Abstract
Bagasse is a residue obtained from the processing of sugarcane (Saccharum officinarum). The aim of this study was to investigate the temperature profile, biochar yield and product quality of a locally designed thermochemical process for the conversion of sugarcane bagasse (SCB) and low-density polyethylene waste into biochar. Product quality was evaluated using Fourier transform infrared spectroscopy, scanning electron microscopy and Branueur–Emmett–Teller analyses. Product yield was 16.67 wt% and 45.46 wt% at 349°C and 250°C peak temperatures for SCB and hybrid biochar, respectively. Both SCB biochar and hybrid had a heterogeneous surface morphology and was mesoporous. The specific surface area of the SCB and hybrid biochar was 533.6 m2/g and 510.5 m2/g, respectively. The process has a three-pronged advantage of product development, waste management and resource conservation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Energy and environmental sustainability are some of the major challenges of humans for the long-term future [1]. Biomass is a material with a high energy content [2, 3], and its utilisation could assuage the impending energy crisis [4]. One of the most produced biomass globally is sugarcane, and it is a potential solution to these energy issues [5].
Sugarcane (Saccharum officinarum) was first cultivated in Southeast Asia and Western India [6]. Globally, the annual production of sugarcane is 1.6 billion tons, generating about 279 million metric tons of biomass residues [7]. Brazil is currently the largest producer of sugarcane in the world. Bagasse is a residue obtained from the processing of sugarcane via the crushing and extraction of the juice [7]. The bagasse constitutes about 35% of the total cane weight [8]. A minimum of 50% of the generated bagasse can be utilised to generate heat and power for the sugar processing [9], and the rest is stacked for further processing into the value-added process (though this is not always the case) [10].
Moreover, low-density polyethylene (LDPE) is the main component of urban solid waste in our major urban centres; it is non-biodegradable and its volume generated is directly proportioned to the intensity of civic and social activities. Current information shows the startling rate at which plastic pollution from quantities of municipal solid waste (MSW) is ravaging the world [11]. For instance, in the USA, 39.3 million tons of LDPE is being generated annually, which is about 11% of the total MSW, apart from other forms of the plastic genre [11, 12]. In this case, the rate of its generation cannot catch up with the rate of recycling giving the presently available technologies if novel technology is not put in place for combining plastic with biomass wastes in doping magnitude [13]. Similar concerns about plastic pollution have also been raised in other continents like Africa, Europe, and Asia [14]. LDPE is selected in this study because it is one of the most significant plastic constituent in solid-waste streams [15, 16].
Researchers have studied several types of thermochemical processes for the conversion of energy from SCB [17]. These include pyrolysis, steam reforming [18, 19], supercritical water gasification [1], air gasification [20, 21] and hydrothermal carbonisation [22, 23]. The concept of recycling the heat from the controlled combustion of biomass for production of biochar from other biomass feedstock was earlier pitched by Abdelhafez, Abbas and Hamed [24] but was improved by other researchers [25,26,27]. In this study, a novel low-cost carbonisation process based on this process is employed that incorporates a retort heating system using controlled combustion of biomass fuel for heat generation. These contemplations make this study significant in the pursuit of energy and environmental sustainability in developing African countries.
The aim of this study is to investigate the temperature profile, biochar yield and product quality of a locally designed thermochemical process for the conversion of sugarcane bagasse (SCB) and low-density polyethylene (LDPE) waste. The biomass was converted in a singular process and a hybrid process with plastics. Product quality was evaluated using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and Branueur–Emmett–Teller (BET) analyses. The relevance of this study is justified given the need to convert waste materials into a more useful product via energy-efficient processes. Furthermore, it is important for solid waste management and energy and environmental sustainability endeavours. The novelty of the study is justified in the types of the process employed. It is low-cost, easy to implement and has no electrical power requirement [25].
2 Materials Methodology
2.1 Feedstock
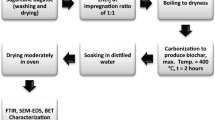
Sugarcane bagasse (Saccharum officinarum) was sourced from the Hausa community in Oja Gboro, Ilorin, Nigeria. Low-density polyethylene (LDPE) waste was sourced from a solid waste stream in Tanke, Ilorin, Nigeria. It was rinsed in distilled water to remove adhering dust and dirt. Both wastes were sundried for 2 days to remove moisture. The combustion fuel used were dry stems of Daniella oliveri picked also within the campus of the University of Ilorin, Nigeria.
2.2 Reactor Configuration
The reactor utilised was of a top-lit, updraft configuration. The concept was earlier pitched by Abdelhafez, Abbas and Hamed [24] but was improved by the current research group. In this study, a novel low-cost carbonisation process is employed that incorporate a retort heating system using controlled combustion. The description of the reactor used in this study is given in Fig. 1. Full dimensions of the reactor sizes have been described elsewhere [26, 27]. The heating process is self-regulating as recycled heat from the controlled combustion of biomass (stems of Daniella oliveri) in the heating gap is used to convert the biomass feed in the inner chamber. The configuration is ‘top-lit and updraft’. The heating gap is ignited at the top, and suction of air to the combustion zone occurs at the bottom air holes. The process is completed when the combustion fuel is used up. The process is easy to implement and has no electrical power requirement hence can be used in remote locations.
2.3 Process Description
Two experiments were conducted: SCB conversion and co-conversion of SCB-LDPE waste. The proportion of LDPE was 30 g for 900 g of the combined feed (giving a plastic content of 3.3%). This is to give allowance for plastic nature of LDPE and to allow effective co-conversion; similar position has been previously employed [28]. This is not as imbalanced as it seems when the effective density of the plastics is considered (as they use up much more space within the reactor). The SCB conversion was conducted in 48.5 cm high reactor with full dimensions described elsewhere [26]. The SCB-LDPE co-conversion was conducted in the 53-cm-high reactor with full dimensions described elsewhere [27]. A CASON CA380 infra-red thermometer (Accuracy; ± 0.1°C, Max; 380°C) was used for monitoring the process temperature so as the temperature profile can be achieved.
2.4 Biochar Yield
The degree of the feed carbonisation in the reactor used (Fig. 1) is expressed as the fraction of the biochar obtained relative to the feed converted. The biochar yield for the conversion process was computed using Eqn. 1–3. Similar expressions were previously employed [26, 27].
where M1 = mass of feed chamber + feed (in grams), M2 = mass of feed chamber alone (in grams), M3 = Weight of feed chamber + product (in grams). For the single conversion of sugarcane bagasse, the values obtained were M1 = 600 g, M2 = 480 g, M3 = 500 g. For the co-conversion of sugarcane bagasse with LDPE waste, the values obtained were M1 = 900g (plastic = 30g), M2 = 625 g and M3 = 750 g. Computing these values, we obtain biochar yields summarised in Table 1.
2.5 Product Characterisation
Product quality was evaluated using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and Branueur–Emmett–Teller (BET) analyses. FTIR (Shimadzu, FTIR-8400S, Japan) spectrum was recorded using the transmittance in the 4000–650 cm-1 region with 30 sample scans. SEM (SEM, Phenom ProX, Phenom-World BV, Netherlands) was done with an acceleration voltage of the microscope set to 15 kV. BET (Quantachrome NovaWin ©1994–2013, Quantachrome Instruments v11.03) was done by N2 adsorption test at 77K.
3 Results and Discussion
3.1 Temperature Profile
The temperature profile for the process was monitored for both SCB conversion and the hybrid co-conversion (and shown in Fig. 2a, b, respectively). \(T_{a}\), \(T_{b}\), \(T_{c}\) and \(T_{d}\) are the temperatures obtained at the base (side), middle (side), top (side) and centre of the reactors, respectively. The \(T_{d}\) gives a better representation of the temperature within the reactor chamber whilst \(T_{a}\), \(T_{b}\) and \(T_{c}\) help in the monitoring of the movement of the combustion zone within the heating space as it proceeds from the top to the bottom of the reactor. Higher \(T_{d}\) in both profiles indicates a synergistic heating effect of the circular heating gap on the central chamber. The progression of each peak through \(T_{c}\) to \(T_{b}\) then \(T_{a}\) indicates the downward progression of the combustion zone within the heating gap. The peak temperatures of 349°C and 250°C for the biomass and hybrid conversion can be observed from Fig. 2a, b, respectively. The peak temperatures and process times are different because the process is self-regulating. The heating effects are dependent on the volume and heating value of the combustion fuel utilised in the process.
3.2 Biochar Yield
It can be observed from Table 1 that the biochar yield was 16.67 and 45.46 wt% at 349 and 250°C peak temperatures for SCB and hybrid biochar, respectively. The biochar yield for the biomass conversion of SCB is higher than 6.98 wt% (peak temperature of 220°C) for plantain fibres [27] and 14.29 wt% (peak temperature of 300°C) for elephant grass [26] obtained using the same apparatus. The noticeable difference in peak temperature and process time occurs because the process is self-regulating and does not involve external parameter controls. Future explorations will be needed in this regard to achieve the requisite optimality and true generalisation of process conditions.
Moreover, biochar yield from the hybrid process is higher than that of the biomass process due to the effect of the plastics in the reactor. This is due to the carbon content of plastics (composed mainly of carbon and hydrogen) [11] being higher than for biomass (composed mainly of carbon, oxygen and hydrogen) [29]. The higher oxygen in the system due to biomass leads to the synthesis of more oxidised conversion products (CO and CO2). These oxidised products take up carbon atoms with them, thereby reducing the char yield. Another reason could be the higher peak temperature for the SCB conversion leading to a greater intensity of thermal degradation hence lesser solid product.
3.3 Biochar Functional Groups
The FTIR spectra for the SCB biochar and the hybrid biochar are presented in Fig. 3. The spectra are presented in transmission mode ranging from 4000 to 650 cm−1 wavelength. The possible functional groups attributed to the peaks observed are shown in Table 2.
The peaks in the region of 3600–3850 cm−1 are attributed to the –OH group of the water, alcohols and phenol present in the biomass samples [30,31,32]. The peak 3278 cm−1 observed in both samples indicate the presence of amide (N–H) groups [33]. The peaks 2920 cm−1 and 2850 cm−1 observed in SCB biochar correspond to the aliphatic C–H stretch of alkanes [32]. The intensity of these two peaks was observed to increase with the addition of LDPE. This may be attributed to the fact that LDPE is characterised by the presence of asymmetric and symmetric -CH2 (ethyl) stretch commonly observed at these peaks [34,35,36]. The peak 1603 cm−1 observed in the SCB biochar correspond to the stretching vibrations of the alkene group (C=C) and was sheen to shift to 1615 cm-1 and increase in intensity with the addition of LDPE to the biochar mixture. The shift and increased intensity may be due to the aromatic carbon content of LDPE [37, 38].
The peak observed at 1358 cm-1 for the SCB biochar is attributed to the C–O stretch of ether [30]. This peak was seen to shift to 1358 cm−1 with the addition of LDPE in the biochar production. The peak may also indicate the presence of C–N since nitrogen possible accumulated in the plant during cultivation [33]. The peaks ranging from 2622 to 1988 cm−1 were not assigned in past works of sugarcane bagasse within the author’s search scope. However, peaks in this range are commonly assigned to unsaturated carbon groups alkenes (C=C) and alkynes (C≡C), which may be bonded by nitrogen (C≡N, C≡C).
3.4 Biochar Morphology
The SEM micrograph of the SCB biochar and hybrid biochar is shown in Fig. 4a, b, respectively. Both SCB biochar and hybrid have a heterogeneous surface morphology with globular features. These globular features led to the generation of interstices on the surface of the char. These interstices are likely to lead to biochar with large specific surface area [25] which could also impact on it a potential catalytic activity and adsorptive capacity [41]. The high specific surface area was confirmed by the BET analysis.
3.5 Biochar Porous Properties
The results of the Branueur–Emmett–Teller (BET) analysis of the SCB biochar and hybrid biochar is summarised in Table 3. It can be observed that the specific surface area of the SCB and hybrid biochar is 533.6 and 510.5 m2/g, respectively. This is relatively high for a biochar sample obtained without the use of any chemical or physical activation process. Moreover, the summary of the reactor performance as presented in Table 1 confirms the variance in operating parameters for SCB and hybrid biochar production; in this case, SCB was produced at the lower temperature. Notwithstanding the fact that the surface area of hybrid biochar was slightly lower than that of the SCB, it was found to be more porous (Table 3). The pores in the hybrid biochar were found to be larger than that of SCB, as further confirmed in Fig. 4a, b.
This further underlines the usefulness of this biochar for various applications. From the values of the pore diameter, it can be observed that the biochar samples are mesoporous. They are > 2 nm but < 50 nm. Both samples had technically impressive pore volumes appropriate for numerous applications. This confirms that the doping LDPE with bagasse (at 3.3%) in biochar production improves the surface area and other textural properties of the produced biochar. A recent report of similar category equally confirmed that [28].
3.6 Comparison with Other Feedstock
Care was taken not to draw comparisons with other feedstock in other areas of the study due to the intricacies of the current research approach. There would, of course, be several reasons why there would be differences in such comparison (besides the nature of the feed composition). In this section, a comparison is made for investigations where the same apparatus/set-up was used. Table 4 shows a comparison of the biochar obtained in this study with those from other biomass material also performed by the current research team using the same process (and arranged in order of decreasing BET specific surface area). It can be observed that SCB feedstock gives biochar with the highest specific surface area than others like elephant grass, plantain fibres, orange peels and orange albedo. This is a pointer towards the inherent suitability of the biomass in obtaining high-quality biochar from the process. Furthermore, the yield for the SCB alone was also higher than for elephant grass and plantain fibres. The pore diameter obtained in the current study was intermediate albeit mesoporous like the others.
3.7 Practical Implications of the Study
Some important advantages can be derived from the current study approach which makes it impactful in developing African countries (of which this research is domiciled). The current design and experimental methodology did not require electrical powers as energy from biomass controlled combustion are recycled to carbonise the feed. This ensures the process is low cost and can be used in remote locations. This is of great advantage especially in agricultural applications where large quantities of biochar are needed in remote location [42]. Sugarcane bagasse and LDPE are waste materials with no competitive use in contemporary Nigeria. Valorisation of this biomass for biochar production gives a three-pronged advantage.
There is the conversion of energy sources into more useful forms that can be applied in other energy, agricultural and environmental processes. Several environmental [43], energy and agricultural issues [42] have been reported in developing Nigeria making the findings of this study relevant in the local context.
It is also relevant in solid waste management and resource conservation. The study has successfully implemented a low-cost technology for biochar production from biomass.
It is important in environmental pollution abatement as LDPE is a non-biodegradable waste material.
4 Conclusion
In this study, the temperature profile, biochar yield and product quality during the thermochemical conversion of sugarcane bagasse (SCB) and low-density polyethylene (LDPE) waste into biochar was investigated. Product quality was evaluated using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and Branueur–Emmett–Teller (BET) analyses. The product yield was 16.67 wt% and 45.46 wt% at 349 and 250°C peak temperatures for SCB and hybrid biochar, respectively. FTIR analysis revealed that the intensity of some of the peaks was increased by LDPE addition to the feed. Both SCB biochar and hybrid had a heterogeneous surface morphology with globular features. The specific surface area of the SCB and hybrid biochar is 533.6 m2/g and 510.5 m2/g, respectively, and they were observed to be mesoporous. It was observed that SCB as the feedstock gave biochar with the highest specific surface area than others like elephant grass, plantain fibres, oil palm fibres, orange peels and orange albedo using the same experimental apparatus and methodology. The process had a three-pronged advantage of product development, waste management and resource conservation. It is recommended that such technology be implemented in the urban and rural context for waste management, product development and other endeavours targeted at achieving environmental sustainability. In terms of the experiments, the effect of varying LDPE doping of the feedstock on product quality can also be investigated.
References
Sheikhdavoodi, M.J.; Almassi, M.; Ebrahimi-Nik, M.; Kruse, A.; Bahrami, H.: Gasification of sugarcane bagasse in supercritical water; evaluation of alkali catalysts for maximum hydrogen production. J. Energy Inst. 88(4), 450–458, 2015
Tsai, W.; Lee, M.; Chang, Y.: Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J. Anal. Appl. Pyrolysis 76(1–2), 230–237, 2006
Adeniyi, A.G., Ighalo, J.O., Marques, G.: Utilisation of machine learning algorithms for the prediction of syngas composition from biomass bio-oil steam reforming. Int. J. Sustain. Energy (2020). https://doi.org/10.1080/14786451.2020.1803862
Yin, C.-Y.: Prediction of higher heating values of biomass from proximate and ultimate analyses. Fuel 90(3), 1128–1132, 2011
Scaramucci, J.A.; Perin, C.; Pulino, P.; Bordoni, O.F.; Da Cunha, M.P.; Cortez, L.A.: Energy from sugarcane bagasse under electricity rationing in Brazil: a computable general equilibrium model. Energy Policy 34(9), 986–992, 2006
Hofsetz, K.; Silva, M.A.: Brazilian sugarcane bagasse: energy and non-energy consumption. Biomass Bioenergy 46, 564–573, 2012
Chandel, A.K.; da Silva, S.S.; Carvalho, W.; Singh, O.V.: Sugarcane bagasse and leaves: foreseeable biomass of biofuel and bio-products. J. Chem. Technol. Biotechnol. 87(1), 11–20, 2012
De Moraes Rocha, G.J.; Nascimento, V.M.; Goncalves, A.R.; Silva, V.F.N.; Martín, C.: Influence of mixed sugarcane bagasse samples evaluated by elemental and physical–chemical composition. Indust. Crops Prod. 64, 52–58, 2015
Sun, J.-X.; Sun, R.; Sun, X.-F.; Su, Y.: Fractional and physico-chemical characterization of hemicelluloses from ultrasonic irradiated sugarcane bagasse. Carbohydr. Res. 339(2), 291–300, 2004
Peng, F.; Ren, J.-L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.-C.: Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J. Agric. Food Chem. 57(14), 6305–6317, 2009
Morét-Ferguson, S.; Law, K.L.; Proskurowski, G.; Murphy, E.K.; Peacock, E.E.; Reddy, C.M.: The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar. Pollut. Bull. 60(10), 1873–1878, 2010
Dahlbo, H.; Poliakova, V.; Mylläri, V.; Sahimaa, O.; Anderson, R.: Recycling potential of post-consumer plastic packaging waste in Finland. Waste Manage. 71, 52–61, 2018
Block, C.; Ephraim, A.; Weiss-Hortala, E.; Minh, D.P.; Nzihou, A.; Vandecasteele, C.: Co-pyrogasification of plastics and biomass, a review. Waste Biomass Valoriz. 10(3), 483–509, 2019
Lourenço, P.M.; Serra-Gonçalves, C.; Ferreira, J.L.; Catry, T.; Granadeiro, J.P.: Plastic and other microfibers in sediments, macroinvertebrates and shorebirds from three intertidal wetlands of southern Europe and west Africa. Environ. Pollut. 231, 123–133, 2017
Adeniyi, A.G.; Eletta, O.A.A.; Ighalo, J.O.: Computer aided modelling of low density polyethylene pyrolysis to produce synthetic fuels. Nigerian J. Technol. 37(4), 945–949, 2018
Adeniyi, A.G.; Ighalo, J.O.: Simulation of low density polyethylene (LDPE) pyrolysis and optimisation of pyro-oil yield. Int. Polym. Process. 35(2), 229–235, 2020
Adeniyi, A.G.; Ighalo, J.O.: Computer-aided modeling of thermochemical conversion processes for environmental waste management. In: Hussain, C.M. (ed.) Handbook of Environmental Materials Management, pp. 1–16. Springer, Berlin (2020)
Waheed, Q.M.; Williams, P.T.: Hydrogen production from high temperature pyrolysis/steam reforming of waste biomass: rice husk, sugar cane bagasse, and wheat straw. Energy Fuels 27(11), 6695–6704, 2013
Adeniyi, A.G.; Ighalo, J.O.; Abdulsalam, A.: Modelling of integrated processes for the recovery of the energetic content of sugarcane bagasse. Biofuels Bioprod. Biorefin. 13(4), 1057–1067, 2019
Gómez, E.O.; Cortez, L.s.A.B., Lora, E.S., Sanchez, C.G., and Bauen, A. : Preliminary tests with a sugarcane bagasse fueled fluidized-bed air gasifier. Energy Convers. Manage. 40(2), 205–214, 1999
Jayaraman, K.; Gokalp, I.; Petrus, S.; Belandria, V.; Bostyn, S.: Energy recovery analysis from sugar cane bagasse pyrolysis and gasification using thermogravimetry, mass spectrometry and kinetic models. J. Anal. Appl. Pyrolysis 132, 225–236, 2018
Iryani, D.A.; Kumagai, S.; Nonaka, M.; Sasaki, K.; Hirajima, T.: Characterization and production of solid biofuel from sugarcane bagasse by hydrothermal carbonization. Waste Biomass Valoriz. 8(6), 1941–1951, 2017
Chai, X.; He, H.; Fan, H.; Kang, X.; Song, X.: A hydrothermal-carbonization process for simultaneously production of sugars, graphene quantum dots, and porous carbon from sugarcane bagasse. Bioresource Technol. 282, 142–147, 2019
Abdelhafez, A.A., Abbas, M.H.H., and Hamed, M.H.: Biochar: A Solution For Soil Lead (Pb) Pollution’, in Editor (Ed.)(Eds.): ‘Book Biochar: A Solution For Soil Lead (Pb) Pollution’ (Assiut University Center for Environmental Studies-Egypt, 2016, edn.), pp.
Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V.: Biochar from the thermochemical conversion of orange (Citrus sinensis) peel and albedo: product quality and potential applications. Chem. Africa 3(2), 439–448, 2020
Adeniyi, A.G., Ighalo, J.O., Onifade, D.V.: Production of biochar from elephant grass (Pernisetum purpureum) using an updraft biomass gasifier with retort heating. Biofuels (2019). https://doi.org/10.1080/17597269.2019.1613751
Adeniyi, A.G., Ighalo, J.O., Onifade, D.V.: Production of bio-char from plantain (Musa paradisiaca) fibers using an Updraft Biomass Gasifier with retort heating. Combust. Sci. Technol. (2019). https://doi.org/10.1080/00102202.2019.1650269
Adelodun, A.A.; Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V.; Arowoyele, L.T.: Thermochemical conversion of oil palm Fiber-LDPE hybrid waste into biochar. Bioprod. Biorefin., Biofuels (2020)
Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.: An overview of the chemical composition of biomass. Fuel 89(5), 913–933, 2010
Naik, D.K.; Monika, K.; Prabhakar, S.; Parthasarathy, R.; Satyavathi, B.: Pyrolysis of sorghum bagasse biomass into bio-char and bio-oil products. J. Thermal Anal. Calorim. 127(2), 1277–1289, 2017
Yin, R.; Liu, R.; Mei, Y.; Fei, W.; Sun, X.: Characterization of bio-oil and bio-char obtained from sweet sorghum bagasse fast pyrolysis with fractional condensers. Fuel 112, 96–104, 2013
Saleh, M.E.; Hedia, R.M.: Mg-Modified Sugarcane Bagasse Biochar for Dual Removal of Ammonium and Phosphate Ions from Aqueous Solutions. Alexandria Sci. Exchange J. 39, 74–91, 2018
Hafshejani, L.D.; Hooshmand, A.; Naseri, A.A.; Mohammadi, A.S.; Abbasi, F.; Bhatnagar, A.: Removal of nitrate from aqueous solution by modified sugarcane bagasse biochar. Ecol. Eng. 95, 101–111, 2016
Hadi, J.A.; Najmuldeen, F.G.; Ahmed, I.: Quality restoration of waste polyolefin plastic material through the dissolution-reprecipitation technique. Chem. Ind. Chem. Eng. Quart. 20(2), 163–170, 2014
Doğan, F.; Şirin, K.; Kolcu, F.; Kaya, İ: Conducting polymer composites based on LDPE doped with poly (aminonaphthol sulfonic acid). J. Electrostat. 94, 85–93, 2018
Moreno-Bayona, D.A., Gómez-Méndez, L.D., Blanco-Vargas, A., Castillo-Toro, A., Herrera-Carlosama, L., Poutou-Pinales, R.A., Salcedo-Reyes, J.C., Díaz-Ariza, L.A., Castillo-Carvajal, L.C., Rojas-Higuera, N.S.: Simultaneous bioconversion of lignocellulosic residues and oxodegradable polyethylene by Pleurotus ostreatus for biochar production, enriched with phosphate solubilizing bacteria for agricultural use. PloS ONE (2019). https://doi.org/10.1371/journal.pone.0217100
Rodier, L.; Bilba, K.; Onésippe, C.; Arsène, M.-A.: Utilization of bio-chars from sugarcane bagasse pyrolysis in cement-based composites. Ind. Crops Prod. 141, 111731, 2019
Li, X.; Zhang, H.; Li, J.; Su, L.; Zuo, J.; Komarneni, S.; Wang, Y.: Improving the aromatic production in catalytic fast pyrolysis of cellulose by co-feeding low-density polyethylene. Appl. Catal. A General 455, 114–121, 2013
Pereira, P.H.F.; Voorwald, H.C.J.; Cioffi, M.O.H.; Mullinari, D.R.; Da Luz, S.M.; Da Silva, M.L.C.P.: Sugarcane bagasse pulping and bleaching: Thermal and chemical characterization. BioResources 6(3), 2471–2482, 2011
Ahmad, S.; Wong, Y.; Veloo, K.: Sugarcane bagasse powder as biosorbent for reactive red 120 removals from aqueous solution. In: Mohammad Razi, M.A. (ed.) Book Sugarcane Bagasse Powder as Biosorbent for Reactive Red 120 Removals from Aqueous Solution, p. 012027, IOP Publishing (2018)
Ighalo, J.O.; Adeniyi, A.G.: A Mini-Review of the Morphological Properties of Biosorbents Derived from Plant Leaves. SN Appl. Sci. 2(3), 509, 2020
Ighalo, J.O.; Adeniyi, A.G.: Biomass to biochar conversion for agricultural and environmental applications in nigeria: challenges, peculiarities and prospects. Mater. Int. 2(2), 111–116, 2020
Ighalo, J.O.; Adeniyi, A.G.: A comprehensive review of water quality monitoring and assessment in Nigeria. Chemosphere 260, 127569, 2020
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Rights and permissions
About this article
Cite this article
Adeniyi, A.G., Abdulkareem, S.A., Ighalo, J.O. et al. Thermochemical Co-conversion of Sugarcane Bagasse-LDPE Hybrid Waste into Biochar. Arab J Sci Eng 46, 6391–6397 (2021). https://doi.org/10.1007/s13369-020-05119-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05119-9