Abstract
Quercetin-7-O-β-D-glucoside (1) and patuletin-7-O-β-D-glucoside (2) isolated from Achillea biebersteinii and different extracts of aerial parts of the plant were investigated for antioxidant and anticholinesterase effects. The ethyl acetate extract possessed the highest inhibitory activity against acetylcholinesterase (67.2 ± 1.32 %), whereas it showed lower inhibitory activity against butyrylcholinesterase (50.3 ± 1.03 %) as compared with neostigmine (100 %), at 200 μg/mL. Compound 2 exhibited the strongest inhibition to acetylcholinesterase and compound 1 did so to butyrylcholinesterase with IC50 values of 1.77 and 2.24 µM, respectively. Additionally, compound 1 showed the strongest antioxidant capacity. Docking simulations revealed that compounds 1 and 2 targeted both the catalytic and the peripheral active sites of acetylcholinesterase and butyrylcholinesterase with many hydrogen bond interactions. The ethyl acetate extract has the richest phenolic content (205.7 GAE/mg ext), and the best antioxidant capacity results were observed with it among the tested extracts. The mean amount of total flavonoids was 3.39 % (calculated as rutin).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acetylcholine is the chemical transmitter for nerves of the parasympathetic, somatic, preganglionic sympathetic, and parts of the central nervous system. After acetylcholine interacts with the cholinergic receptor, it is rapidly hydrolyzed by the enzyme acetylcholinesterase (AChE). Neurodegenerative diseases have become a major age-associated health problem, especially in industrialized countries, in which the proportion of the elderly population is rising (Uc and Rizzo, 2008) and the prevalence of Alzheimer’s disease (AD) is considered as the most common form of dementia. The main symptoms associated with AD include a decline in cognitive function, primary memory loss and in the later stages of the disease language deficits, depression, agitation, mood disturbances, and psychosis (Houghton and Howes, 2005). Since the discovery of cholinergic deficits in patients suffering from AD, AChE has been the main target for its treatment (Hodges, 2006). Acetylcholine has a functional key role in cognitive functions including learning and memory, arousal, and attentional processes in the brain. AChE is the key enzyme in the breakdown of acetylcholine; therefore, the inhibition of AChE is considered one of the treatment strategies against several neurological disorders such as AD, senile dementia, ataxia, and myasthenia gravis (Hoozesmans et al., 2006). However, current drugs are not able to stop AD, and can only slow the progress of these diseases by symptomatic treatment. Consequently, there is still a need to discover new drug candidates, and plants are attractive sources for the discovery of these new candidates.
The genus Achillea L. (Asteraceae) comprises 85 species worldwide and 50 species in the flora of Turkey with more than 50 % endemism (Huber-Morath, 1975). They have rich sesquiterpene lactone, lignan, and flavonoid content, (Ulubelen et al., 1990; Mahmoud et al., 2012) and possess antiemetic, antiviral, antiallergic, and antiirritant effects (Goren et al., 1988; Orth et al., 2000; Pecetti et al., 2012). Many studies on antioxidant and anticholinesterase activities have been conducted on Achilllea species (Baris et al., 2011; Keser et al., 2011; Vitalini et al., 2011). Achillea biebersteinii is called ‘Sarı çiçek’ in Turkey, and is known to be used in folk remedies for various purposes such as the treatment of abdominal pain, particularly stomachache (Sezik et al., 2001).
Oxidative stress disorders are caused by free radicals and related species. They are mostly derived from oxygen (reactive oxygen species/ROS) and nitrogen (reactive nitrogen species/RNS), and are associated with many acute and chronic diseases such as inflammation and neurodegenerative conditions including AD (Hoozesmans et al., 2006).
In this context, we decided to investigate the antioxidant, AChE and butyrylcholinesterase (BuChE) inhibitory activities of the petroleum ether, chloroform, ethyl acetate, n-butanol, and water extracts from A. biebersteinii with their total flavonoid and phenolic contents. Additionally, we isolated major secondary metabolites and intended to fulfill their antioxidant capacity, molecular docking, and in vitro AChE and BuChE inhibitory effects.
Materials and methods
General
1H and 13C NMR spectra were recorded on a Varian Mercury Plus 400 MHz for proton and 100 MHz for carbon by using TMS as the internal standard. The solvent was CD3OD. Absorption data for the antioxidant activity were measured using Thermo Scientific Multiskan GO microplate and cuvette spectrophotometer. Silica gel 60 (0.063–0.200 mm, Merck) and Sephadex LH-20 (Fluka) were used for open column chromatographic separations. Lichroprep RP-18 (25-40 µm, Merck) material was used for vacuum liquid chromatography. TLC was carried out on pre-coated Kieselgel 60 F254 aluminum sheets (Merck), and compounds were detected under UV fluorescence and sprayed with 1 % vanillin-H2SO4 reagent, followed by heating at 105 °C. HRESIMS was performed on Agilent 1200/6210 TOF.
Rutin hydrate, riboflavin, tween 20, gallic acid monohydrate, sodium carbonate, Folin–Ciocalteu’s phenol reagent, aluminum chloride, methionine, sodium phosphate dibasic, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), nitroblue tetrazolium (NBT), the stable free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH), iron (II) chloride, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), ammonium thiocyanate, potassium persulfate, l-ascorbic acid, linoleic acid, and α-tocopherol were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). For determination of AChE and BChE inhibitory activities 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB), acetylthiocholine iodide (ATCI), and butyrylthiocholine iodide (BTCI) were purchased from Sigma-Aldrich as well.
Plant material
Aerial parts of A. biebersteinii Afan. were collected from Erzurum, between Erzurum–Cat, Dutcu Village in August 2012. The taxonomic identification of plant material was confirmed by Dr. Meryem Sengul Koseoglu, a senior plant taxonomist at the Biology Department of Atatürk University, Erzurum, Turkey. The voucher specimens of the plant have been deposited in the herbarium of Atatürk University Faculty of Science, Erzurum, Turkey (ATA 9872).
Extraction and isolation
Under shadow, dried and powdered 500 g of the aerial parts of A. biebersteinii Afan. were extracted with methanol at 40 °C (4 × 2 L). After filtration, the methanol evaporated in vacuum until it became dry (methanol extract 74.4 g, 14.9 %). The extract was then dissolved in 150 mL water and successively partitioned with petroleum ether (4 × 0.5 L), chloroform (2 × 0.5 L), ethyl acetate (5 × 0.5 L), and n-butanol (11 × 0.5 L) to obtain 15.7 g (3.1 %), 2.2 g (0.4 %), 5.9 g (1.2 %), and 30.4 g (6.1 %), respectively. The remaining water extract was 7.2 g (1.4 %).
The ethyl acetate extract was subjected to CC on silica gel using chloroform with increasing amounts of methanol (100:0 → 0:100, v/v). Fractions 10–16 (2173 mg A) were pooled after controlling with TLC. Fr. A was applied to a Sephadex LH-20 column and eluted with MeOH to give Fr. 3–14 (1770 mg A1) which was fractionated by column chromatography over reverse-phased material using MeOH-H2O mixtures (0 → 100 %) to afford Fr. 47–52 (13 mg A2). Fr. A2 was applied to a Sephadex LH-20 column and eluted with MeOH to give Fr. 9–20 (10 mg) and gave compound 1.
The n-butanol extract was subjected to a silica gel column which was eluted with a mixture of CHCl3-MeOH (100:0 → 30:70, v/v). The fractions were combined after monitoring with TLC as Fr. 12–17 (3894 mg A). Fr. A was subjected to a Sephadex LH-20 column with MeOH to give Fr. 4–15 (2830 mg A1). Fr. A1 was fractionated by column chromatography over reverse-phased material using MeOH-H2O mixtures (0 → 100 %) to yield Fr. 24–32 (125 mg A2). Fr. A2 was applied to a Sephadex LH-20 column and eluted with MeOH to give Fr. 14–34 (26 mg) and gave compound 2.
Characterization of the compounds
The structure of the isolated compounds was identified by spectral methods, such as UV, 1H NMR, 13C NMR, COSY, HMQC, HMBC, and HRESIMS.
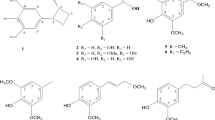
Quercetin-7-O-β-D-glucoside ( 1 )
Yellow amorphous powder. R f: 0.22 (S). Dark purple fluorescent spot by UV365 light turned to yellow-orange color with 1 % vanillin-H2SO4.
UV-spectral data: λ max (nm) (MeOH): 256, 372. 1H NMR (CD3OD, 400 MHz): δ 7.75 (1H, d, J = 2.1 Hz, H-2′), 7.65 (1H, dd, J = 8.5, 2.1 Hz, H-6′), 6.88 (1H, d, J = 8.5 Hz, H-5′), 6.73 (1H, d, J = 2.1 Hz, H-8), 6.45 (1H, d, J = 2.1 Hz, H-6), 5.10 (1H, d, J = 7.3 Hz, H-1′′), 3.93 (1H, dd, J = 12.1, 2.1 Hz, Hb-6′′), 3.72 (1H, dd, J = 12.1, 5.8 Hz, Ha-6′′), 3.60-3.30 (4H, m, H-2′′, H-3′′, H-4′′, H-5′′). 13C NMR (CD3OD, 100 MHz): δ 176.1 (C, C-4), 163.0 (C, C-7), 160.7 (C, C-5), 156.3 (C, C-2), 147.7 (C, C-9), 147.4 (C, C-4′), 144.9 (C, C-3′), 136.2 (C, C-3), 122.4 (C, C-1′), 120.5 (CH, C-6′), 114.9 (CH, C-2′), 114.7 (CH, C-5′), 104.9 (C, C-10), 100.3 (CH, C-1′′), 98.8 (CH, C-6), 94.1 (CH, C-8), 76.9 (CH, C-5′′), 76.5 (CH, C-3′′), 73.3 (CH, C-2′′), 69.9 (CH, C-4′′), 61.1 (CH2, C-6′′). HRESIMS: m/z 465.10113 [M + H]+ (calcd. for C21H20O12, 464.37630). 1H NMR and 13C NMR agree with data given in the literature (Li et al., 2011).
Patuletin-7-O-β-D-glucoside ( 2 )
Yellow amorphous powder. R f: 0.26 (S). Dark purple fluorescent spot by UV365 light turned to yellow-orange color with 1 % vanillin-H2SO4.
UV-spectral data: λ max (nm) (MeOH): 257, 365. 1H NMR (CD3OD, 400 MHz): δ 7.70 (1H, d, J = 1.7 Hz, H-2′), 7.59 (1H, dd, J = 8.4, 1.7 Hz, H-6′), 6.84 (1H, d, J = 8.4 Hz, H-5′), 6.74 (1H, s, H-8), 5.08 (1H, d, J = 7.3 Hz, H-1′′), 3.96 (1H, dd, J = 12.1, 10.3 Hz, Hb-6′′), 3.90 (3H, s, O-CH 3), 3.75 (1H, dd, J = 12.1, 6.0 Hz, Ha-6′′), 3.60-3.30 (4H, m, H-2′′, H-3′′, H-4′′, H-5′′). 13C NMR (CD3OD, 100 MHz): δ 175.9 (C, C-4), 156.0 (C, C-7), 151.5 (C, C-5, C-9), 147.7 (C, C-4′), 147.5 (C, C-2), 144.8 (C, C-3′), 135.9 (C, C-3), 131.7 (C, C-6), 122.4 (C, C-1′), 120.6 (CH, C-6′), 114.9 (CH, C-5′), 114.7 (CH, C-2′), 105.1 (C, C-10), 100.6 (CH, C-1′′), 93.9 (CH, C-8), 77.0 (CH, C-5′′), 76.5 (CH, C-3′′), 73.4 (CH, C-2′′), 69.9 (CH, C-4′′), 61.2 (CH2, C-6′′), 60.2 (CH3, O-CH3). HRESIMS: m/z 495.40228 [M + H]+ (calcd. for C22H22O13, 494.40228). 1H NMR and 13C NMR agree with data given in the literature (Bicha et al., 2013).
(S): CHCl3:MeOH:H2O (80:20:2, v/v/v)
Determination of total phenolic content in the extracts
Total soluble phenolic compounds in the petroleum ether, chloroform, ethyl acetate, n-butanol, and water extracts were determined with Folin–Ciocalteu reagent (Slinkard and Singleton, 1977). The total concentration of phenolic compounds in the extracts were calculated as gallic acid equivalents, where an equation from the standard gallic acid graph was used.
Determination of total flavonoid content over rutin
The flavonoid content of A. biebersteinii was determined as rutin equivalent by using the method of Lar’kina et al. (2009) with slight modifications (Guvenalp et al., 2010).
Antioxidant capacity assays
Antioxidant activities of petroleum ether, chloroform, ethyl acetate, n-butanol, water extracts, and isolated compounds were determined by using these tests: DPPH free radical scavenging activity (Blois, 1958), ABTS radical cation decolorization assay (Re et al., 1999), superoxide anion radical scavenging activity (Zhishen et al., 1999), and total antioxidant activity by ferric thiocyanate method (Mitsuda et al., 1966) which were carried out as described in their respective references.
Determination of AChE and BChE inhibitory activities
The AChE and BuChE inhibitory activities of the extracts and compounds were evaluated using Ellman’s colorimetric method (Ellman et al., 1961) with some modifications using commercially available neostigmine bromide as the reference compound (Yerdelen and Tosun, 2015).
Molecular docking studies
The docking study was performed using Surflex-Dock in Sybyl-X 2.0 by Tripos Associates. 3D structure of compounds was constructed using the Sybyl sketcher module. The structures were minimized using the steepest descent conjugated gradient method until the gradient was 0.001 kcal/mol. Maximum number of iteration was kept at 1000 with the Tripos force field with the Gasteiger Huckel charge. The simulation system was built on the crystal structures of 1ACJ and 1P0I, which were obtained from the Protein Data Bank. At the commencement of docking, water and ligands were removed and the random hydrogen atoms were added. Docking calculations using Surflex-Dock for 1ACJ and 1P0I were performed through protomol generation by ligand. The threshold and bloat parameters were set to 0.5 and 0, respectively.
Statistical analysis
The experiments were repeated at least three times. Since the results of each experiments were similar, statistical analysis was performed on the data recorded. For all parameters, the Student’s t test was used to assess significant differences between control and plant.
Results and discussion
Since ancient times, medicinal plant extracts have been used in traditional medicine for treatment of various diseases. The pharmaceutical industry requires potential novel drug sources, and plants are attractive sources for drug research and discovery. Besides, consumers have preferred to use herbal medicinal products to protect their health benefits. In elderly people, AD represents the most frequently occurring form of dementia, especially if considered alongside concomitant cerebrovascular disease (Bullock, 2004). AChE inhibitors are the only agents approved by the Food and Drug Administration for the treatment of AD.
Several findings suggest that oxidative stress may play an important role in the pathogenesis of AD. First, the brains of patients with AD contain lesions that are typically associated with exposure to free radicals. In addition, oxidative stress in the brains of AD patients is indicated by elevated cerebral levels of endogenous antioxidants that scavenge free radicals. Moreover, in vitro studies suggest that exogenous antioxidants reduce the toxicity of Aβ in the brains of AD patients. Antioxidants may decrease the level of oxidative stress in the brain and thereby reduce the amount of DNA damage, neuronal cell death, and aggregation of Aβ within the brain (Markesbery, 1997).
Antioxidant capacity assays
In this context, the antioxidant capacities of methanol (ABM), petroleum ether (ABP), chloroform (ABC), ethyl acetate (ABE), n-butanol (ABB), and water (ABW) extracts of herbs were determined by DPPH free radical, superoxide anion radical, and ABTS cation radical decolorization assays and their total antioxidant capacity by ferric thiocyanate method with their total flavonoid and phenolic contents. Ascorbic acid, trolox, α-tocopherol, and gallic acid were used as reference compounds for the antioxidant assays. Among them ethyl acetate extract (ABE) showed the best results with 67.7 % scavenging of DPPH radical, 97.4 % scavenging of ABTS cation radical, 83.1 % scavenging of superoxide anion radical, and 47.5 % inhibition of lipid peroxidation, while DPPH was scavenged with 59.9, 94.7, and 91.8 % by the standard compounds α-tocopherol, trolox, and ascorbic acid, respectively. ABTS cation radical was scavenged by gallic acid 96.7 %, trolox 97.4 %, and α-tocopherol 48.9 %. Trolox inhibited 52.7 % of lipid peroxidation and scavenged superoxide anion radical by 69.5 %. The results showed us scavenging capacity of ethyl acetate extract and standard substances were close. The ferric thiocyanate method measures the amount of peroxide, which is the primary product of oxidation produced during the initial stages of oxidation (Ak and Gulcin, 2008). Ethyl acetate extract exhibited effective antioxidant activity in the linoleic acid emulsion system at 60th h (Table 1).
Total phenol and flavonoid contents
The amount of total phenol content was determined by using the Folin–Ciocalteu method and was expressed as gallic acid equivalents. The gallic acid standard graph is shown in Fig. 1, and the mean amount of total phenolics in the extracts shown in Table 2. The ethyl acetate extract possessed the richest phenolic content (205.72 GAE/mg ext) among the tested extracts. Total flavonoid content in the A. biebersteinii was expressed as rutin equivalent. The mean amount of total flavonoids was 3.39 mg (calculated as rutin in dry raw material in percent).
Secondary metabolites
From this view point, we decided to isolate major secondary metabolites from ethyl acetate and n-butanol extracts. The isolation of the compounds was carried out using several and repeated chromatographic techniques. From the aerial parts of the plant, two flavonoid glycosides [quercetin-7-O-β-D-glucoside (1) and patuletin-7-O-β-D-glucoside (2)] were isolated. Known compounds were identified by comparing their spectroscopic data with that of those reported in the literature. The structures of the compounds were given in Fig. 2.
Antioxidant capacities of isolated secondary metabolites were determined by DPPH, superoxide anion, ABTS cation radical scavenging, and lipid peroxidation inhibitory assays. Their IC50 values are given in Table 3. According to our results, in all tests of DPPH, ABTS, superoxide anion radical scavenging, and lipid peroxidation inhibitory assay methods, compound 1 showed the best antioxidant capacity among the isolated compounds.
Molecular docking studies
Biological activity tests on molecules have been carried out by using in vitro or in vivo methods without having preliminary studies. In this study, in silico screening for AChE and BuChE inhibitory effects of compounds 2 and 1 were preferred, respectively, as a preliminary assessment mechanism for reducing the cost and time required for drug discovery.
The binding of the most potent compounds to Torpedo californica (AChE-1ACJ) and HuBuChE (1P0I) was performed between the most active compound 2 with AChE and compound 1 with BuChE, using the Surflex-Dock molecular modeling software. The docking results revealed that compounds 1 and 2 played roles in multiple hydrogen bonding interactions in catalytic active sites (CAS) and peripheral active sites (PAS) of the related enzymes.
1ACJ-compound 2 complex, substituted groups on tetrahydropyran ring created many hydrogen bonding interactions with the CAS amino acid residues such as His440, Glu199, Gly118, Gly119, and Ser200. 3′-substituted hydroxyl group (–OH) on tetrahydropyran ring created a hydrogen bonding interaction with carbonyl group of His440 (1.94 Å) residue in CAS. Two hydrogen bonding interactions occurred between 5′-substituted hydroxyl group and 6′-substituted hydroxymethyl group on tetrahydropyran ring with –NH group of Gly118 residue, and distances of 2.57 and 2.26 Å, respectively. The most potent hydrogen bonding interaction occurred between 6′-substituted hydroxymethyl group on tetrahydropyran ring and hydroxyl group of Ser200 (1.74 Å) residue in CAS of AChE. The keto group of chromen-4-one ring created a hydrogen bond with hydroxyl group of Tyr334 (1.78 Å) residue in PAS. Also 3-substituted hydroxyl group on chromen ring displayed two hydrogen bond interactions with the carbonyl groups of Ser81 (2.29 Å) and Asn85 (2.10 Å) residues in PAS. In addition, phenolic hydroxyl groups made two hydrogen bonds with Gln69 (2.15 Å) and Tyr70 (1.74 Å) residues. The molecular simulation results show that tetrahydropyran and chromen-4-one rings are primarily responsible for hydrogen bonding interactions with PAS and CAS of AChE, respectively, shown in Fig. 3.
The most potent BuChE inhibitor, compound 1, displayed multiple binding patterns with Glu197, Tyr128, Tyr332, Trp430, Trp82, and Pro285 residues of 1P0I, as shown in Fig. 4. In the 1P0I-compound 1 complex, 3′- and 4′-substituted hydroxyl groups on tetrahydropyran ring created hydrogen bonds with C–O group of Glu197 (1.94–2.24 Å). In addition, the 4′- and 5′-substituted –OH groups displayed hydrogen bond interaction with –OH group of Tyr128 residue, at distances of 1.83 and 1.91 Å. The keto group of chromen-4-one ring could interact with Tyr332 (1.98 Å) in PAS. The other –H bond interactions in PAS occurred between 5-substituted hydroxyl group on chromen-4-one ring and –NH groups of Trp430 (2.57 Å) and indole moiety of Trp82 (2.38 Å). Para-substituted phenolic –OH group showed an –H bond interaction with carbonyl group of Pro285 (2.05 Å) residue. The results indicate that the chromen-4-one ring is an essential structural part for the interaction with PAS on BuChE inhibition.
AChE and BuChE inhibitory activities
Promising results for AChE and BuChE inhibitory potentials of compounds 1 and 2 were obtained from our in silico findings, and further studies were designed as in vitro AChE and BuChE inhibitory assays. In our study, as shown in Table 4, the ethyl acetate and n-butanol extracts which contain compounds 1 and 2 possessed significant inhibitory activity against AChE (67.2 ± 1.32 %) and (52.5 ± 1.36 %), while lower inhibitory activity against BuChE (50.3 ± 1.03 %) and (37.6 ± 1.12 %), respectively, as compared with a reference compound neostigmine (100 %) at 200 μg/mL. Neostigmine inhibited AChE and BuChE by 100 % at 50, 100, and 200 μg/mL concentrations. The IC50 values of all tested compounds and their selectivity index for AChE are summarized in Table 5. Compound 2 exhibited the strongest inhibition to AChE, and compound 1 exhibited the strongest inhibition to BuChE with IC50 values of 1.77 and 2.24 µM, respectively.
Conclusion
To the best of our knowledge, the current study is the first investigation on the antioxidant and AChE and BuChE inhibitory activities of a wide range of concentration from different polarity extracts of A. biebersteinii as well as the AChE and BuChE inhibitory activities of quercetin-7-O-β-D-glucoside and patuletin-7-O-β-D-glucoside.
In this study, in vitro and in silico methods were used simultaneously for quercetin-7-O-β-D-glucoside and patuletin-7-O-β-D-glucoside for the first time, and both of them showed significant results with these methods. Our results suggest that quercetin-7-O-β-D-glucoside and patuletin-7-O-β-D-glucoside may become a novel therapeutic candidate for the treatment of AD.
References
Ak T, Gulcin İ (2008) Antioxidant and radical scavenging properties of curcumin. Chem-Biol Interact 174:27–37
Baris D, Kizil M, Aytekin C, Kizil G, Yavuz M, Ceken B, Ertekin AS (2011) Antimicrobial and antioxidant activity of ethanol extract of three Hypericum and three Achillea species from Turkey. Int J Food Prop 14:339–355
Bicha S, Amrani A, Benaissa O, León F, Zama D, Brouard I, Benayache S, Bentamene A, Benayache F (2013) A flavonoid with high antioxidant effect from Centaurea acaulis L. Der Pharm Lett 5:24–30
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Bullock R (2004) Galantamine: use in Alzheimer’s disease and related disorders. Expert Rev Neurother 4:153–163
Ellman GL, Courtney KD, Andres V, Featherstone J, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Goren N, Oksuz S, Ulubelen A (1988) A sesquiterpene lactone, sintenin from Achillea sintenisii. Phytochemistry 27:2346–2347
Guvenalp Z, Ozbek H, Yuzbasioglu M, Kuruuzum-Uz A, Demirezer LÖ (2010) Flavonoid quantification and antioxidant activities of some Pimpinella species. Rev Anal Chem 29:233–240
Hodges JR (2006) Alzheimer’s centennial legacy: origins landmarks and the current status of knowledge concerning cognitive aspects. Brain A J Neurol 129:2811–2822
Hoozesmans JJM, Veerhuis R, Rozemuller JM, Eikelenboom P (2006) Neuroinflammation and regeneration in the in the early stages of Alzheimer’s disease pathology. Int J Dev Neurosci 24:157–165
Houghton P, Howes MJ (2005) Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease. Neurosignals 14:6–22
Huber-Morath A (1975) Achillea L. In: Davis PH (ed) Flora of Turkey and the East Aegean Islands, vol 5. Edinburgh University Press, Edinburgh, pp 224–252
Keser S, Celik S, Turkoglu S, Yilmaz O, Turkoglu I (2011) Determination of antioxidant properties of ethanol and water extracts of Achillea millefolium L. (Yarrow). Asian J Chem 23:3172–3176
Lar’kina MS, Kadyrova TV, Ermilova EV, Krasnov EA (2009) Quantitative determination of flavonoids from the aerial part of greater knapweed (Centaurea scabinosa L.). Pharm Chem J 43:14–17
Li Y, Qiao W, Yuan K (2011) Isolation and structural elucidation of chemical constituents of Mussaenda hainanensis Merr. J Med Plants Res 5:1459–1465
Mahmoud AA, Al-Shihry SS, Hegazy MEF (2012) A new epimeric sesquiterpene lactone from Achillea ligustica. Rec Nat Prod 6:21–27
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 23:134–147
Mitsuda H, Yasumoto K, Iwami K (1966) Antioxidation action of indole compounds during the autoxidation of linoleic acid. Eiyo to Shokuryo 19:210–214
Orth M, Juchelka D, Mosandl A, Czygan FC (2000) Chiral monoterpenes as taxonomically useful markers for Achillea species differentiation. Pharmazie 55:456–459
Pecetti L, Tava A, Romani M, Cecotti R, Mella M (2012) Variation in terpene and linear-chain hydrocarbon content in yarrow (Achillea millefolium L.) germplasm from the Rhaetian Alps, Italy. Chem Biodivers 9:2282–2294
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231
Sezik E, Yesilada E, Honda G (2001) Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J Ethnopharmacol 75:95–115
Slinkard K, Singleton VL (1977) Total phenol analyses: automation and comparison with manual methods. Am J Enology and Viticult 28:49–55
Uc EY, Rizzo M (2008) Driving and neurodegenerative diseases. Curr Neurol Neurosci 8:377–383
Ulubelen A, Oksuz S, Schuster A (1990) A sesquiterpene lactone from Achillea millefolium subsp. millefolium. Phytochemistry 29:3948–3949
Vitalini S, Beretta G, Iriti M, Orsenigo S, Basilico N, Acqua S, Lorizzi M, Fico G (2011) Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim Pol 58:203–219
Yerdelen KÖ, Tosun E (2015) Synthesis, docking and biological evaluation of oxamide and fumaramide analogs as potential AChE and BuChE inhibitors. Med Chem Res 24:588–602
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Acknowledgments
This work was supported by The Foundation of Atatürk University (2013/274).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sevindik, H.G., Güvenalp, Z., Yerdelen, K.Ö. et al. Research on drug candidate anticholinesterase molecules from Achillea biebersteinii Afan. using by molecular docking and in vitro methods. Med Chem Res 24, 3794–3802 (2015). https://doi.org/10.1007/s00044-015-1423-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1423-8