 and Ar (0.15:1:2) was used at a total flow rate of 315 SCM, with a temp of 1000 °C for 20 min. HRTEM and Raman spectroscopy confirmed that graphene can be produced on Ni only when cooling rates are moderate, while high and low cooling rates seem to have adverse effects on graphene synthesis. A thermal CVD process was developed further to produce graphene on a 1 cm2 area of Cu foil [53]. A high-quality and uniform graphene was thus produced. A variety of applications have also been enabled by the transfer of graphene to different substrates through two different and simple methods. It has been confirmed that the growth of graphene by thermal CVD is reproducible and that the material can be transferred to a wide variety of media, including Si, glass, and PDMS. Graphene will now be able to be applied in new and exciting ways thanks to these developments. In order to create more interest in actual applications, it is necessary to solve problems such as growing graphene on wafer size substrates and controlling successfully the number of layers.
While this method has advantages, there are disadvantages as well, such as high production costs, low throughput, and additional purification to eliminate residual catalysts [94]. Most researchers have aimed to decrease the temperature and pressure of GNPs synthesis while maintaining its quality in order to overcome these setbacks [89, 95]. It is not unusual for graphene to be synthesized through plasma enhanced CVD (PECVD) contemporaneous with that of exfoliation. Among the first reports, Obraztsov et al. [96] published a PECVD method using a dc discharge to produce nanostructured graphite-like carbon (NG). A surface wave PECVD method was used by Kalita et al. [97], to synthesize GNPs coating at 450 °C. Through this method, the growth temperature and deposition time were significantly lowered (5 min), which further enhanced the synthesis and scalability of the process as a whole [62]. An improved process has been developed by Shang et al. [98] to synthesize multilayered graphene nanoflakes (MGNF) on silicon (Si) substrates by using microwave PECVD (MW-PECVD). As a result of this method, GNPs produced had a highly graphitized knife-edge structure, which had sharp edges of 2–3 nm thickness. A comparable method was used by Yuan et al. [99] to synthesize high quality GNPs sheets on stainless steel substrates at 500 °C using MW-PECVD. The crystallinity of GNPs produced by this method was found to be improved. In a recent study by Meškinis et al. [100] using MW-PECVD to synthesize GNPs directly on Si (100) substrate. The result was observed that temperature and synthesis time reduce the number of GNPs layers, as carbon desorbs more quickly at higher temperatures. GNPs can also be synthesized using the thermal CVD process.
A scalable route was used to synthesize atomically thin graphene membranes with nanoporous large surfaces by roll-to-roll CVD [101]. Monolayer GNPs of uniform high quality were synthesized through this process. An investigation of the temperature dependence of the GNP structure during thermal CVD was analyzed [102]. According to various analyses, the fabricated structure formed a porous low-density network with multilayered GNPs connected to each other. In addition to its versatility in synthesizing graphene on any substrate, PECVD has shown promise in expanding its field of application. Graphene layer thickness can be controlled better with this method in the future, allowing larger scale production. Unzipping CNTs is one of the most common techniques in this category. With CNTs as a starting material, lithium and ammonia can be intercalated, followed by sequential exfoliation in acid and heating to open or "unzip" them vertically [103]. A multi-walled CNTs (MWCNTs), a nanoribbon, and pure graphene flakes are produced during this process. A common method to synthesize graphene from nanoribbons is plasma etching [104].
2.3 Feedstock for GNPs Synthesis
GNPs synthesis would be economically feasible subject to the type of feedstock, since a continuous supply of carbon precursors would be necessary for mass production [105]. Top–down graphene synthesis is most commonly conducted with graphite as a feedstock. The abundance of this resource in nature makes it relatively inexpensive. GNPs synthesis has been tested using more sustainable carbon precursors besides graphite as a feedstock, both from top-down and bottom-up approaches. In arc discharge synthesis, asphalt has been explored as a starting material for GNPs production, rather than graphite, to reduce graphene production costs. Biomass materials may also be used instead of graphite in oxidative exfoliation [106, 107]. It has been shown that biomass feedstocks such as wood, leaf, bagasse, fruit, newspaper, bone, and cow dung are capable of replacing graphite in oxidative exfoliation reduction processes [108].
Researchers have recently developed and proposed green synthesis techniques based on environmentally friendly biomass resources. To produce graphene, green synthesis processes have been introduced to use biomass precursors (Fig. 7) such as chitosan [109], glucose [110], alfalfa plants [111], populous wood [112], sugar [113], tea [114], rice husk [115], plastic waste [116], pulping black liquor [117], coconut shell [118], sugarcane bagasse, and orange peel [119], and palm oil fuel ash [120]. By utilizing nontoxic chemicals and natural precursors, green production aims to reduce toxic waste [121]. Carbon percentiles of 45wt% to 50wt% make biomass materials promising materials for producing graphene, replacing fossil fuels and mined graphite [122]. In several literature sources, approaches to synthesize GNPs without catalysts were reported using environmentally friendly biomass resources as carbon sources [123,124,125,126]. In contrast to the conventional carbon source, which utilizes high-purity hydrocarbon gas, this method alternatively uses solid raw materials that are used as carbon sources of GNPs precursors, such as food and sugar, without requiring the use of catalysts. As a result, environmental burdens during GNPs synthesis can be reduced, costs can be reduced, and material yields can be improved.
Biomass-derived graphene [268]
The first step in producing GNPs is to dehydrate biomass materials and then crystallize them at high temperatures. Unlike the ideal two-dimensional layered graphene sheets, GNPs that come from biomass are usually made up of nanographene domains that are lined up. Nanographene domains can have unique functional groups, diverse structures, and a wide range of physical and chemical properties [127]. Primo et al. [109] fabricated single and multilayer GNPs using chitosan. Multi- or single-layer GNPs were obtained by spin coating chitosan to produce high-quality and conformal films on arbitrary substrate and carbonizing it at 600–800 °C. Long et al. [128] used pyrolysis to align the cellulose into 2D crystal structures produced multilayered 2D GNPs from sugarcane bagasse cellulose. Researches have suggested the possibility of generating 2D layered GNPs sheets from layer-structured biomass materials. A multilayer graphene was synthesized using wheat straws by Chen et al. [129]. To make large multilayer graphene sheets, Purkait et al. [130] used peanut shells as a starting material. The multilayer graphene was also obtained by heat treatment of camphor leaves [131]. Carbonized brown-rice husk was used to synthesize GNPs, which are then activated with potassium hydroxide (KOH) in a one-stage process.
Nanosheets obtained from this method display an ultra-thin crumpled-silk-veil-wave structure, a high surface area of 1225 m2 g−1 and a high porosity [132]. Rice husk biomass was used by Sekar et al. [133] to produce corrugated graphene nanosheets via KOH activation. A large specific surface area was observed for the 700 °C activated nanosheets. Muramatsu et al. [124] demonstrate a novel synthetic method for producing large amounts of GNPs using rice husks with chemical activation. By using KOH as the activation agent, rice husk char was converted into GNPs. It was then mixed and compacted into a mullite crucible container, which was then covered with ceramic wool. Afterward, the crucible was placed in a larger crucible covered in carbon powder and ceramic wool. A fully covered carbon powder prevented material oxidation at high temperatures by keeping it from being exposed to air. As shown in Fig. 8, the crucible set was annealed at 900 °C for 2 h in air inside a muffle furnace. In addition, Table 1 shows a summary of previous research on GNP synthesis methods and feedstocks.
3 Surface Modification of GNPs
GNPs surfaces have been functionalized or modified indiscriminately by adding various types of functional groups that act as reaction sites for subsequent modifications. The terms “functionalization” and “surface modification” have been used widely and indiscriminately to describe this process [134]. By functionalizing GNPs, its intrinsic properties are preserved and agglomeration is prevented. By modifying GNPs, new functional groups can be added to enhance its excellent properties. A variety of methods for functionalizing GNPs are currently available, including covalent, noncovalent, and elemental methods [135]. One of the main limitations of graphene application in oil and gas industry is its agglomeration in aqueous media. There has been no significant progress in the field of hydrophobic graphite and graphene sheets in water, with the absence of dispersing agents. The most unique properties of graphene sheets can be achieved only when the sheets are individually assembled, so prevention of aggregate formation is crucial [136,137,138].
The covalent functionalization of a compound enhances its solubility in solvents by attaching a diverse chemical moiety. As such a method involves high temperatures and hazardous chemicals such as neat acids, it can be considered aggressive [139]. Attaching functional groups causes a change in GNPs structure as well as altering its properties. The noncovalent functionalization of graphene is, in contrast more appealing because it allows the attachment of various groups without affecting its structure or properties [140]. Surfactants, commonly known as surface-active agents, have been used to disperse carbon nanomaterials in noncovalent methods. An actual surfactant is composed of two parts, one of which is a hydrophobic tail. In this case, it is a chain of hydrocarbons. It also contains a polar hydrophilic head, which could be anionic, cationic, or nonionic [141]. The hydrophilic moiety of nonionic surfactants forms a large solvation shell outside of the NPs, which facilitates their dispersion. The dispersion of NPs is stabilized by electrostatic attraction between the micellar domains of ionic surfactants. Compared with nonionic surfactants, ionic surfactants are less thermally and chemically stable, toxic, and resistant to environmental degradation. There have been several studies that demonstrate how some surfactants stabilize aqueous GNPs dispersions [140,141,142,143]. Further, high-quality GNPs dispersion containing surfactants of various kinds could allow it to be used in a wider variety of applications. A few of the important different types of surfactants used with graphene nanofluids are presented in Fig. 9.
Nanofluids also exhibit thermo-physical properties that are affected by surfactants. Mehrali et al. [144] used 4-(1,1,3,3-tetramethylbutyl) phenyl-polyethylene glycol (PEG) (Triton X-100) surfactant to disperse nitrogen-doped graphene (NDG) by forming a shell around the surface due to its benzene ring structure. According to the authors, nanofluids were stable for six months after ultra-sonication for one hour with Triton X-100 as a surfactant. GNPs modified nanomaterials are slightly improved in thermal stability when nonionic surfactants are used as dispersion agents [145]. In comparison with pristine GNPs, noncovalently functionalized GNPs dispersed better in aqueous media. Pu et al. [146] explored ionic and nonionic surfactants dispersion of hydrophobic graphene in aqueous solutions, showing the nonionic surfactant has the best dispersion rate as confirmed by UV spectroscopy and a material sedimentation test. Based on their findings, different surfactants altered the homogeneity of GNPs dispersion in aqueous media. In a study performed by Sarsam et al. [147], four surfactants were used to achieve 0.1 weight percent stability for GNPs nanofluids samples: SDBS, sodium dodecyl sulfate (SDS), and cetyl triammonium bromide (CTAB). SDBS surfactant dispersed in water increased graphene nanofluid thermal conductivity, and maximum stability value was achieved within 60 min of probe time.
3.1 Factor Influencing Surface Modification of GNPs
The high aggregation and agglomeration features of GNPs coupled with their physical instability and sedimentation in aqueous media have limited their applications. GNPs suffer from material instability because of their large surface area, chemical reactivity, and high surface energy [148]. The surface modification of GNPs is therefore necessary to ensure their compatibility. In the presence of van der Waals interactions, graphene sheets will assemble irreversibly or even restack to form graphite unless properly separated from one another. In previous attempts to produce GNPs in large quantities by thermal expansion/reduction or chemical conversion, this problem has been encountered [137, 149, 150]. As graphene sheets have many distinct properties that are unique to each sheet, preventing aggregation is of particular significance. By attaching other molecules or polymers to the sheets, aggregate formation can be drastically reduced [136, 137]. The production of graphene sheets in bulk quantity should take into account new strategies for maintaining their individual separation while being relatively clean. As a result of surface modification of GNPs, the material will achieve several primary objectives, including increasing or enhancing the dispersion, increasing the surface activity of GNPs, enriching the physicochemical and mechanical properties of GNPs, enhancing their thermographic strength, and enhancing their biocompatibility [151]. GNP surface modification is affected by a number of variables in different ways. Dispersion fluid properties such as temperature, pH, and ionic strength may determine how GNP surfaces interact with particles, whereas surface modification may depend on surface charge and size of particles in a dispersion fluid [152]. Surface modification of GNPs is currently the most effective method for solving these problems. In this way, both agglomeration and aggregation problems are solved while anti-friction and anti-wear performance are improved.
3.1.1 Temperature
It was discovered that the process of GNP adsorption is spontaneous and exothermic when the temperature is variable [153]. It was indicated that the surface of the adsorbent was heterogeneous based on the Freundlich and D–R isotherms in the temperature range 25–55 °C. It has been found that temperature plays a key role in the modification of the surface of NPs, despite the fact that little research has been done on the subject. NPs are typically surface-modified at ambient temperature while bond formation is influenced by temperature, although high temperatures can also cause bond cleavage, resulting in materials losing their original properties [152]. To facilitate the bonding with the molecules of the grafting process, NPs must therefore undergo surface modification within a controlled temperature range, so that the original structure of the particles will not be altered in any way. It has been demonstrated by Qamar et al. [154] that modifying graphene nanosheets at ambient temperature can prevent graphene agglomeration, and thus improve the quality and stability of dispersions. Adsorption isotherm analysis confirmed the effect of polymer adsorption on graphene surfaces. At temperatures ranging from 60 to 100 °C, UV–VIS spectral analysis confirmed graphene's conversion to surface-modified graphene. Based on Fourier transform infrared spectroscopy (FTIR) and XPS analysis, it was confirmed that the surface-modified graphene was successfully formed [155].
3.1.2 Surface Charge and pH
It is the net electrostatic charge of the particles that determines the surface charge of NPs. It can be classified by its zeta potential (ZP), which serves as a qualitative indicator of dispersion stability [156]. As one of the most critical variables affecting ZP in aqueous media, pH plays a crucial role. When more acid is added, a positive charge is built up. Electrostatic interactions between particles increase pH sensitivity due to changes in electrostatic interactions, with attraction playing a role in low pH environments while repulsion plays a role in high pH environments [157]. Simple protonation differences rather than irreversible chemical changes are what cause this phenomenon because particles react electrostatically differently at various pH levels. There is no reversible reaction between assembly and disassembly during pH-sensitive assembly [158]. A study was conducted by Hadadian et al. [159] to examine graphene nanosheets and zinc oxide NPs for effective adsorption. Based on the results, a maximum desorption percentage of 90.32% was achieved at pH 3.6. According to the thermodynamic study, the adsorption of zinc oxide on graphene was spontaneous and endothermic. In Sham and Notley [160], polyethylenimine (PEI) is employed to modify the surface properties of graphene. Multi-layers form when PEI molecules are oriented and arranged in a specific way because the charge of graphene sheets hardly varies in pH despite basic or acidic conditions.
3.1.3 Ionic strength
There have been extensive studies exploring a variety of capping agents used to improve GNPs solution stability. Studies conducted on NP suspension stability have also shown that increasing cation valence has a substantial influence [161]. Stability is a result of both steric and electrostatic repulsions. Another important factor that affects suspension stability is ionic species-specific surface adsorption [162]. A study was conducted on the effect of noncovalently functionalized GNPs dispersion [140]. The best dispersion stability was achieved through Triton X-100, a nonionic surfactant, followed by SDS, an anionic surfactant, and DTAB, a cationic surfactant. Following this review, it can be concluded that GNP dispersion affects the rheological properties of water-based drilling fluids. Graphene dispersion in aqueous solutions has been reported to be effective with nonionic block copolymers. UV–visible spectroscopy, rheology, and conductivity studies are used to investigate the stability of the aqueous graphene dispersions [154]. It has been discovered that Lugalvan BNO12 stabilizes graphene better than Triton X series dispersants because the latter contains two aromatic rings that act as anchoring groups and stabilize graphene dispersion as compared to the Triton X series that has just one aromatic group.
3.2 Surface Modification Methods
The stacking of π–π between GNPs sheets, however, results in the formation of multi-layers. Dispersible GNPs can be achieved by a variety of chemical methods, including exfoliation of graphite, chemical or thermal reduction of graphite, intercalation expansion of graphite, and CVD [163,164,165]. GNPs sheet is a hydrophobic material, preventing its dissolution in solvents. For GNPs sheets to be useful in the future, functionalization of GNP is essential. The surface is modified using a variety of methods to make it soluble in common solvents, so as to avoid stacking. The surface-modified structures of GNPs are shown in Fig. 9. The structure is a honeycomb-like arrangement of crystalline carbon atoms that is arranged in a hexagonal lattice or several coupled layers of crystalline carbon atoms arranged in a hexagonal lattice [166]. It can be covalently modified, noncovalently modified, and doped with elements due to the presence of functional groups on its surface. The synergy between GNPs and their modifiers produces remarkable properties and characteristics in modified GNPs. In this way, it is possible to resolve the problems related to its manufacture, storage, and handling, as well as their application in engineering and applied sciences [167]. Covalent or noncovalent functionalization of GNPs is possible. In covalent bonding, different chemical moieties are attached to each other to make the compound more soluble in a solvent. Additionally, it alters and modifies GNPs, changing some of its properties [139, 140]. It is attractive to use noncovalent functionalization because it allows a variety of groups to attach to the GNPs while maintaining its physical properties without affecting its structure [140, 168].
3.2.1 Covalent Surface Modification
GNP surfaces can be covalently bonded to functional groups, resulting in enhanced performance [169]. The carbon structure can be functionalized by covalently bonding functional groups with its edges or basal planes. Through covalently introducing functional groups, GNPs aromatic character can be tuned, improving its solubility, stability and band-gap opening, and improving its electronic properties [170]. The extended aromatic character can be perturbed whenever organic molecules covalently attach to its surface, making its electronic properties more controllable. Researchers [171] used reactive intermediates such as radicals, nitrenes, carbenes, and arynes to covalently functionalize graphene. By adding free radicals, inserting CH, or performing cycloaddition reactions, these reactive species can modify GNPs covalently. A covalent modification results in band gap opening and changes in conductivity by breaking extended conjugation. The addition of functional groups also allows additional molecules and materials to be conjugated to it, allowing for the control of chemical properties. As a result of covalent modification, GNP materials become even more soluble and processable. XPS, solid–state 29Si NMR spectra, and FTIR measurements confirmed the successful preparation of covalently functionalized graphene nanosheets [171]. According to SEM images, modified sheets had stronger interfacial bonds with epoxy matrix than untreated sheets [172].
3.2.2 Noncovalent Surface Modification
In noncovalent interactions, molecules need to be physically adsorbed on GNP surfaces to form interactions such as hydrophobic, π–π, van der Waals, and electrostatic interactions. In noncovalent functionalization, composites are formed, polymers are wrapped, surfactants or small aromatic molecules are absorbed, and porphyrins or bio-molecules, such as DNA and peptides, are interacting. Surface modification of carbon-based nanomaterials by noncovalent functionalization is well known [173]. In contrast to covalent modification, noncovalent modification of GNPs is more feasible [174]. As a result of noncovalent functionalization of GNPs, composite materials with specific functions are synthesized by generating H-bonding and electrostatic interactions between the material and the functional molecules. Through noncovalent interactions, surface modification ensures both bulk structure and quality preservation as well as optimal dispersibility of the products [175]. A significant benefit of noncovalent modification of GNPs is that it is a simple, mild process, which preserves its structure and properties [176].
The ability to attach different groups to graphene's surface without altering its structure and properties is one of the leading reasons why noncovalent functionalization is so appealing [140, 168]. In comparison with Triton X, Lugalvan BNO12 is adsorbed on GNPs in greater amounts. Based on thermogravimetric analysis (TGA) and FTIR investigations, polymers chains were grafted to the graphene surfaces. Adsorption isotherms have indicated that dispersions made up of polymers at optimal concentrations have lower viscosities and conductivities. In a study by Uddin et al. [155], various surfactants were tested to determine their effects on GNPs dispersion stability. To investigate the effects of ionic and nonionic surfactants, SDBS, SDS, and Triton X-100 were used. Water dispersion test showed that SDBS functionalized GNPs had the best dispersive stability. Using atomic force microscopy, different surfactants made a significant difference in the thickness of the functionalized GNPs. Furthermore, functionalized GNPs thermal properties were found to be influenced by the surfactant used.
3.2.3 Elemental Doping of GNPs
Doping GNPs with heteroatoms (N, S, and P) is another way to modify them. The graphitic lattice of GNPs can be altered by doping it with heteroatoms, increasing its chemical and physical properties, and improving its applications [177,178,179,180]. It has been shown that to obtain graphene sheets with desired properties, a high level of doping is often required [181,182,183,184]. Doping graphitic surfaces has been accomplished by various methods to date. A doping process can be classified into two basic types: synthesis-related and post-synthesis-related. Doping is achieved by annealing the material with the appropriate dopant agent sources after synthesis, which is a universal post-synthesis method. With this method, a better understanding of doped graphene materials, different levels and configurations of doping, as well as synergy effects from co-dopants, is examined [185]. By thermal annealing, high-concentration boron can be doped into the GNPs. Yeom et al. [186] showed that high-concentration boron ensures that GNPs can be used in a variety of energy storage and conversion applications. A maximum boron concentration is observed in B-doped GNPs prepared at 1000 °C, making it the most concentrated of all the B-doped GNPs examined so far. Doping is achieved by mixing GO with g-B2O3 in a well-mixed dopant gram-scale production. It is easy to control the nitrogen content by varying the molar ratio of reactants over a wide range. GNPs structures are efficiently restored during the reaction as a result of this investigation.
There are a number of applications where pH has an important effect on the stability of modified GNPs. As a consequence of strong repulsive forces, pH control can increase stability [187]. As functional groups are reduced in a nanomaterial, its stability decreases in water, with pH being the greatest factor [188]. Graphene's surface chemical properties were found to be affected by pH in an aqueous solution, affecting its adsorption [189]. An experiment in which GNPs were modified by washing them several times with distilled water until they reached a neutral pH was performed [190]. A different study showed that changing the covalent PEGylation of PE-CVD graphene sheets provided better colloidal stability and overcame its hydrophobicity [191]. Cabello-Alvarado et al. [192] used chemically modified GNPs as a method for improving uremic toxin absorption. A study on the impact of pH on graphene's colloidal stability in water revealed that all samples had values below − 30 mV, the stability threshold for a stable suspension. The ZP of graphene made from graphite flakes ranged from − 32.3 to − 37.5 mV, but the milled sample consistently registered lower values of − 43.1 and − 42.9 mV at pH 7–9 [193]. Thus, it can be said that pH is very important for the stability of modified GNPs. However, the exact change and application may have an impact on how pH affects the stability of modified GNPs.
3.3 Application of GNPs in Oil Well Drilling
There have been several years of investigations into the use of nanotechnology as an additive in oil well drilling with varied successes [194]. Nanotechnology is seen as a lasting solution to solve the long-standing problems in oil and gas operations. To determine how it may improve drilling fluid rheology, FL, or shale stability [195,196,197]. The use of graphene nanomaterial has been extensively studied for addressing all of the aforementioned oil well drilling problems [13, 198, 199]. It is important to note that rheological effects desired from additives in drilling fluids vary widely depending on the ultimate objective: whether to clean holes (high low-shear rate), reduce equivalent circulating densities (ECDs) by lowering PV or YP, or minimize temperature effects [200].
3.4 GNPs as a Drilling Fluid Additive
Graphene-based nanomaterials have been developed for many applications in the oil and gas industry in the past few years. There are several applications for this technology, including drilling, cementing, enhanced oil recovery, desalination, oil spill cleanup, and emulsion stabilization, to name a few. Hydrocarbons are recovered from the earth through drilling, and drilling fluids play a key role in this process [201]. As drilling fluid circulates through the annulus, cuttings from the wellbore are carried and transported to surface facilities. Environmentally benign, water-based muds can be customized to suit a variety of purposes. With specific additives, their efficiency can be enhanced through enhanced rheology, improved lubricity, shale stability, clay and cuttings dispersion inhibition, and a high penetration rate. Moreover, the downhole torque problems can be reduced as well by minimizing bit accretion [202,203,204]. Certain drilling-fluid properties may be enhanced by the presence of GNPs while others may be degraded by their presence, depending on their concentration, formulation, and condition. PV, YP, gel strength (GS), filtration properties, fluid lubricity are some examples of these properties.
3.4.1 Drilling Fluid Plastic Viscosity (PV)
A fluid’s PV determines how resistant it is to flow in a given direction. Bingham's model can be interpreted mathematically to calculate PV as a measure independent of shear stresses [205, 206]. Drilling fluid suspension capacity is assessed by PV. The PV and GS of drilling fluids are increased by a high solids content, particularly when low-density clays are present. There is a correlation between thicker filter cakes and slower drilling speeds when drilling fluids contain a high percentage of solids. A drilling fluid that contains large amounts of sand may be abrasive, damaging pumps, tubulars, and downhole tools and motors [207]. Increasing fluid cleaning will reduce PV by diluting drilling fluids with water, or by reducing solids content in the fluids [206, 208]. In drilling operations, bentonite is typically used as an additive to improve the rheological properties of drilling fluid. However, a variety of NPs can also be used to improve the rheological properties of drilling fluid. In drilling fluids, bentonite is widely used as an additive since it is inexpensive and provides rheological improvements [209]. Numerous rheological properties, especially PV, have been significantly improved by GNPs [210]. There was no difference in the PV of an ester-based drilling fluid when in-house GNPs, commercial GNPs, or graphene nanopowder were used [211]. Graphene from industrial waste was studied by Putra et al. [212] for its rheological properties at different temperatures and concentrations. An increase in PV was found when GNPs were added at a small concentration.
3.4.2 Drilling Fluid Yield Point (YP)
There is a measure of resistance to fluid flow called YP that depends on underlying chemical conditions. Chemical treatments can lead to a decrease in attractive forces, which in turn leads to a decrease in yield stress. The apparent viscosity (AV) also decreases with decreasing yield stress [213]. The AV is calculated by extrapolating the yield stress to the zero shear rate of the Bingham plastic rheological model. The YP is calculated by subtracting PV from the reading at 300 revolutions/minute (rpm) from viscometer measurements. If the YP of a solution (of the same density) is high, then the solution will be more capable of suspending drill cuttings than if it has a low value of YP [214]. When drilling fluid contains halite, anhydrite, gypsum, cement, hydrogen sulfide, or carbon dioxide, the YP of the drilling fluid tends to increase. Furthermore, if barite, sodium carbonate and bicarbonate additives are added, YP tends to increase. The YP can go down when mud thinners or deflocculants (like lignite, phosphates, and lignosulfonates) are used to get rid of solid phases. Also, the removal of pollutants through chemical neutralization or the addition of water to the fluid can also result in a decrease in YP [215]. A drilling fluid which contains GNPs can have a better YP. In their study, an increase of 17% and 36% in PV and YP, respectively, was observed with GNPs addition with a concentration of 0.2 ppb [13]. There is no doubt that using different kinds of NPs can substantially improve YP. Coconut grease with GNPs has been investigated for its rheological characteristics and its ability to improve YP substantially. The results proved that GNP concentration and thickener concentration were found to increase yield stress [216].
3.4.3 Drilling Fluid Gel Strength (GS)
Bingham's plastic rheological model revolves around GS. The indicator indicates how fast gels form, and how strong they are under static conditions. As GS increases, particles will be suspended in fluids for a longer period of time and will not easily settle due to gravity. The static shear stresses resulting from solid phases in suspension do not permit the fluids to remain suspended without sufficient fluid GS. Based on American petroleum institute (API) standard procedures, measurements are usually taken after 10 s and 10 min, although they may also be taken 30 min or 16 h later [213]. The GS of the formulated mud sample with GNP recorded a change from the base fluid of 11 to 15 1b/100ft2 after 10 s. For the 10-min measurement, the GS increased from 12 to 18 1b/100ft2 [217].
3.4.4 Drilling Fluid Filtration Properties
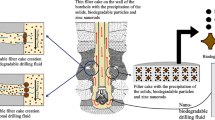
Measurements of FL and characterization of filter cakes determine the filtration properties. An efficient drilling fluid system should have low FL. Additionally, a correlation has been shown between filter cake characteristics and differential sticking. Filter cakes that are thinner have less differential sticking [218]. It is generally recommended that thin filter cakes be less than 2 mm API, while thicker filter cakes be between 4 and 6 mm API [219]. A drilling fluid's filtration control can be positively or negatively affected by GNPs additives. WMB with industrial waste graphene shows improved filtration properties, but as concentration increases, the properties deteriorate. At API conditions, 0.05 ppb is considered the optimal concentration as it reduces filtrate loss by 8.43%; at 200 °F, 225 °F, and 250 °F, it reduces the filtrate loss by 18.57%, 13.82%, and 14.06%, respectively [220]. GNPs treated drilling fluids showed an improved result in terms of filtration properties, with a reduction in FL of 38.96% and 34.36% under low pressure low temperature (LPLT) and high pressure high temperature (HPHT) conditions, respectively, at a concentration of 0.08wt% [221]. A drilling fluid was studied for its filtration ability and rheology through the investigation of the morphology and graphitization of carbon nanomaterials. The study confirms that, at a concentration of 0.007wt% GNP reduced filtrate loss and filter cake thickness by 20% and 25%, respectively [211]. A typical FL control mechanism for drilling fluid in a drilled formation is depicted in Fig. 10, where the filter cake made of bentonite and polymer minimizes the passage of water from the drilling fluid into the drilled formation [222].
Insight into the filtration control of a drilling fluid system [222]
3.4.5 Drilling Fluid Lubricity
Tests to determine the lubricity of drilling fluids are performed to determine their efficiency in reducing friction. The drill pipe spins at a certain speed and bears pressure against the hole wall at a certain pressure [223]. A number of factors affect the coefficient of friction (CoF), including mud quality, filter cake, lubricant type and concentration, and contact-surface roughness [224]. CoF increases with increasing temperature above 50 °C. In response, it was suggested that laboratory as well as field CoF values be specified along with surface roughness and temperature [225]. Swaco [194], conducted a study to investigate the tribological behavior of GNPs as nanosolid lubricants. A study also evaluates the application of GNP to micro-drilling of carbon fiber-reinforced plastic (MD-CFRP) [226]. The results showed that GNPs increased the lubrication action in terms of surface quality (delamination and uncut fibers) and tool wear of the base fluid. With 0.1wt% of palm oil and GNPs, palm oil and GNPs, together exhibited synergistic anti-friction properties that reduced grinding energy consumption by 91.78% compared to dry cutting. Compared to commercially available LB2000, the cutting energy was reduced by 80.25% at the same percentage of GNPs [227].
The latest advances in NP technology have led to numerous studies evaluating NPs' use in the petroleum industry [228,229,230]. It has been concluded from the experimental results that NPs can be used as an additive to modify the properties of drilling fluids [230, 231]. Adding NPs to drilling fluids improves their rheological properties, minimizes the loss of drilling fluid, reduces CoF, enhances heat transfer, stabilizes shale, and inhibits gas hydrate formation. Due to their very high surface area to volume ratio, NPs provide improved material properties that are surface-dependent [232, 233]. A significant amount of research has been conducted on graphene nanomaterials as an additive for improving the properties of drilling fluids. In their study, Taha and Lee [9] used WBM containing GNP to reduce torque, improve lubricity, enhance bit lifetime, and reduce bit balling at high temperatures. The result shows an improvement in the rate of penetration (ROP), while the filtrate loss and torque were as well minimized. Based on the results of a study by Aftab et al. [210], GNPs were used to enhance the rheological properties of mud blends. As a result of the addition of GNPs, the FL volumes (API and HPHT) were also reduced. When compared to MWCNTs and nanosilica (NS) at the same concentration, GNP has a better performance than MWCNTs and NS. A number of studies have shown that the addition of GNP to drilling fluids effectively improves the mud properties, as shown in Table 2.
3.5 GNPs and Modified GNPs as Shale Swelling Inhibitors in WBM
Shale instability causes wellbore instability due to clay minerals swelling capacity and their high sensitivity to water. It is common to experience drilling problems related to shale and unconventional hydrocarbon formations, as well as drilling in deep water or in strata that have been depleted. As a result of their morphology, consolidation, mineral content, grain dispersion, and geological characteristics, clay-rich reservoirs exhibit a wide range of properties [3]. In this regard, describing and generalizing the characteristics of shale rock in response to drilling fluids is difficult. Minerals such as smectite, montmorillonite, illite, chlorites, vermiculites, cristobalite, and kaolinite can be found in shale with varying particle diameters and characteristics [3, 234]. The complex physical and chemical differences in shale formations can be responsible for swelling, sloughing, loss of circulation, pipe sticking, hole collapse, and highly pressurized formations [235]. Furthermore, gas kicks may occur, resulting in a blowout of the well. The drilling mud must have the ability to act as a shale barrier to address these concerns, resulting in a lower amount of mud filtrate and improved wellbore stability. Drilling fluid additives can be used to stabilize shale and improve wellbore integrity, either by blocking shale pores or coating the shale surface to avoid water penetration [236]. They can also effectively penetrate shale strata to plug the shale nanopores and fractures [237].
A number of studies have investigated the use of GNPs and modified GNPs as shale swelling inhibitors for WBMs [12, 210, 213, 238,239,240,241,242,243,244,245,246,247]. It has been demonstrated that adding GNPs increases the rheological properties of WBM, decreases the fluid loss volume, and improves their shale inhibition behavior [210, 238, 243]. When GNPs are used, shale swelling can be minimized and mud rheological properties can be improved [210]. GNPs are not the only nanoparticles investigated for their capacity to inhibit shale swelling; nanosilica and MWCNT have been discovered to be effective as well [210, 244]. Using nanosilica and GNPs in WBM formulations has been found to increase the mud's ability to inhibit clay swelling in unconventional shale reservoirs [238]. By testing immersion and cutting dispersion against the Woodford shale, the nanoparticle-based muds were shown to inhibit the shale reservoir formation by physically plugging the shale pore throats [238].
The study by Aftab et al. [210], examined how well GNP worked in KCl mud systems to improve the rheological properties of drilling fluid and keep clay particles from getting wet. The results show that GNP exhibited a higher level of inhibition in KCl mud systems when compared to polymeric, nanosilica, and MWCNT. The presence of GNP in KCl mud systems resulted in a minimum 20% decrease in shale volume, as compared to mud systems with equivalent concentrations of nanosilica or MWCNT. Additionally, the incorporation of GNP resulted in enhanced rheological characteristics of KCl-based drilling fluids and mitigated fluid loss during filtration test. In another study, Wang et al. [172] determined how to improve GNP in WBMs to slow down the hydration of shale. In contrast to commonly used shale inhibitors such as KCl and nanosilica, GNP has shown superior efficacy in effectively obstructing nano- and micron-scale holes, impeding water infiltration into shale formations, suppressing the swelling of clay minerals during hydration, and preserving the mechanical integrity of shale. GNP sheets have the ability to produce extensive, uninterrupted films that serve as a protective barrier for shale. Moreover, the flexible nature of these graphene sheets allows for effortless deformation, enabling them to effectively seal and occupy various shale pore configurations. Hence, it can be inferred that GNP has significant promise in safeguarding and enhancing the stability of shale formations in drilling fluid systems.
The introduction of additional functional groups to GNP would enhance the number of available chemical reaction sites, thereby promoting shale stabilization. Electrochemical exfoliation was used to make nanocomposites by mixing biopolymers with graphene-modified gum Arabic (GrO–ArG). According to the findings, this significantly lessens clay swelling in WBMs found in shale-rich formations that are susceptible to water damage [242]. Based on the results, the authors suggest that GrO–ArG could be a useful additive for improving the rheological properties of WBM, limiting fluid loss, and inhibiting clay from swelling. These properties make GrO–ArG a suitable candidate for the development of environmentally friendly materials in the field of green technology. The effectiveness of shale stabilization via the GrO–ArG pathway is heavily reliant on the plugging efficacy of graphene. Graphene effectively fills the pores that are found on the surface and interlayer spacing of the clay material. Following this, the clay was shielded from the deleterious effects of water [242].
In another study by Zhu et al. [245], choline chloride/graphene composite (Ch–G) was synthesized for application as a shale stabilizer for drilling fluid, and its mechanism of action showed that it efficiently plugged the shale nanopores and coated the shale surface from absorbing water [245]. Water intrusion was further prevented, and the inhibition effect was further enhanced by the intercalation of the Ch–G in montmorillonite. In addition, in terms of shale expansion inhibition, Ch–G was found to be a highly effective, environmentally friendly shale inhibitor. Another high-performance shale inhibitor was developed using reduced graphene oxide (rGO) functionalized with ethylenediamine (EDA). The developed rGO-EDA shale inhibitor was found to be highly effective at plugging shale nanopores and inhibiting clay hydration [244]. Another drilling fluids containing cetyltrimethylammonium-modified graphene (CTAB-Gr) formulation was developed by Rana et al. [246]. According to the authors, adding CTAB-Gr to the drilling mud makes the shale inhibition properties much better than with other shale inhibitors. Additionally, it improves the rheological characteristics of the mud, making it more suitable for drilling purposes. The use of CTAB-Gr-modified WBM resulted in the maximum recovery of shale dispersion (88.5%), surpassing the recovery rates of unmodified drilling mud (74.6%), KCl (50.3%), and water (26.2%). The swelling test was used to assess the stability of clay materials when exposed to the reactive effects of water in the presence of CTAB-Gr. The clay exhibits a swelling capacity of 17% when treated with CTAB-Gr, whereas it shows swelling capacities of 22% and 27% when treated with KCl and water, respectively.
Saleh et al. [247] used polyethylenimine-modified graphene (PEI-Gr) in the development of a drilling mud with shale-swelling inhibitive properties. When comparing the performance of several fluids, namely conventional WBM, KCl, water, and PEI-Gr, it was observed that PEI-Gr exhibited the most significant enhancement in shale dispersion recovery, achieving a recovery rate of 91%. In contrast, the recovery rates for conventional WBM, KCl, and water were 75%, 50%, and 26%, respectively. Furthermore, PEI-Gr exhibited superior water-inhibition durability at the 24-h mark when compared to conventional WBM, water alone, and KCl. During the process of clay pore clogging, the interaction between polyethylenimine-graphene (PEI-Gr) and hydroxyl groups present on the clay surface facilitates the attachment of the amine groups within PEI to the hydroxyl groups, hence impeding clay swelling. Saleh et al. (268) and Rana et al. (267) used dispersion recovery tests and inhibition stability tests to observe how well the modified graphene could hinder the reaction between shale and drilling fluid. It was also shown that the WBM with the modified graphene had better dispersion recovery and longer inhibitory durability than both the base mud and the WBM based on KCl [247]. The incorporation of modified graphene into the WBM resulted in a significant enhancement of shale inhibition and an improvement in the rheological characteristics, as reported by Rana et al. [246].
According to the studies that examined how GNP and modified GNP-based fluids can stop clay from swelling, it was found that graphene can efficiently plug shale surfaces. This plugging capacity is attributed to the flexible nanosheet-like structure of graphene, which effectively prevents water from interacting with shale and consequently impedes shale swelling. Furthermore, apart from the physical phenomenon of graphene clogging the pores in the clay, the inhibitors also hindered hydration by means of chemical interaction between the modified groups and the clay particles. PEI has positively charged amines that make it easier for PEI-Gr to interact with the clay's hydroxyl groups. Moreover, the amine groups with positive charges in CTAB facilitated the binding of CTAB-Gr to the clay surface via hydrogen bonding. The introduction of modified graphene effectively hindered the ingress of water molecules into the clay nanopores, thereby minimizing the swelling caused by hydration.
3.6 Modified GNPs in Drilling Fluids
Due to their tendency toward aggregation and difficulty in processing, unmodified GNPs may not be ideal in some cases [248]. As a result of unmodified GNPs poor dispersion in aqueous media, fluids containing it may exhibit flocculation and aggregation. The use of modified GNPs in drilling fluids would be more feasible and more stable as confirmed by a patent on the modification of graphene surfaces [199]. The intrinsic hydrophobicity of GNPs also prevents its direct use in water-based drilling fluids. Table 3 highlights some research works were modified GNPs was used as a fluid drilling additives in WBM.
Among other laboratory studies conducted on clay swelling inhibition using graphene, high-performance shale stabilizer of ethylene-diamine-modified graphene (EDA-G) exhibited momentous control in clay swelling [249]. Under some conditions, adding EDA-G to drilling fluid resulted in the lowest filtration volume. Nanopores of ultralow permeability shale could be plugged by using EDA-G solution. Moreover, clay's hydration was inhibited by EDA-G solution as well. A concentration of 0.2 wt% by carbon content of EDA-G solution was found to be highly effective as an inhibitor. It was observed that EDA-G solution both exhibited excellent nanopore plugging capability and inhibited clay hydration. In a study performed by Tian et al. [250] on organic-sulfonate functionalized graphene in water-based drilling fluids, graphene was found to be highly dispersive in drilling fluids. A high degree of stability can be achieved after the drilling fluids are aged at 240 °C with the help of SDBS/graphene. As a result of the SDBS/graphene slurry, the CoF and wear rate have been reduced by 76% and 59%, respectively, compared to the base slurry.
3.7 Well Tabular Corrosion Control
As long as the oil and gas industry has existed, corrosion has been a major concern. Any type of downhole equipment that is not plastic is susceptible to this condition. This includes pipelines, production tubing, casing, and just about every other nonplastic equipment. Downhole metal equipment has been coated with GNPs to prevent corrosion as illustrated in Fig. 11. As a coating material, GNPs offer strong mechanical and electrical properties, as well as low chemical reactivity and impermeability to gases and liquids [201]. GNPs and metal oxides were used to functionalize titanium alloy surfaces [251]. On top of the thin GNPs, a multilayer film of Al2O3 and TiO2 is deposited. Micro-Raman and X-ray diffraction analysis confirmed the amorphous structure of the metal oxide films.
Graphene acts an anti-corrosion barrier [269]
3.8 Well Cementing
A further application for GNPs is as an additive for cementing jobs during the compilation stages of oil and gas wells. GNPs are being used in cement formulations to enhance cement properties and long-term reliability [252]. By using GNPs as a cement additive, the study aims to reduce gas leakage potential and improve wellbore integrity. As a result of its ability to modify the microstructure of hydration products within cement, the material may improve cement durability. This investigation found that adding GNPs to the cement systems improved the mechanical properties of the cement by 10% and 30%, respectively, resulting in improved compressive and tensile strength. A major part of the chemical shrinkage of cement-containing systems can be attributed to the high surface area and aspect ratio of GNPs. Sun et al. [253] CNFs and GNPs were used to quantify the rheology, curing temperature, and mechanical performance of the cementing process of oil wells. These composites were characterized in terms of their thermal performance, surface functional groups, morphology, and mechanical performance. Fresh composite cement slurry showed increased yield stresses when CNFs and GNPs were added, and temperature had a significant influence on them. By adding CNFs and GNPs to cement slurry composites, both flexural and compressive strength were enhanced. By slightly modifying existing casting methods, GNPs were introduced into cement paste to produce cement nanocomposites [254]. By monitoring light absorbance over time, sonicated GNPs were quantitatively assessed for stability in suspension. In the study, 1% GNP suspension remained stable for 6 h after 1 h of sonication with 15% surfactant. A mercury intrusion test showed that GNP reduced effective porosity by 37% and the critical pore diameter by 30%. An illustration of the cementing operation in a producing reservoir is shown in Fig. 12.
3.9 Environmental and Economic Aspects of GNPs
Graphene-based NPs known as GNPs have potential uses in a number of industries, including the automobile, biomedical sciences, supercapacitors, sensors, and building materials. According to Malhotra et al. [255], the environment, aquatic life, and people may all be harmed by the waste material released by these companies. Research on the effects of GNPs on the environment has revealed that GNPs can inevitably release themselves into the environment at various stages of their life cycles [256]. There might be some concerns about the role of GNPs in a sustainable future if they are released into the environment. Size, layers, and surface chemistry all play a role in the potential environmental impact of GNPs [257]. According to a comparative life cycle evaluation of GNPs-reinforced poly(propylene) composites and glass fiber-reinforced poly(propylene) composites, nanocomposite reduces the environmental impact of AP composites by 17–75%, based on their impact category [258]. The incorporation of GNPs into soil and the influence they have on microbiomes can be facilitated through water-based transport techniques such as precipitation and surface runoff [259]. Hence, understanding the environmental aspects of GNPs is important to minimize their negative effects on the environment.
The unique properties and applications of GNPs have attracted significant attention in the last few years. Razali et al. [211] found that GNPs could enhance the rheological properties of drilling fluids such as PV, YP, and GS. The drilling process can be improved as a result, and there will be less waste generated. The filtration capability of drilling fluids can also be improved by GNPs [12]. As a result, they can reduce the impact of drilling on the environment by preventing the loss of drilling fluids into the formation. Studies have shown that GNPs can contribute to flocculation in drilling fluid systems by improperly dispersing NPs [260]. The drilling fluid's effectiveness can be reduced, and waste generation may increase as a result. In order to mitigate the agglomeration issue, the surface of GNP needs to be modified prior to application, which will in turn improve the drilling fluid characteristics. Carbon emissions from the construction industry may be reduced by using GNPs. The carbon dioxide emissions from cementitious composites using graphene are expected to be reduced in a circular economy [261]. Lovén et al. [262] examined workplace emissions and exposure to GNPs to understand their potential environmental effects. As a result of incorporating GNPs into bio-based and biodegradable blends, they may be able to have a positive impact on the environment [263]. Due to their low toxicity and low production/use amounts, some researchers have argued that GNPs are not an environmental threat [264]. The economic potential of nanomaterials lies in their ability to improve the mechanical properties of materials, which will reduce the cost of materials and services in industries such as construction and manufacturing [265].Tavares et al. [263] found that the use of GNPs in bio-based and biodegradable blends enabled the composite's physicochemical, thermal, rheological, and mechanical properties to be improved, which was cost-effective [263]. Various materials can benefit from using GNPs as nanofillers, improving their properties and introducing new applications [266]. Economic growth and job creation can be achieved in the nanotechnology industry through the development and commercialization of GNPs.
In general, GNPs can have a positive impact on both the environment and the economy. The necessary precautions should be taken to minimize any negative effects, but more study is needed to determine their potential environmental impact.
4 Challenges and Prospects
GNP is a good conductor of electricity in the electrical domain, but it is difficult to turn off. It is also vulnerable to an oxidative environment. Research on GNP preparation and use has advanced at an unprecedented rate in the oil and gas sector because of its special qualities. However, producing high-quality GNP is a costly and time-consuming process. Since GNPs were first successfully fabricated in 2004, the preparation process has reached a mature stage, owing to widespread interest in the material. Dispersions can be difficult to obtain, and GNP flakes can have significant damage if they are not completely exfoliated into single- or few-layer material with a reasonable lateral dimension. Graphene nanosheets can easily be modified for a variety of applications using chemical and physical methods due to its oxygen-containing functional groups. There has been detailed discussion in the current review on how GNPs can be functionalized by various techniques. A study of graphene-based NPs in hybrid nanofluids holds great potential for research. GNPs have demonstrated their immense potential in the drilling industry, where they have provided new changes and improvements. In spite of this, the following observations ought to be taken into account for the future direction of GNP utilization:
-
1.
The development of new synthesis methods that use robust, cost-effective, and environmentally friendly precursors would make producing graphene in mass more feasible compared to current methods requires advance studies.
-
2.
GNPs require improved studies on nanofluid stability. Despite numerous studies, it has not been possible to eliminate from unset the agglomeration and sedimentation of graphene nanomaterial. To enhance drilling fluid properties, size and dispersion rate are essential.
-
3.
GNPs production must be scaled up with green methods, and drilling fluid properties can be optimized with cost-effective nanomaterials. It will reduce the use of unnecessary and expensive drilling fluid additives while improving the functionality of the fluid. It is thus possible to protect the environment while reducing drilling costs. To generate stable nanofluids in large quantities by surface modification of GNPs and the preparation of stable nanofluids, economic considerations must be taken into account.
-
4.
Changing the surface of graphene nanomaterials can improve the flow properties of a drilling fluid that is based on water by making it easier for additives to mix. This is why more research is needed. As a result, cutting transport efficiency might be improved during a reservoir drilling operation.
-
5.
GNPs can be modified at low temperatures and functionalized at high temperatures without failure, but no research has been done to demonstrate that high temperatures are ineffective for surface modification. The modification process becomes more efficient at higher temperatures because more bonds can form because of activation energy.
-
6.
Even though changing the surface of GNPs has been shown to make them more stable through ZP analysis at different pH levels, this has only been shown in the laboratory. Nevertheless, the effects of reservoir pH on modified GNPs' stability need to be considered in drilling fluid field applications.
-
7.
Both surface-modified and unmodified GNPs have been widely used in drilling fluid applications. Previous studies have mostly focused on the physical interactions between modified GNPs and drilling fluid additives and also on the formation of the well rock. At varying pH and temperature, researchers may be able to understand how GNPs improve drilling fluid properties by observing interactions between modified nano-enhanced drilling fluids and well reservoir fluids.
-
8.
GNP is still in the laboratory stage in terms of modification, characterization, and applications as a drilling fluid additive. There are no substantial field trials in a proper drilling environment where GNP and modified GNPs have been applied. Thus, proper GNP assessment in the oil and gas field is necessary.
-
9.
Nanotechnology will play a major role in oil and gas success in the future; thus, further studies on nanomaterials, such as GNP, could be vital to achieving greater output. Laboratory results indicate that GNP could be suitable for field application. Thus, conducting and facilitating the field application of GNP-based drilling fluid requires collaboration between oil industries and academics or research institutes.
5 Conclusions
This review presents vital information on GNPs modification and application for oil well drilling and completion operations. The synthesis methods, feedstocks and the challenges of GNPs modification were discussed in detail. The research's findings led to the following significant conclusions.
-
1.
Bottom-up approaches are more advantageous than top-down approaches because they are likely to produce nanostructures with fewer defects, more consistent chemical alignment, and improved short-range and long-range organization.
-
2.
The results indicate that the right synthesis method can significantly lower growth temperature and deposition time, which can further enhance the synthesis and scalability of the process as a whole.
-
3.
Biomass feedstocks such as wood, leaf, bagasse, fruit, newspaper, bone, and cow dung are capable of replacing graphite in oxidative exfoliation reduction processes.
-
4.
The review indicates that it is attractive to use noncovalent functionalization because it allows a variety of groups to attach to the GNP while maintaining its physical properties without affecting its structure.
-
5.
The review also shows that the addition of GNP to drilling fluids effectively improved the mud properties, ROP, filtrate loss control, and reduced torque and drag. It shows that 0.1–2.5 g of GNP reduced FL volume by 40–89%.
-
6.
In addition, the review indicates that adding GNPs to cement systems can improve the mechanical properties of the cement by 10–30%, thereby resulting in improved compressive and tensile strength.
Abbreviations
- API:
-
American petroleum institute
- APTS:
-
3-Aminopropyl triethoxysilane
- CNFs:
-
Cellulose nanofibers
- CNTs:
-
Carbon nanotubes
- CoF:
-
Coefficient of friction
- CTAB:
-
Cetyl triammonium bromide
- CVD:
-
Chemical vapor deposition
- FL:
-
Fluid loss
- FTIR:
-
Fourier transform infrared spectroscopy
- GNPs:
-
Graphene nanoplatelets
- GS:
-
Gel strength
- HOPG:
-
Highly oriented pyrolytic graphite
- HPHT:
-
High pressure and high temperature
- KOH:
-
Potassium hydroxide
- LPLT:
-
Low pressure and low temperature
- LPE:
-
Liquid-phase exfoliation
- MGNF:
-
Multilayered graphene nanoflakes
- MWCNTs:
-
Multi-walled carbon nanotubes
- MW-PECVD:
-
Microwave plasma-enhanced chemical vapor deposition
- NDG:
-
Nitrogen-doped graphene
- N2O4 :
-
Dinitrogen tetroxide
- NPs:
-
Nanoparticles
- NS:
-
Nanosilica
- OBM:
-
Oil-based muds
- °C:
-
Degree celsius
- °:
-
Degrees
- PEG:
-
Polyethylene glycol
- PEI:
-
Polyethylenimine
- pH:
-
Hydrogen potential
- PECVD:
-
Plasma-enhanced chemical vapor deposition
- PFLG:
-
Planar few layer graphene
- PV:
-
Plastic viscosity
- SDBS:
-
Sodium dodecyl benzene sulfate
- SDS:
-
Sodium dodecyl sulfate
- TEM:
-
Transmission electron microscopy
- WBM:
-
Water-based muds
- YP:
-
Yield point
- ZP:
-
Zeta potential
References
Oseh, J.O.; Norddin, M.M.; Ismail, I.; Gbadamosi, A.O.; Agi, A.; Mohammed, H.N.: A novel approach to enhance rheological and filtration properties of water–based mud using polypropylene–silica nanocomposite. J. Pet. Sci. Eng. 181, 106264 (2019)
Gbadamosi, A.O.; Junin, R.; Abdalla, Y.; Agi, A.; Oseh, J.O.: Experimental investigation of the effects of silica nanoparticle on hole cleaning efficiency of water-based drilling mud. J. Pet. Sci. Eng. 172, 1226–1234 (2019). https://doi.org/10.1016/j.petrol.2018.09.097
Oseh, J.O.; Mohd Norddin, M.N.A.; Ismail, I.; Gbadamosi, A.O.; Agi, A.; Ismail, A.R.: Experimental investigation of cuttings transportation in deviated and horizontal wellbores using polypropylene–nanosilica composite drilling mud. J. Pet. Sci. Eng. 189, 106958 (2020). https://doi.org/10.1016/j.petrol.2020.106958
Jiang, G., et al.: Novel water-based drilling and completion fluid technology to improve wellbore quality during drilling and protect unconventional reservoirs. Engineering 18, 129–142 (2021)
Agi, A., et al.: Application of polymeric nanofluid in enhancing oil recovery at reservoir condition. J. Pet. Sci. Eng. 194, 107476 (2020)
Agi, A.; Junin, R.; Gbonhinbor, J.; Onyekonwu, M.: Natural polymer flow behaviour in porous media for enhanced oil recovery applications: a review. J. Pet. Explor. Prod. Technol. 8(4), 1349–1362 (2018)
Agi, A., et al.: Dynamic stabilization of formation fines to enhance oil recovery of a medium permeability sandstone core at reservoir conditions. J. Mol. Liq. 371, 121107 (2022)
Yakasai, F.; Jaafar, M.Z.; Sidek, M.A.; Bandyopadhyay, S.; Agi, A.; Ngouangna, E.N.: Co-precipitation and grafting of (3-aminopropyl) triethoxysilane on Ferro nanoparticles to enhance oil recovery mechanisms at reservoir conditions. J. Mol. Liq. 371, 121007 (2023)
Taha, N. M.; Lee, S.: Nano graphene application improving drilling fluids performance. Presented at the International Petroleum Technology Conference, OnePetro, (2015). https://doi.org/10.2523/IPTC-18539-MS
Tavakkoli, O.; Kamyab, H.; Shariati, M.; Mohamed, A.M.; Junin, R.: Effect of nanoparticles on the performance of polymer/surfactant flooding for enhanced oil recovery: a review. Fuel 312, 122867 (2022)
Tofighy, M.A.; Mohammadi, T.: Barrier, diffusion, and transport properties of rubber nanocomposites containing carbon nanofillers. In: Carbon-Based Nanofillers and Their Rubber Nanocomposites, pp. 253–285. Elsevier, The Netherlands (2019)
Ridha, S.; Ibrahim, A.; Shahari, R.; Fonna, S.: Graphene nanoplatelets as high-performance filtration control material in water-based drilling fluids. In: IOP Conference Series: Materials Science and Engineering, IOP Publishing, p 012025 (2018)
Arain, A.H.; Ridha, S.; Ilyas, S.U.; Mohyaldinn, M.E.; Suppiah, R.R.: Evaluating the influence of graphene nanoplatelets on the performance of invert emulsion drilling fluid in high-temperature wells. J. Pet. Explor. Prod. Technol. 12(9), 1–25 (2022)
Alkhamis, M.; Imqam, A.: New cement formulations utilizing graphene nano platelets to improve cement properties and long-term reliability in oil wells. In: SPE Kingdom of Saudi Arabia annual technical symposium and exhibition, OnePetro (2018)
Tabatabaei, M.; Santos L.; Al Hassan, A. A.; Dahi Taleghani, A.: Limiting deteriorative impacts of oil-based mud residuals on cement bonding. In: SPE Annual Technical Conference and Exhibition, OnePetro (2022)
Srivastava, M.; Singh, J.; Kuila, T.; Layek, R.K.; Kim, N.H.; Lee, J.H.: Recent advances in graphene and its metal-oxide hybrid nanostructures for lithium-ion batteries. Nanoscale 7(11), 4820–4868 (2015)
Aftab, A., et al.: Influence of tailor-made TiO2/API bentonite nanocomposite on drilling mud performance: towards enhanced drilling operations. Appl. Clay Sci. 199, 105862 (2020)
Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S.: Synthesis of graphene and its applications: a review. Crit. Rev. Solid State Mater. Sci. 35(1), 52–71 (2010). https://doi.org/10.1080/10408430903505036
Cataldi, P.; Athanassiou, A.; Bayer, I.S.: Graphene nanoplatelets-based advanced materials and recent progress in sustainable applications. Appl. Sci. 8(9), 1438 (2018)
Castillo, A.D.R., et al.: High-yield production of 2D crystals by wet-jet milling. Mater. Horiz. 5(5), 890–904 (2018)
Novoselov, K.S., et al.: Electric field effect in atomically thin carbon films. Science (2004). https://doi.org/10.1126/science.1102896
Kuzmenko, A.B.; Van Heumen, E.; Carbone, F.; Van Der Marel, D.: Universal optical conductance of graphite. Phys. Rev. Lett. 100(11), 117401 (2008)
Geim, A.K.; Novoselov, K.S.: The rise of graphene. In: Nanoscience and Technology: a Collection of Reviews from Nature Journals, pp. 11–19. World Scientific, Singapore (2010)
Ferrari, A.C., et al.: Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 7(11), 4598–4810 (2015)
Liang, M.; Luo, B.; Zhi, L.: Application of graphene and graphene-based materials in clean energy-related devices. Int. J. Energy Res. 33(13), 1161–1170 (2009)
Gaikwad, A.V., et al.: Carbon nanotube/carbon nanofiber growth from industrial by-product gases on low-and high-alloy steels. Carbon 50(12), 4722–4731 (2012)
Wang, H.; Liang, Y.; Mirfakhrai, T.; Chen, Z.; Casalongue, H.S.; Dai, H.: Advanced asymmetrical supercapacitors based on graphene hybrid materials. Nano Res. 4(8), 729–736 (2011)
Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G.: Graphene CVD growth on copper and nickel: role of hydrogen in kinetics and structure. Phys. Chem. Chem. Phys. 13(46), 20836–20843 (2011)
Ahmad, M.S., et al.: Effect of reaction conditions on the lifetime of SAPO-34 catalysts in methanol to olefins process–a review. Fuel 283, 118851 (2021)
Filipponi, L.; Sutherland, D.: Filipponi: NANOYOU teachers training kit in nanotechnologies-Google Scholar https://scholar.google.com/scholar_lookup?title=NANOYOU%20teachers%20training%20kit%20in%20nanotechnologies&author=L.%20Filipponi&publication_year=2010 (2010). Accessed 19 Aug 2022
Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P.: Experimental observation of the quantum hall effect and Berry’s phase in graphene. Nature 438(7065), 201–204 (2005)
Lee, C.; Wei, X.; Kysar, J.W.; Hone, J.: Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321(5887), 385–388 (2008)
Truong, Q.-T.; Pokharel, P.; Song, G.S.; Lee, D.-S.: Preparation and characterization of graphene nanoplatelets from natural graphite via intercalation and exfoliation with tetraalkylammoniumbromide. J. Nanosci. Nanotechnol. 12(5), 4305–4308 (2012)
Kumar, H.P.; Xavior, M.A.: Graphene reinforced metal matrix composite (GRMMC): a review. Procedia Eng. 97, 1033–1040 (2014)
Wolf, E.L.: Applications of graphene: an overview. Springer, New York (2014)
Lang, B.: A LEED study of the deposition of carbon on platinum crystal surfaces. Surf. Sci. 53(1), 317–329 (1975). https://doi.org/10.1016/0039-6028(75)90132-6
Kotov, N.A.: Carbon sheet solutions. Nature 442(7100), 254–255 (2006)
Nieto, A.; Lahiri, D.; Agarwal, A.: Synthesis and properties of bulk graphene nanoplatelets consolidated by spark plasma sintering. Carbon 50(11), 4068–4077 (2012)
Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S.: The chemistry of graphene oxide. Chem. Soc. Rev. 39(1), 228–240 (2010)
Madhad, H.V.; Mishra, N.S.; Patel, S.B.; Panchal, S.S.; Gandhi, R.A.; Vasava, D.V.: Graphene/graphene nanoplatelets reinforced polyamide nanocomposites: a review. High Perform. Polym. 33(9), 981–997 (2021). https://doi.org/10.1177/09540083211011216
Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H.: Recent advances in graphene based polymer composites. Prog. Polym. Sci. 35(11), 1350–1375 (2010)
Park, S.; Ruoff, R.S.: Chemical methods for the production of graphenes. Nat. Nanotechnol. (2009). https://doi.org/10.1038/nnano.2009.58
Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D.: Graphene-based materials and their composites: a review on production, applications and product limitations. Compos. Part B Eng. 142, 200–220 (2018)
Fu, H.; Zhang, S.; Chen, H.; Weng, J.: Graphene enhances the sensitivity of fiber-optic surface plasmon resonance biosensor. IEEE Sens. J. 15(10), 5478–5482 (2015)
Kausar, A.: Trends in graphene reinforced polyamide nanocomposite for functional application: a review. Polym.-Plast. Technol. Mater. 58(9), 917–933 (2019)
Madhad, H.V.; Vasava, D.V.: Review on recent progress in synthesis of graphene–polyamide nanocomposites. J. Thermoplast. Compos. Mater. 35(4), 570–598 (2022)
Jiménez-Suárez, A.; Prolongo, S.G.: Graphene nanoplatelets. Appl. Sci. 10(5), 1753 (2020)
Wang, Z., et al.: Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: a comprehensive utilization strategy. ACS Appl. Mater. Interfaces 8(2), 1434–1439 (2016). https://doi.org/10.1021/acsami.5b10660
Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K.: A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 36(5), 638–670 (2011)
Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S.: The mechanics of graphene nanocomposites: a review. Compos. Sci. Technol. 72(12), 1459–1476 (2012)
Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M.: Electrochemistry of graphene and related materials. Chem. Rev. 114(14), 7150–7188 (2014)
Edwards, R.S.; Coleman, K.S.: Graphene synthesis: relationship to applications. Nanoscale 5(1), 38–51 (2013)
Li, X., et al.: Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324(5932), 1312–1314 (2009)
Chua, C.K.; Pumera, M.: Chemical reduction of graphene oxide: a synthetic chemistry viewpoint. Chem. Soc. Rev. 43(1), 291–312 (2014)
Emiru, T.F.; Ayele, D.W.: Controlled synthesis, characterization and reduction of graphene oxide: a convenient method for large scale production. Egypt. J. Basic Appl. Sci. 4(1), 74–79 (2017)
Güler, Ö.; Güler, S.H.; Selen, V.; Albayrak, M.G.; Evin, E.: Production of graphene layer by liquid-phase exfoliation with low sonication power and sonication time from synthesized expanded graphite. Fuller. Nanotub. Carbon Nanostruct. 24(2), 123–127 (2016)
Haar, S., et al.: Liquid-phase exfoliation of graphite into single-and few-layer graphene with α-functionalized alkanes. J. Phys. Chem. Lett. 7(14), 2714–2721 (2016)
Dimiev, A.M.; Khannanov, A.; Vakhitov, I.; Kiiamov, A.; Shukhina, K.; Tour, J.M.: Revisiting the mechanism of oxidative unzipping of multiwall carbon nanotubes to graphene nanoribbons. ACS Nano 12(4), 3985–3993 (2018)
Huang, H.; Chen, S.; Wee, A.T.S.; Chen, W.: Epitaxial growth of graphene on silicon carbide (SiC). In: Graphene, pp. 177–198. Elsevier, The Netherlands (2014)
Yazdi, G.R.; Iakimov, T.; Yakimova, R.: Epitaxial graphene on SiC: a review of growth and characterization. Crystals 6(5), 53 (2016)
Yang, Y., et al.: Bottom-up fabrication of graphene on silicon/silica substrate via a facile soft-hard template approach. Sci. Rep. 5(1), 1–7 (2015)
Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S.: Graphene based materials: past, present and future. Prog. Mater. Sci. 56(8), 1178–1271 (2011)
Jayasena, B.; Subbiah, S.: A novel mechanical cleavage method for synthesizing few-layer graphenes. Nanoscale Res. Lett. 6(1), 95 (2011). https://doi.org/10.1186/1556-276X-6-95
Chen, J.; Duan, M.; Chen, G.: Continuous mechanical exfoliation of graphene sheets via three-roll mill. J. Mater. Chem. 22(37), 19625–19628 (2012)
Yi, M.; Shen, Z.: A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 3(22), 11700–11715 (2015)
Viculis, L.M.; Mack, J.J.; Kaner, R.B.: A chemical route to carbon nanoscrolls. Science 299(5611), 1361–1361 (2003)
Lapshin, R.V.: STM observation of a box-shaped graphene nanostructure appeared after mechanical cleavage of pyrolytic graphite. Appl. Surf. Sci. 360, 451–460 (2016). https://doi.org/10.1016/j.apsusc.2015.09.222
Kim, K.S., et al.: Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457(7230), 706–710 (2009)
Reina, A., et al.: Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 9(1), 30–35 (2009)
Eda, G.; Fanchini, G.; Chhowalla, M.: Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 3(5), 270–274 (2008)
Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K.: Liquid-phase exfoliation of graphite towards solubilized graphenes. Small 5(16), 1841–1845 (2009)
Green, A.A.; Hersam, M.C.: Solution phase production of graphene with controlled thickness via density differentiation. Nano Lett. 9(12), 4031–4036 (2009). https://doi.org/10.1021/nl902200b
Hamilton, C.E.; Lomeda, J.R.; Sun, Z.; Tour, J.M.; Barron, A.R.: High-yield organic dispersions of unfunctionalized graphene. Nano Lett. 9(10), 3460–3462 (2009)
Hernandez, Y., et al.: High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 3(9), 563–568 (2008)
Jankovskỳ, O.; Kučková, ŠH.; Pumera, M.; Šimek, P.; Sedmidubskỳ, D.; Sofer, Z.: Carbon fragments are ripped off from graphite oxide sheets during their thermal reduction. New J. Chem. 38(12), 5700–5705 (2014)
Somanathan, T.; Prasad, K.; Ostrikov, K.; Saravanan, A.; Mohana Krishna, V.: Graphene oxide synthesis from agro waste. Nanomaterials 5(2), 826–834 (2015)
Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R.: High-efficient synthesis of graphene oxide based on improved hummers method. Sci. Rep. 6(1), 1–7 (2016)
Yoon, G.; Seo, D.-H.; Ku, K.; Kim, J.; Jeon, S.; Kang, K.: Factors affecting the exfoliation of graphite intercalation compounds for graphene synthesis. Chem. Mater. 27(6), 2067–2073 (2015)
Khan, M.S.; Shakoor, A.; Khan, G.T.; Sultana, S.; Zia, A.: A study of stable graphene oxide dispersions in various solvents. J. Chem. Soc. Pak. 37(1), 62–67 (2015)
Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E.: Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 430, 108–112 (2014)
Ciesielski, A.; Samorì, P.: Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 43(1), 381–398 (2014)
Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N.: Measurement of multicomponent solubility parameters for graphene facilitates solvent discovery. Langmuir 26(5), 3208–3213 (2010)
Lotya, M., et al.: Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 131(10), 3611–3620 (2009)
Si, Y.; Samulski, E.T.: Synthesis of water soluble graphene. Nano Lett. 8(6), 1679–1682 (2008)
Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B.: High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 4(1), 25–29 (2009)
Tung, V.C., et al.: Low-temperature solution processing of graphene- carbon nanotube hybrid materials for high-performance transparent conductors. Nano Lett. 9(5), 1949–1955 (2009)
Nethravathi, C.; Rajamathi, M.: Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 46(14), 1994–1998 (2008)
Somani, P.R.; Somani, S.P.; Umeno, M.: Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 430(1–3), 56–59 (2006)
Campos-Delgado, J., et al.: CVD synthesis of mono-and few-layer graphene using alcohols at low hydrogen concentration and atmospheric pressure. Chem. Phys. Lett. 584, 142–146 (2013)
Min, B.H.; Kim, D.W.; Kim, K.H.; Choi, H.O.; Jang, S.W.; Jung, H.-T.: Bulk scale growth of CVD graphene on Ni nanowire foams for a highly dense and elastic 3D conducting electrode. Carbon 80, 446–452 (2014)
Brownson, D.A.; Banks, C.E.: The electrochemistry of CVD graphene: progress and prospects. Phys. Chem. Chem. Phys. 14(23), 8264–8281 (2012)
Lee, X.J.; Lee, L.Y.; Foo, L.P.Y.; Tan, K.W.; Hassell, D.G.: Evaluation of carbon-based nanosorbents synthesised by ethylene decomposition on stainless steel substrates as potential sequestrating materials for nickel ions in aqueous solution. J. Environ. Sci. 24(9), 1559–1568 (2012)
Yu, Q.; Lian, J.; Siriponglert, S.; Li, H.; Chen, Y.P.; Pei, S.-S.: Graphene segregated on Ni surfaces and transferred to insulators. Appl. Phys. Lett. 93(11), 113103 (2008)
Gupta, B.; Notarianni, M.; Mishra, N.; Shafiei, M.; Iacopi, F.; Motta, N.: Evolution of epitaxial graphene layers on 3C SiC/Si (1 1 1) as a function of annealing temperature in UHV. Carbon 68, 563–572 (2014)
Jang, J., et al.: Low-temperature-grown continuous graphene films from benzene by chemical vapor deposition at ambient pressure. Sci. Rep. 5(1), 1–7 (2015)
Obraztsov, A.N.; Zolotukhin, A.A.; Ustinov, A.O.; Volkov, A.P.; Svirko, Y.; Jefimovs, K.: DC discharge plasma studies for nanostructured carbon CVD. Diam. Relat. Mater. 12(3–7), 917–920 (2003)
Kalita, G., et al.: Low temperature deposited graphene by surface wave plasma CVD as effective oxidation resistive barrier. Corros. Sci. 78, 183–187 (2014)
Shang, N.G., et al.: Catalyst-free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. Adv. Funct. Mater. 18(21), 3506–3514 (2008)
Yuan, G.D., et al.: Graphene sheets via microwave chemical vapor deposition. Chem. Phys. Lett. 467(4–6), 361–364 (2009)
Meškinis, Š; Vasiliauskas, A.; Guobienė, A.; Talaikis, M.; Niaura, G.; Gudaitis, R.: The direct growth of planar and vertical graphene on Si (100) via microwave plasma chemical vapor deposition: synthesis conditions effects. RSC Adv. 12(29), 18759–18772 (2022)
Kidambi, P.R., et al.: A scalable route to nanoporous large-area atomically thin graphene membranes by roll-to-roll chemical vapor deposition and polymer support casting. ACS Appl. Mater. Interfaces 10(12), 10369–10378 (2018)
Kim, C.-D.; Kim, M.; Jo, Y.: Study on the temperature dependence of three-dimensional graphene structure by thermal chemical vapor deposition. New Phys.: Sae Mulli 72, 329–335 (2022)
Cano-Márquez, A.G., et al.: Ex-MWNTs: graphene sheets and ribbons produced by lithium intercalation and exfoliation of carbon nanotubes. Nano Lett. 9(4), 1527–1533 (2009)
Jiao, L.; Zhang, L.; Wang, X.; Diankov, G.; Dai, H.: Narrow graphene nanoribbons from carbon nanotubes. Nature 458(7240), 877–880 (2009)
Zhong, Y.L.; Tian, Z.; Simon, G.P.; Li, D.: Scalable production of graphene via wet chemistry: progress and challenges. Mater. Today 18(2), 73–78 (2015)
Dato, A.; Frenklach, M.: Substrate-free microwave synthesis of graphene: experimental conditions and hydrocarbon precursors. New J. Phys. 12(12), 125013 (2010)
Li, Y.; Chen, Q.; Xu, K.; Kaneko, T.; Hatakeyama, R.: Synthesis of graphene nanosheets from petroleum asphalt by pulsed arc discharge in water. Chem. Eng. J. 215, 45–49 (2013)
Akhavan, O.; Bijanzad, K.; Mirsepah, A.: Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv. 4(39), 20441–20448 (2014)
Primo, A.; Atienzar, P.; Sanchez, E.; Delgado, J.M.; García, H.: From biomass wastes to large-area, high-quality, N-doped graphene: catalyst-free carbonization of chitosan coatings on arbitrary substrates. Chem. Commun. 48(74), 9254–9256 (2012)
Hallaj, T.; Amjadi, M.; Manzoori, J.L.; Shokri, R.: Chemiluminescence reaction of glucose-derived graphene quantum dots with hypochlorite, and its application to the determination of free chlorine. Microchim. Acta 182(3), 789–796 (2015)
Qu, J.; Luo, C.; Zhang, Q.; Cong, Q.; Yuan, X.: Easy synthesis of graphene sheets from alfalfa plants by treatment of nitric acid. Mater. Sci. Eng. B 178(6), 380–382 (2013)
Ekhlasi, L.; Younesi, H.; Rashidi, A.; Bahramifar, N.: Populus wood biomass-derived graphene for high CO2 capture at atmospheric pressure and estimated cost of production. Process. Saf. Environ. Prot. 113, 97–108 (2018)
Gupta, S.S.; Sreeprasad, T.S.; Maliyekkal, S.M.; Das, S.K.; Pradeep, T.: Graphene from sugar and its application in water purification. ACS Appl. Mater. Interfaces 4(8), 4156–4163 (2012)
Wang, Y.; Shi, Z.; Yin, J.: Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. ACS Appl. Mater. Interfaces 3(4), 1127–1133 (2011)
Ismail, M.S., et al.: Synthesis and characterization of graphene derived from rice husks. Malays. J. Fundam. Appl. Sci. 15(4), 516–521 (2019). https://doi.org/10.11113/mjfas.v15n4.1228
Pandey, S., et al.: Bulk synthesis of graphene nanosheets from plastic waste: an invincible method of solid waste management for better tomorrow. Waste Manag. 88, 48–55 (2019)
Ding, Z., et al.: Green synthesis of chemical converted graphene sheets derived from pulping black liquor. Carbon 158, 690–697 (2020)
Sun, L., et al.: From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 1(21), 6462 (2013). https://doi.org/10.1039/c3ta10897j
Hashmi, A.; Singh, A.K.; Jain, B.; Singh, A.: Muffle atmosphere promoted fabrication of graphene oxide nanoparticle by agricultural waste. Fuller. Nanotub. Carbon Nanostruct. 28(8), 627–636 (2020)
Ayub, M.; Othman, M.H.D.; Yusop, M.Z.M.; Khan, I.U.; Zakria, H.S.: Facile and economical, single-step single-chemical method for conversion of palm oil fuel ash waste into graphene nanosheets. Appl. Mater. Today 25, 101193 (2021)
Singh, P.; Bahadur, J.; Pal, K.: One-step one chemical synthesis process of graphene from rice husk for energy storage applications. Graphene 6(3), 61–71 (2017)
Schlesinger, W.H.; Bernhardt, E.S.: Biogeochemistry: an analysis of global change. Academic press, Cambridge (2013)
Arifin, N.F.T., et al.: Comparison of different activated agents on biomass-derived graphene towards the hybrid nanocomposites with zeolitic imidazolate framework-8 for room temperature hydrogen storage. J. Environ. Chem. Eng. 9(2), 105118 (2021). https://doi.org/10.1016/j.jece.2021.105118
Muramatsu, H., et al.: Rice husk-derived graphene with nano-sized domains and clean edges. Small 10(14), 2766–2770 (2014)
Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; Zatile, E.; Palomares, F.J.; Aranda, P.: Supported graphene from natural resources: easy preparation and applications. Wiley Online Library, New Jersey (2011)
Zhu, C.; Guo, S.; Fang, Y.; Dong, S.: Reducing sugar: new functional molecules for the green synthesis of graphene nanosheets. ACS Nano 4(4), 2429–2437 (2010)
Kong, X., et al.: Synthesis of graphene-like carbon from biomass pyrolysis and its applications. Chem. Eng. J. 399, 125808 (2020)
Long, S.-Y.; Du, Q.-S.; Wang, S.-Q.; Tang, P.-D.; Li, D.-P.; Huang, R.-B.: Graphene two-dimensional crystal prepared from cellulose two-dimensional crystal hydrolysed from sustainable biomass sugarcane bagasse. J. Clean. Prod. 241, 118209 (2019)
Chen, F.; Yang, J.; Bai, T.; Long, B.; Zhou, X.: Facile synthesis of few-layer graphene from biomass waste and its application in lithium ion batteries. J. Electroanal. Chem. 768, 18–26 (2016)
Purkait, T.; Singh, G.; Singh, M.; Kumar, D.; Dey, R.S.: Large area few-layer graphene with scalable preparation from waste biomass for high-performance supercapacitor. Sci. Rep. 7(1), 1–14 (2017)
Shams, S.S.; Zhang, L.S.; Hu, R.; Zhang, R.; Zhu, J.: Synthesis of graphene from biomass: a green chemistry approach. Mater. Lett. 161, 476–479 (2015)
Sankar, S., et al.: Ultrathin graphene nanosheets derived from rice husks for sustainable supercapacitor electrodes. New J. Chem. 41(22), 13792–13797 (2017)
Sekar, S.; Aqueel Ahmed, A.T.; Sim, D.H.; Lee, S.: Extraordinarily high hydrogen-evolution-reaction activity of corrugated graphene nanosheets derived from biomass rice husks. Int. J. Hydrog. Energy (2022). https://doi.org/10.1016/j.ijhydene.2022.02.233
Wang, C.-I.; Periasamy, A.P.; Chang, H.-T.: Photoluminescent C-dots@ RGO probe for sensitive and selective detection of acetylcholine. Anal. Chem. 85(6), 3263–3270 (2013)
Bhunia, P., et al.: A non-volatile memory device consisting of graphene oxide covalently functionalized with ionic liquid. Chem. Commun. 48(6), 913–915 (2012)
Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S.: Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly (sodium 4-styrenesulfonate). J. Mater. Chem. 16(2), 155–158 (2006)
Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C.: Solution properties of graphite and graphene. J. Am. Chem. Soc. 128(24), 7720–7721 (2006)
Stankovich, S., et al.: Graphene-based composite materials. Nature 442(7100), 282–286 (2006)
Hirsch, A.: A review of carbon materials and nanotechnology. Mol. Cryst. Liq. Cryst. (2011). https://doi.org/10.1080/15421406.2011.590375
Ibrahim, A.; Ridha, S.; Amer, A.; Shahari, R.; Ganat, T.: Influence of degree of dispersion of noncovalent functionalized graphene nanoplatelets on rheological behaviour of aqueous drilling fluids. Int. J. Chem. Eng. 2019, 1–11 (2019). https://doi.org/10.1155/2019/8107168
Morrison, I.D.; Ross, S.: Colloidal dispersions: suspensions, emulsions, and foams. Wiley-Interscience, New York (2002)
Hao, R.; Qian, W.; Zhang, L.; Hou, Y.: Aqueous dispersions of TCNQ-anion-stabilized graphene sheets. Chem. Commun. 48, 6576–6578 (2008). https://doi.org/10.1039/B816971C
Zhang, M., et al.: Production of graphene sheets by direct dispersion with aromatic healing agents. Small 6(10), 1100–1107 (2010). https://doi.org/10.1002/smll.200901978
Mehrali, M., et al.: Preparation, characterization, viscosity, and thermal conductivity of nitrogen-doped graphene aqueous nanofluids. J. Mater. Sci. 49(20), 7156–7171 (2014). https://doi.org/10.1007/s10853-014-8424-8
Wan, Y.-J., et al.: Improved dispersion and interface in the graphene/epoxy composites via a facile surfactant-assisted process. Compos. Sci. Technol. 82, 60–68 (2013)
Pu, N.-W.; Wang, C.-A.; Liu, Y.-M.; Sung, Y.; Wang, D.-S.; Ger, M.-D.: Dispersion of graphene in aqueous solutions with different types of surfactants and the production of graphene films by spray or drop coating. J. Taiwan Inst. Chem. Eng. 43(1), 140–146 (2012)
Sarsam, W.S.; Amiri, A.; Kazi, S.N.; Badarudin, A.: Stability and thermophysical properties of non-covalently functionalized graphene nanoplatelets nanofluids. Energy Convers. Manag. 116, 101–111 (2016)
Mohammadi, A.; Barikani, M.; Barmar, M.: Effect of surface modification of Fe3O4 nanoparticles on thermal and mechanical properties of magnetic polyurethane elastomer nanocomposites. J. Mater. Sci. 48(21), 7493–7502 (2013)
McAllister, M.J., et al.: Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 19(18), 4396–4404 (2007)
Stankovich, S., et al.: Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7), 1558–1565 (2007)
Zhu, G.; Liu, Y.; Sweeney, S.; Chen, S.: Functionalization and grafting of nanoparticle surfaces. J. Encycl. Interfacial Chem. pp. 711–724 (2018)
Ngouangna, E.N.; Zaidi Jaafar, M.; Norddin, M.; Agi, A.; Oseh, J.O.; Mamah, S.: Surface modification of nanoparticles to improve oil recovery mechanisms: a critical review of the methods, influencing parameters, advances and prospects. J. Mol. Liq. 360, 119502 (2022). https://doi.org/10.1016/j.molliq.2022.119502
Rao, R.A.K.; Singh, S.; Singh, B.R.; Khan, W.; Naqvi, A.H.: Synthesis and characterization of surface modified graphene–zirconium oxide nanocomposite and its possible use for the removal of chlorophenol from aqueous solution. J. Environ. Chem. Eng. 2(1), 199–210 (2014). https://doi.org/10.1016/j.jece.2013.12.011
Qamar, S.; Yasin, S.; Ramzan, N.; Iqbal, T.; Akhtar, M.N.: Preparation of stable dispersion of graphene using copolymers: dispersity and aromaticity analysis. Soft Mater. 17(2), 190–202 (2019). https://doi.org/10.1080/1539445X.2019.1583673
Uddin, M.E.; Kuila, T.; Nayak, G.C.; Kim, N.H.; Ku, B.-C.; Lee, J.H.: Effects of various surfactants on the dispersion stability and electrical conductivity of surface modified graphene. J. Alloys Compd. 562, 134–142 (2013)
Paek, H.-J., et al.: Modulation of the pharmacokinetics of zinc oxide nanoparticles and their fates in vivo. Nanoscale 5(23), 11416–11427 (2013). https://doi.org/10.1039/C3NR02140H
Zhang, C.; Uchikoshi, T.; Liu, L.; Kikuchi, M.; Ichinose, I.: Effect of surface modification with TiO2 coating on improving filtration efficiency of whisker-hydroxyapatite (HAp) membrane. Coatings 10(7), 670 (2020)
Park, S., et al.: Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 9(4), 1593–1597 (2009). https://doi.org/10.1021/nl803798y
Hadadian, M.; Goharshadi, E.K.; Fard, M.M.; Ahmadzadeh, H.: Synergistic effect of graphene nanosheets and zinc oxide nanoparticles for effective adsorption of Ni (II) ions from aqueous solutions. Appl. Phys. A 124(3), 239 (2018). https://doi.org/10.1007/s00339-018-1664-8
Sham, A.Y.W.; Notley, S.M.: Layer-by-layer assembly of thin films containing exfoliated pristine graphene nanosheets and polyethyleneimine. Langmuir 30(9), 2410–2418 (2014). https://doi.org/10.1021/la404745b
Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M.: Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 44(4), 1260–1266 (2010)
Kamiya, H., et al.: Characteristics and behavior of nanoparticles and its dispersion systems. In: Nanoparticle Technology Handbook, pp. 113–176. Elsevier, The Netherlands (2008)
Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G.: Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. (2008). https://doi.org/10.1038/nnano.2007.451
Dreyer, D.R.; Murali, S.; Zhu, Y.; Ruoff, R.S.; Bielawski, C.W.: Reduction of graphite oxide using alcohols. J. Mater. Chem. 21(10), 3443–3447 (2011)
Zhu, J.: New solutions to a new problem. Nat. Nanotechnol. 3(9), 528–529 (2008)
Marcano, D.C., et al.: Improved synthesis of graphene oxide. ACS Nano 4(8), 4806–4814 (2010)
Liu, J.; Tang, J.; Gooding, J.J.: Strategies for chemical modification of graphene and applications of chemically modified graphene. J. Mater. Chem. 22(25), 12435–12452 (2012)
Kumar, A.; Dixit, C.K.: Methods for characterization of nanoparticles. In: Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids, pp. 43–58. Elsevier, The Netherlands (2017)
Chua, C.K.; Pumera, M.: Covalent chemistry on graphene. Chem. Soc. Rev. 42(8), 3222–3233 (2013)
Urade, A.:Graphene functionalization: covalent versus non-covalent. AZoNano.com. https://www.azonano.com/article.aspx?ArticleID=6097 (2022). Accessed 30 Nov 2022
Park, J.; Yan, M.: Covalent functionalization of graphene with reactive intermediates. Acc. Chem. Res. 46(1), 181–189 (2013). https://doi.org/10.1021/ar300172h
Jiang, K.G.; Li, X.; Luckham, P.F.: Study of graphene oxide to stabilize shale in water-based drilling fluids. Colloids Surf., A 606, 125457 (2020). https://doi.org/10.1016/j.colsurfa.2020.125457
Georgakilas, V., et al.: Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 116(9), 5464–5519 (2016). https://doi.org/10.1021/acs.chemrev.5b00620
Choi, E.-Y., et al.: Noncovalent functionalization of graphene with end-functional polymers. J. Mater. Chem. 20(10), 1907–1912 (2010)
Lee, D.-W.; Kim, T.; Lee, M.: An amphiphilic pyrene sheet for selective functionalization of graphene. Chem. Commun. 47(29), 8259–8261 (2011)
Song, P.; Xu, Z.; Wu, Y.; Cheng, Q.; Guo, Q.; Wang, H.: Super-tough artificial nacre based on graphene oxide via synergistic interface interactions of π-π stacking and hydrogen bonding. Carbon 111, 807–812 (2017)
Sheng, Z.-H.; Gao, H.-L.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H.: Synthesis of boron doped graphene for oxygen reduction reaction in fuel cells. J. Mater. Chem. 22(2), 390–395 (2012)
Stephan, O., et al.: Doping graphitic and carbon nanotube structures with boron and nitrogen. Science 266(5191), 1683–1685 (1994)
Wang, X., et al.: N-doping of graphene through electrothermal reactions with ammonia. Science 324(5928), 768–771 (2009)
Wu, Z.-S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.-M.: Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 5(7), 5463–5471 (2011)
Panchakarla, L.S., et al.: Synthesis, structure, and properties of boron-and nitrogen-doped graphene. Adv. Mater. 21(46), 4726–4730 (2009)
Rao, C.N.R.; Gopalakrishnan, K.; Govindaraj, A.: Synthesis, properties and applications of graphene doped with boron, nitrogen and other elements. Nano Today 9(3), 324–343 (2014)
Tang, Y.-B., et al.: Tunable band gaps and p-type transport properties of boron-doped graphenes by controllable ion doping using reactive microwave plasma. ACS Nano 6(3), 1970–1978 (2012)
Zhao, L., et al.: Visualizing individual nitrogen dopants in monolayer graphene. Science 333(6045), 999–1003 (2011)
Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P.: Heteroatom-doped graphene materials: syntheses, properties and applications. Chem. Soc. Rev. 43(20), 7067–7098 (2014)
Yeom, D.-Y., et al.: High-concentration boron doping of graphene nanoplatelets by simple thermal annealing and their supercapacitive properties. Sci. Rep. (2015). https://doi.org/10.1038/srep09817
Le Ba, T.; Mahian, O.; Wongwises, S.; Szilágyi, I.M.: Review on the recent progress in the preparation and stability of graphene-based nanofluids. J. Therm. Anal. Calorim. 142(3), 1145–1172 (2020). https://doi.org/10.1007/s10973-020-09365-9
Chowdhury, I.; Hou, W.-C.; Goodwin, D.; Henderson, M.; Zepp, R.G.; Bouchard, D.: Sunlight affects aggregation and deposition of graphene oxide in the aquatic environment. Water Res. 78, 37–46 (2015)
Li, Z.; Jiang, H.; Wang, X.; Wang, C.; Wei, X.: Effect of pH on adsorption of tetracycline antibiotics on graphene oxide. Int. J. Environ. Res. Public Health 20(3), 2448 (2023). https://doi.org/10.3390/ijerph20032448
Andrade-Guel, M., et al.: Surface modification of graphene nanoplatelets by organic acids and ultrasonic radiation for enhance uremic toxins adsorption. Materials (2019). https://doi.org/10.3390/ma12050715
Alhourani, A.; Førde, J.-L.; Eichacker, L.A.; Herfindal, L.; Hagland, H.R.: Improved pH-responsive release of phenformin from low-defect graphene compared to graphene oxide. ACS Omega 6(38), 24619–24629 (2021). https://doi.org/10.1021/acsomega.1c03283
Cabello-Alvarado, C., et al.: Graphene nanoplatelets modified with amino-groups by ultrasonic radiation of variable frequency for potential adsorption of uremic toxins. Nanomaterials (2019). https://doi.org/10.3390/nano9091261
Mancillas-Salas, S.; Reynosa-Martinez, A.C.; Barroso-Flores, J.; Lopez-Honorato, E.: Impact of secondary salts, temperature, and pH on the colloidal stability of graphene oxide in water. Nanoscale Adv. 4(11), 2435–2443 (2022). https://doi.org/10.1039/D2NA00070A
SWACO, M.: MI SWACO joins Rice University in Nanotech Research Program. Press Release Oct. vol. 28 (2009)
Murtaza, M., et al.: Performance evaluation of iron oxide and graphite nanoparticles in water-based drilling muds at hpht conditions. Presented at the Middle East Oil, Gas and Geosciences Show, OnePetro (2023). https://doi.org/10.2118/213963-MS
Blkoor, S.O., et al.: Enhanced cutting transport performance of water-based drilling muds using polyethylene glycol/nanosilica composites modified by sodium dodecyl sulphate. Geoenergy Sci. Eng. 230, 212276 (2023)
Oseh, J.O., et al.: Rheological and filtration control performance of water-based drilling muds at different temperatures and salt contaminants using surfactant-assisted novel nanohydroxyapatite. Geoenergy Sci. Eng. 228, 211994 (2023). https://doi.org/10.1016/j.geoen.2023.211994
Ahmad, M.; Ali, I.; Bins Safri, M.S.; Bin Mohammad Faiz, M.A.I.; Zamir, A.: Synergetic effects of graphene nanoplatelets/tapioca starch on water-based drilling muds: enhancements in rheological and filtration characteristics. Polymers 13(16), 2655 (2021). https://doi.org/10.3390/polym13162655
Saleh, T. A.; Al-Arfaj, M. K.; Rana, A. A.: Modified graphene shale inhibitors, US11230657B2 [Online]. Available: https://patents.google.com/patent/US11230657B2/en (2022). Accessed 19 Sept 2023
Xuan, Y.; Jiang, G.; Li, Y.: Nanographite oxide as ultrastrong fluid-loss-control additive in water-based drilling fluids. J. Dispers. Sci. Technol. 35(10), 1386–1392 (2014). https://doi.org/10.1080/01932691.2013.858350
Neuberger, N.; Adidharma, H.; Fan, M.: Graphene: a review of applications in the petroleum industry. J. Pet. Sci. Eng. 167, 152–159 (2018). https://doi.org/10.1016/j.petrol.2018.04.016
Cripps, S. J., et al.: Disposal of oil-based cuttings. Rogalandsforskning, [Online]. Available: https://norceresearch.brage.unit.no/norceresearch-xmlui/handle/11250/2712444 (1998). Accessed 20 Apr 2022
Gentzis, T.; Deisman, N.; Chalaturnyk, R.J.: Effect of drilling fluids on coal permeability: Impact on horizontal wellbore stability. Int. J. Coal Geol. 78(3), 177–191 (2009)
Marsh, R.: A database of archived drilling records of the drill cuttings piles at the north west Hutton oil platform. Mar. Pollut. Bull. 46(5), 587–593 (2003)
Bridges, S.; Robinson, L.: A practical handbook for drilling fluids processing. Gulf Professional Publishing, Houston (2020)
Guo, B.; Liu, G.: Applied drilling circulation systems: hydraulics, calculations and models. Gulf Professional Publishing, Houston (2011)
Gong, W., et al.: 2021 Synthesis, characterization, and performance evaluation of starch-based degradable temporary plugging agent for environmentally friendly drilling fluid. Lithosphere 1, 6679756 (2021)
Sajjadian, M.; Sajjadian, V.A.; Rashidi, A.: Experimental evaluation of nanomaterials to improve drilling fluid properties of water-based muds HP/HT applications. J. Pet. Sci. Eng. 190, 107006 (2020)
Caenn, R.; Darley, H.C.; Gray, G.R.: Composition and properties of drilling and completion fluids. Gulf professional publishing, Houston (2011)
Aftab, A.; Ismail, A.R.; Ibupoto, Z.H.: Enhancing the rheological properties and shale inhibition behavior of water-based mud using nanosilica, multi-walled carbon nanotube, and graphene nanoplatelet. Egypt. J. Pet. 26(2), 291–299 (2017). https://doi.org/10.1016/j.ejpe.2016.05.004
Razali, S.Z., et al.: Effects of morphology and graphitization of carbon nanomaterials on the rheology, emulsion stability, and filtration control ability of drilling fluids. J. Mater. Res. Technol. 21, 2891–2905 (2022)
Putra, P. H. M.; Jan, B. M.; Patah, M. F. A.; Junaidi, M. U. M.: Effect of temperature and concentration of industrial waste graphene on rheological properties of water based mud. In: IOP Conference Series: Materials Science and Engineering, IOP Publishing, pp. 012120 (2020)
Caenn, R.; Darley, H.C.H.; Gray, G.R.: Composition and properties of drilling and completion fluids, 7th edn. Gulf Professional Publishing, United Kingdom (2017)
Abduo, M.I.; Dahab, A.S.; Abuseda, H.; AbdulAziz, A.M.; Elhossieny, M.S.: Comparative study of using water-based mud containing multiwall carbon nanotubes versus oil-based mud in HPHT fields. Egypt. J. Pet. 25(4), 459–464 (2016). https://doi.org/10.1016/j.ejpe.2015.10.008
Elkatatny, S.; Tariq, Z.; Mahmoud, M.: Real time prediction of drilling fluid rheological properties using Artificial Neural Networks visible mathematical model (white box). J. Pet. Sci. Eng. 146, 1202–1210 (2016)
Bhat, S.A.; Charoo, M.S.: Rheological characteristics of coconut grease with graphene nanoplatelets. Biomass Convers. Biorefinery 13, 1–8 (2022)
Madkour, T.M.; Fadl, S.; Dardir, M.M.; Mekewi, M.A.: High performance nature of biodegradable polymeric nanocomposites for oil-well drilling fluids. Egypt. J. Pet. 25(2), 281–291 (2016)
Degeare, J.; Haughton, D.; Mcgurk, M.: 5-pipe sticking. Guide Oilw. Fish. Oper. Gulf Prof. Publ. Burlingt., pp. 12–22 (2003)
Courteille, J. M.; C. Zurdo, C.: A new approach to differential sticking. In: SPE Annual Technical Conference and Exhibition, OnePetro (1985)
Putra, P.H.M.; Jan, B.M.; Patah, M.F.A.; Junaidi, M.U.M.: Effect of temperature and concentration of industrial waste graphene on rheological properties of water based mud. IOP Conf. Ser. Mater. Sci. Eng. 778(1), 012120 (2020). https://doi.org/10.1088/1757-899X/778/1/012120
Gudarzifar, H.; Sabbaghi, S.; Rezvani, A.; Saboori, R.: Experimental investigation of rheological & filtration properties and thermal conductivity of water-based drilling fluid enhanced. Powder Technol. 368, 323–341 (2020)
Collins R.: Drilling Fluids: It’s About the Chemistry. In: Trenchless Technology. https://trenchlesstechnology.com/directional-drilling-fluids-chemistry/ (2014). Accessed 08 Apr 2023
Sönmez, A.; Kök, M.V.; Özel, R.: Performance analysis of drilling fluid liquid lubricants. J. Pet. Sci. Eng. 108, 64–73 (2013)
Maidla, E.E.; Wojtanowicz, A.K.: Laboratory study of borehole friction factor with a dynamic-filtration apparatus. SPE Drill. Eng. 5(03), 247–255 (1990)
Livescu, S.; Craig, S.; Watkins, T.: Smaller coiled tubing diameter achievable by the use of lubricants. In: International Petroleum Technology Conference, OnePetro (2014)
Kim, J.W.; Nam, J.; Jeon, J.; Lee, S.W.: A study on machining performances of micro-drilling of multi-directional carbon fiber reinforced plastic (MD-CFRP) based on nano-solid dry lubrication using graphene nanoplatelets. Materials 14(3), 685 (2021)
Ibrahim, A.M.M.; Li, W.; Xiao, H.; Zeng, Z.; Ren, Y.; Alsoufi, M.S.: Energy conservation and environmental sustainability during grinding operation of Ti–6Al–4V alloys via eco-friendly oil/graphene nano additive and minimum quantity lubrication. Tribol. Int. 150, 106387 (2020)
Amanullah, M.; Al-Tahini, A. M.: Nano-technology-its significance in smart fluid development for oil and gas field application. In: SPE Saudi Arabia Section Technical Symposium, OnePetro (2009)
Zoveidavianpoor, M.; Samsuri, A.: The use of nano-sized Tapioca starch as a natural water-soluble polymer for filtration control in water-based drilling muds. J. Nat. Gas Sci. Eng. 34, 832–840 (2016)
Salih, A. H.; Elshehabi, T. A.; Bilgesu, H. I.: Impact of nanomaterials on the rheological and filtration properties of water-based drilling fluids. In: SPE Eastern Regional Meeting, OnePetro (2016)
William, J.K.M.; Ponmani, S.; Samuel, R.; Nagarajan, R.; Sangwai, J.S.: Effect of CuO and ZnO nanofluids in xanthan gum on thermal, electrical and high pressure rheology of water-based drilling fluids. J. Pet. Sci. Eng. 117, 15–27 (2014)
Cheraghian, G.: Effects of nanoparticles on wettability: a review on applications of nanotechnology in the enhanced Oil recovery. Int. J. Nano Dimens. 6(5), 443–452 (2015)
Saffari, H.R.M.; Soltani, R.; Alaei, M.; Soleymani, M.: Tribological properties of water-based drilling fluids with borate nanoparticles as lubricant additives. J. Pet. Sci. Eng. 171, 253–259 (2018)
Sudharsan, J.; Khare, S.K.: Role of nanocomposite additives in well bore stability during shale formation drilling with water based mud–a comprehensive review. Mater. Today: Proc. 62, 6412–6419 (2022)
Saleh, T.A.: Advanced trends of shale inhibitors for enhanced properties of water-based drilling fluid. Upstream Oil Gas Technol. 8, 100069 (2022)
Growcock, F. B.; Kaageson-Loe, N.; Friedheim, J.; Sanders, M. W.; Bruton, J.: Wellbore stability, stabilization and strengthening. In: Offshore mediterranean conference and exhibition, OMC, pp. OMC-2009. (2009) Accessed 18 Oct 2023
Shahri, M. P.: Quantification of wellbore strengthening mechanisms: comprehensive parametric analysis. In: SPE Annual Technical Conference and Exhibition? SPE, pp. D031S033R003. (2015) Accessed 18 Oct 2023
Aramendiz, J.; Imqam, A.: Water-based drilling fluid formulation using silica and graphene nanoparticles for unconventional shale applications. J. Pet. Sci. Eng. 179, 742–749 (2019). https://doi.org/10.1016/j.petrol.2019.04.085
Saleh, T.A.; Ibrahim, M.A.: Advances in functionalized Nanoparticles based drilling inhibitors for oil production. Energy Rep. 5, 1293–1304 (2019)
Muhammed, N.S.; Olayiwola, T.; Elkatatny, S.: A review on clay chemistry, characterization and shale inhibitors for water-based drilling fluids. J. Pet. Sci. Eng. 206, 109043 (2021)
Rana, A.; Saleh, T. A.; Arfaj, M. K.: Improvement in rheological features, fluid loss and swelling inhibition of water-based drilling mud by using surfactant-modified graphene. In: Abu Dhabi International Petroleum Exhibition & Conference, OnePetro (2019)
Rana, A.; Murtaza, M.; Saleh, T.A.; Kamal, M.S.; Mahmoud, M.: Green nanocomposite synthesis and application: Electrochemically exfoliated graphene-modified biopolymer as an effective clay swelling inhibitor for water-based drilling muds. Geoenergy Sci. Eng. 231, 212394 (2023)
Ikram, R.; Mohamed Jan, B.; Vejpravova, J.; Choudhary, M.I.; Zaman Chowdhury, Z.: Recent advances of graphene-derived nanocomposites in water-based drilling fluids. Nanomaterials 10(10), 2004 (2020)
Lv, K., et al.: Study of Janus amphiphilic graphene oxide as a high-performance shale inhibitor and its inhibition mechanism. Front. Chem. 8, 201 (2020)
Zhu, H.; Li, D.; Zhao, X.; Pang, S.; An, Y.: A novel choline chloride/graphene composite as a shale inhibitor for drilling fluid and the interaction mechanism. RSC Adv. 12(47), 30328–30334 (2022). https://doi.org/10.1039/D2RA05085D
Rana, A.; Arfaj, M.K.; Yami, A.S.; Saleh, T.A.: Cetyltrimethylammonium modified graphene as a clean swelling inhibitor in water-based oil-well drilling mud. J. Environ. Chem. Eng. 8(4), 103802 (2020). https://doi.org/10.1016/j.jece.2020.103802
Saleh, T.A.; Rana, A.; Arfaj, M.K.: Graphene grafted with polyethyleneimine for enhanced shale inhibition in the water-based drilling fluid. Environ. Nanotechnol., Monit. Manag. 14, 100348 (2020). https://doi.org/10.1016/j.enmm.2020.100348
Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J.: The chemistry of graphene. J. Mater. Chem. 20(12), 2277–2289 (2010). https://doi.org/10.1039/B920539J
Yuxiu, A.; Guancheng, J.; Yourong, Q.; Xianbin, H.; He, S.: High-performance shale plugging agent based on chemically modified graphene. J. Nat. Gas Sci. Eng. 32, 347–355 (2016). https://doi.org/10.1016/j.jngse.2016.04.048
Tian, X.; Song, N.; Yang, G.; Zhou, C.; Zhang, S.; Zhang, P.: Organic-sulfonate functionalized graphene as a high temperature lubricant for efficient antifriction and antiwear in water based drilling fluid. Tribol. Lett. 70(2), 1–8 (2022)
Mondal, J., et al.: Functionalization of titanium alloy surface by graphene nanoplatelets and metal oxides: corrosion inhibition. J. Nanosci. Nanotechnol. 15(9), 6533–6540 (2015). https://doi.org/10.1166/jnn.2015.10775
Alkhamis, M.; Imqam, A.: New cement formulations utilizing graphene nano platelets to improve cement properties and long-term reliability in oil wells. Presented at the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, OnePetro (2018). https://doi.org/10.2118/192342-MS
Sun, X.; Wu, Q.; Zhang, J.; Qing, Y.; Wu, Y.; Lee, S.: Rheology, curing temperature and mechanical performance of oil well cement: Combined effect of cellulose nanofibers and graphene nano-platelets. Mater. Des. 114, 92–101 (2017). https://doi.org/10.1016/j.matdes.2016.10.050
Du, H.; Pang, S.D.: Dispersion and stability of graphene nanoplatelet in water and its influence on cement composites. Constr. Build. Mater. 167, 403–413 (2018). https://doi.org/10.1016/j.conbuildmat.2018.02.046
Malhotra, N., et al.: Toxicity studies on graphene-based nanomaterials in aquatic organisms: current understanding. Molecules 25(16), 3618 (2020). https://doi.org/10.3390/molecules25163618
Ding, X.; Pu, Y.; Tang, M.; Zhang, T.: Environmental and health effects of graphene-family nanomaterials: potential release pathways, transformation, environmental fate and health risks. Nano Today 42, 101379 (2022). https://doi.org/10.1016/j.nantod.2022.101379
Khan M.: The environmental impact of graphene nanomaterials. AZoNano.com. https://www.azonano.com/article.aspx?ArticleID=6223 (2011). Accessed 13 Sep 2023
Melo de Lima, L.R.; Dias, A.C.; Trindade, T.; Oliveira, J.M.: A comparative life cycle assessment of graphene nanoplatelets- and glass fibre-reinforced poly(propylene) composites for automotive applications. Sci. Total. Environ. 871, 162140 (2023). https://doi.org/10.1016/j.scitotenv.2023.162140
Beloin-Saint-Pierre, D.; Hischier, R.: Towards a more environmentally sustainable production of graphene-based materials: Building on current knowledge to offer recommendations. Int. J. Life Cycle Assess. 26, 327–343 (2021)
Ikram, R.; Jan, B.M.; Ahmad, W.; Sidek, A.; Khan, M.; Kenanakis, G.: Rheological investigation of welding waste-derived graphene oxide in water-based drilling fluids. Materials 15(22), 8266 (2022)
Asim, N., et al.: Application of graphene-based materials in developing sustainable infrastructure: an overview. Compos. Part B Eng. 245, 110188 (2022). https://doi.org/10.1016/j.compositesb.2022.110188
Lovén, K., et al.: Emissions and exposures of graphene nanomaterials, titanium dioxide nanofibers, and nanoparticles during down-stream industrial handling. J. Expo. Sci. Environ. Epidemiol. 31(4), 736–752 (2021)
Tavares, L.; Sousa, L.R.; da Silva, S.M.; Lima, P.S.; Oliveira, J.M.: Effect of Incorporation of graphene nanoplatelets on physicochemical, thermal, rheological, and mechanical properties of biobased and biodegradable blends. Polymers 15(17), 3622 (2023)
Arvidsson, R., et al.: Environmental assessment of emerging technologies: recommendations for prospective LCA. J. Ind. Ecol. 22(6), 1286–1294 (2018)
Devon, J.; Hacking, E.; Wilson, K.; Craciun, M.F.; Vinai, R.: Effects of graphene nanoplatelets inclusion on microstructure and mechanical properties of alkali activated binders. CEMENT 13, 100080 (2023)
Chieng, B.W.; Ibrahim, N.A.; Wan Yunus, W.M.Z.; Hussein, M.Z.; Then, Y.Y.; Loo, Y.Y.: Effects of graphene nanoplatelets and reduced graphene oxide on poly (lactic acid) and plasticized poly (lactic acid): a comparative study. Polymers 6(8), 2232–2246 (2014)
Bøggild, P.: The war on fake graphene. Nature 562(7728), 502–503 (2018). https://doi.org/10.1038/d41586-018-06939-4
Rebecca, P.N.; Durgalakshmi, D.; Balakumar, S.; Rakkesh, R.A.: Biomass-derived graphene-based nanocomposites: a futuristic material for biomedical applications. ChemistrySelect 7(5), e202104013 (2022). https://doi.org/10.1002/slct.202104013
Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z.: A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 373, 104–121 (2019). https://doi.org/10.1016/j.cej.2019.05.034
Wu, W.; Yu, B.: Corn flour nano-graphene prepared by the hummers redox method. ACS Omega 5(46), 30252–30256 (2020). https://doi.org/10.1021/acsomega.0c04722
Zhang, H., et al.: A chemical blowing strategy to fabricate biomass-derived carbon-aerogels with graphene-like nanosheet structures for high-performance supercapacitors. Chemsuschem 12(11), 2462–2470 (2019). https://doi.org/10.1002/cssc.201900267
Mondal, D., et al.: Deep eutectic solvent promoted one step sustainable conversion of fresh seaweed biomass to functionalized graphene as a potential electrocatalyst. Green Chem. 18(9), 2819–2826 (2016)
Cheng, J.; Wan, W.; Chen, X.; Zheng, Q.: Preparation and structural characterization of graphene by rice husk. Trans. Chin. Soc. Agric. Eng. 31(12), 288–294 (2015)
Kumar, M.; Sachdeva, A.; Garg, R.K.; Singh, S.: Synthesis and characterization of graphene prepared from rice husk by a simple microwave process. Nano Hybrids Compos. 29, 74–83 (2020). https://doi.org/10.4028/www.scientific.net/NHC.29.74
Béguerie, T.; Weiss-Hortala, E.; Nzihou, A.: Calcium as an innovative and effective catalyst for the synthesis of graphene-like materials from cellulose. Sci. Rep. (2022). https://doi.org/10.1038/s41598-022-25943-3
Baqiya, M.A., et al.: Structural study on graphene-based particles prepared from old coconut shell by acid–assisted mechanical exfoliation. Adv. Powder Technol. 31(5), 2072–2078 (2020). https://doi.org/10.1016/j.apt.2020.02.039
Azam, M.A.; Mudtalib, N.E.S.A.A.; Seman, R.N.A.R.: Synthesis of graphene nanoplatelets from palm-based waste chicken frying oil carbon feedstock by using catalytic chemical vapour deposition. Mater. Today Commun. 15, 81–87 (2018). https://doi.org/10.1016/j.mtcomm.2018.02.019
Ristiani, D., et al.: Mesostructural study on graphenic-based carbon prepared from coconut shells by heat treatment and liquid exfoliation. Heliyon 8(3), e09032 (2022). https://doi.org/10.1016/j.heliyon.2022.e09032
Husin, H.; Elraies, K.A.; Choi, H.J.; Aman, Z.: Influence of graphene nanoplatelet and silver nanoparticle on the rheological properties of waterbased Mud. Appl. Sci. 8(8), 1386 (2018). https://doi.org/10.3390/app8081386
Rafieefar, A.; Sharif, F.; Hashemi, A.; Bazargan, A.M.: Rheological behavior and filtration of water-based drilling fluids containing graphene oxide: experimental measurement, mechanistic understanding, and modeling. ACS Omega 6(44), 29905–29920 (2021). https://doi.org/10.1021/acsomega.1c04398
Kusrini, E.; Oktavianto, F.; Usman, A.; Mawarni, D.P.; Alhamid, M.I.: Synthesis, characterization, and performance of graphene oxide and phosphorylated graphene oxide as additive in water-based drilling fluids. Appl. Surf. Sci. 506, 145005 (2020). https://doi.org/10.1016/j.apsusc.2019.145005
Perumalsamy, J.; Gupta, P.; Sangwai, J.S.: Performance evaluation of esters and graphene nanoparticles as an additives on the rheological and lubrication properties of water-based drilling mud. J. Pet. Sci. Eng. 204, 108680 (2021). https://doi.org/10.1016/j.petrol.2021.108680
Mohamed, A.; Tirth, V.; Kamel, B.M.: Tribological characterization and rheology of hybrid calcium grease with graphene nanosheets and multi-walled carbon nanotubes as additives. J. Mater. Res. Technol. 9(3), 6178–6185 (2020)
Medhi, S.; Chowdhury, S.; Bhatt, N.; Gupta, D.K.; Rana, S.; Sangwai, J.S.: Analysis of high performing graphene oxide nanosheets based non-damaging drilling fluids through rheological measurements and CFD studies. Powder Technol. 377, 379–395 (2021). https://doi.org/10.1016/j.powtec.2020.08.053
Zheng, Y.; Asif, A.; Amiri, A.; Polycarpou, A.A.: Graphene-based aqueous drilling muds as efficient, durable, and environmentally friendly alternatives for oil-based muds. ACS Appl. Nano Mater. 4(2), 1243–1251 (2021). https://doi.org/10.1021/acsanm.0c02852
Movahedi, H.; Jamshidi, S.; Hajipour, M.: New insight into the filtration control of drilling fluids using a graphene-based nanocomposite under static and dynamic conditions. ACS Sustain. Chem. Eng. 9(38), 12844–12857 (2021). https://doi.org/10.1021/acssuschemeng.1c03563
Saleh, A.; Saleh, T. A.; Arfaj, M. K.:Improvement in rheological features, fluid loss and swelling inhibition of water-based drilling mud by using surfactant-modified graphene. In: Abu Dhabi International Petroleum Exhibition & Conference, OnePetro (2019)
Batiha, M.A.; Dardir, M.M.; Abuseda, H.; Negm, N.A.; Ahmed, H.E.: Improving the performance of water-based drilling fluid using amino acid-modified graphene oxide nanocomposite as a promising additive. Egypt. J. Chem. 64(4), 1799–1806 (2021)
Rana, A.; Arfaj, M.K.; Saleh, T.A.: Graphene grafted with glucopyranose as a shale swelling inhibitor in water-based drilling mud. Appl. Clay Sci. 199, 105806 (2020)
Zhang, H.; Yao, J.; Zhang, S.; Chen, H.: Carboxylized graphene oxide nanosheet for shale plugging at high temperature. Appl. Surf. Sci. 558, 149901 (2021)
Ma, J.; Pang, S.; Zhang, Z.; Xia, B.; An, Y.: Experimental study on the polymer/graphene oxide composite as a fluid loss agent for water-based drilling fluids. ACS Omega 6(14), 9750–9763 (2021)
Pumera, M.: Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 39(11), 4146–4157 (2010). https://doi.org/10.1039/C002690P
Acknowledgements
The authors would like to acknowledge the financial support from the Ministry of Higher Education (MOHE), Malaysia, under the Fundamental Research Grant Scheme (FRGS) via reference number FRGS/PV/2022/03075 and Universiti Teknologi Malaysia for the funding under UTM Fundamental Research (UTMFR) (Q.J130000.21A2.06E09; QJI30000.3046.04M20) and the Fundamental Research Grant Scheme (FRGS) (Ref: FRGS/1/2023/TK09/UTM/02/13; FRGS/1/2021/TKO/UTM/02/7). Engr. Dr. Jeffrey Onuoma Oseh is a researcher at UTM under the postdoctoral fellowship scheme for the project “Evaluation of Modified Hydroxyapatite Nanoparticles for Rheological and Filtration Properties Modification in Field-Applicable Drilling Muds”.
Author information
Authors and Affiliations
Contributions
MNY, MNAMN, and II were involved in the conceptualization; MNY, JOO, and ENN contributed to the methodology; MNY, FY, NR, and SQAM assisted in the formal analysis; MNY and JOO contributed to the investigation; MNAMN, AAAR, and ARR contributed to the resources; MNY, JOO, and RY curated the data; MNY, AA, and JOO assisted in writing—original draft preparation; MNY, JOO, and AAAR were involved in writing—review and editing; MNAMN, AAAR, and ARR acquired the funding.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yahya, M.N., Norddin, M.N.A.M., Ismail, I. et al. Graphene Nanoplatelet Surface Modification for Rheological Properties Enhancement in Drilling Fluid Operations: A Review. Arab J Sci Eng 49, 7751–7781 (2024). https://doi.org/10.1007/s13369-023-08458-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08458-5