Abstract
Conventional Portland cement concrete has been extensively used in the past century due to its superior performance compared to other building materials. Recognizing the environmental impact of cement composites and global pressure toward implementation of sustainable construction materials, geopolymer concrete has been introduced as a potential alternative to conventional cement concrete. This study investigates the properties of metakaolin-based geopolymer concrete by employing various mix design parameters based on locally sourced materials. The test results reported herein comprised 16 mixes divided into three groups to understand the influence of various parameters on the workability and compressive strength of the concrete and hence optimizing the mix proportions. The outcome of this research provided insights into the curing conditions, curing age, sodium hydroxide molarity, sodium silicate content, molar ratios of the mix, and aggregate water absorption effect on the geopolymer concrete behavior. A model is proposed for deciding the water to solids ratio based on the total aggregate percentage for workable geopolymer mixes. In order to produce MK-based geopolymer concrete for structural applications, thresholds are proposed for the three molar ratios, namely sodium oxide to silicon oxide, sodium oxide to aluminum oxide, and water to sodium oxide ratios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Conventional Portland cement concrete has been extensively used in the past century due its superior performance compared to other building materials. However, the environmental impact of the cement concrete production cannot be ignored, e.g., carbon dioxide (CO2) emissions as a result of Portland cement manufacturing accounts for 5–7% of global CO2 emissions of industrial and energy sources [1]. Recognizing the environmental impact of cement composites and global pressure toward implementation of sustainable construction materials, geopolymer concrete has been introduced as a potential alternative to conventional cement concrete. Structural binder in geopolymer concrete is produced by the polymerization process of a source material through potassium based or sodium-based alkaline activators.
A variety of source materials has been investigated in the literature including metakaolin (MK), kaolin [2, 3], fly ash [4, 5], slag [6], rice husk ash [7, 8], and other natural local pozzolans [9, 10]. Extensive research on MK-based geopolymer mortar/paste was conducted to investigate the effect of alkaline activators on the geopolymerization process [11,12,13,14,15] and curing time and/or temperature on mechanical properties [16,17,18,19]. However, limited studies were conducted on MK-based geopolymer concrete and generally with restricted scope, e.g., Pires et al. [20] investigated the fracture behavior of MK-based geopolymer concrete as part of the range of mixes including cement concrete specimens. Mohseni [21] investigated the effect of sodium silicate to NaOH ratio on the mechanical properties of MK polypropylene fiber-reinforced geopolymer concrete. Alanazi et al. [22] assessed the freeze and thaw durability of five MK-based geopolymer concrete mixes with replacement by slag and/or cement. Pouhet and Cyr [23] primarily studied the influence of H2O/Na2O ratio and aggregate percentage on the workability, porosity, density, and compressive strength of MK geopolymer concrete. The experimental program of Xie et al. [24] was based on the blend of slag and MK to study the effect of the blend proportion and the recycled aggregate content on the slump, setting time, compressive strength, toughness, and Poisson’s ratio.
It is clear from the above review that studies on MK-based geopolymer investigated the behavior of MK-based geopolymer pastes or mortar and very limited research was conducted to investigate the mechanical behavior of MK-based geopolymer concrete. Conclusions drawn from the limited literature on MK-based geopolymer concrete are still inconclusive as these studies addressed a restricted range of parameters. Unlike conventional cement concrete, fresh and hardened properties of geopolymer concrete are highly sensitive to the physical and chemical properties of the mixture components, which significantly vary among various studies due to a lack of standards. Therefore, replicating mix proportions and casting procedures of a certain mix design reported in the literature may produce a mix with significant deviation from the originally reported fresh and hardened properties. This can be attributed to the variability in the (1) aggregate properties (strength, grading, shape, specific gravity, absorption, etc.), (2) MK particle size, source (which has implications on the chemical composition), calcination procedure, Blaine surface area, and structure, (3) chemical composition of alkaline solutions, and (4) curing procedure. This study represents part of a comprehensive experimental campaign by the authors with the overall purpose of investigating fresh and mechanical properties of MK-based geopolymer concrete, including parametric studies on various mix design parameters based on locally sourced materials. The test results reported comprised of 16 mixes divided into three groups to understand the effect of various parameters on the workability and compressive strength of concrete, and hence optimizing the mix proportions. Importantly, the outcome of this research provided insights into the influence of curing conditions, curing age, sodium hydroxide molarity, sodium silicate content, molar ratios of the mix, and aggregate water absorption on the geopolymer concrete properties.
2 Experimental Program
2.1 Materials
2.1.1 Geopolymer binder
The aluminosilicate source in the geopolymer concrete was based on metakaolin sourced from local kaolin after calcination at 750 °C for 3 h. The adequacy of the calcination process to produce MK with amorphous structure was confirmed through XRD analysis. The chemical composition of the MK is given in Table 1.
The alkaline solution used to form the geopolymer binder comprised of sodium silicate and sodium hydroxide (NaOH). Sodium silicate solution incorporated Na2O = 14.7%, SiO2 = 29.4% and H2O = 55.9% by mass, with a density of 1.3 g/cm3 at 20 °C. To prepare the NaOH solution, sodium hydroxide solids of 97% purity were dissolved in water a day before concrete mixing. Three molar concentrations were adopted throughout the experimental program, 8, 14, and 20 M.
2.1.2 Aggregates
Coarse aggregate used in the mix designs of this experimental program included: aggregate with a maximum size of 20 mm, aggregate with a maximum size of 10 mm, crushed aggregate with a maximum size of 4.75 mm, and white sand. Aggregate properties, including the fineness modulus and saturated surface dry (SSD) specific gravity, are provided in Table 2. Grading curves of various aggregate sizes are presented in Fig. 1.
2.2 Geopolymer mixes
Mix proportions prepared under this program are categorized into three groups, as shown in Table 3. The conclusions drawn from each group were integrated into the following groups with a target of obtaining economically efficient MK geopolymer mixes characterized by good compressive strength, workability, with the minimum possible amounts of alkaline solutions.
Group 1 incorporated five mixes (A1–A5), which were designed based on experience from the limited literature database of MK geopolymer concrete in addition to insights from research carried out on fly ash-based geopolymer concrete. Mix A1 was a trial with low amounts of alkaline solutions. Mixes A2 and A3 were identical mixes with high amounts of alkaline solutions. The only difference between them was the molarity of the NaOH solution, which were 20 M, and 14 M, respectively. It should be noted that the minor difference in the weight of NaOH solution between A2 and A3 is attributed to the different densities of the solutions (i.e., 20 M and 14 M). Mixes A4 and A5 were prepared to be identical in all aspects except that the sodium silicate in A5 was almost double the amount in A4.
Group 2 included six mixes (B1-B6), in which B1, B2, and B3 were planned to have no water, and 45 kg/m3 of NaOH solution. The quantity of MK in three mixes was 400 kg/m3, 300 kg/m3, and 400 kg/m3, respectively, whereas the planned quantity of sodium silicate solution in three mixes was 90 kg/m3, 90 kg/m3, and 135 kg/m3, respectively. Mixes B4, B5, and B6 were planned to be identical to B1, B2, and B3 but with different molarity of NaOH solutions varying from 8 to 14 M. However, it was expected from the outcome of Group 1 results that issues of workability will be faced during mixing as the planned mixes represented the minimum or close to minimum amounts possible of NaOH solution, sodium silicate solution, and additional water. Hence, for each mix of this group, mix ingredients were added as planned, and adjustments in the liquids were made until just workable condition was reached. This strategy was adopted to optimize the mix proportions with the minimum possible number of trial mixes.
Group 3 comprised a total of five mixes: C1 to C5. Mixes C1 and C3 were designed to have molar ratios (i.e., SiO2/Al2O3, H2O/Na2O, and Na2O/Al2O3) the same as the mix B5 but with metakaolin content of 400 kg/m3, and 350 kg/m3, respectively, and adjusting other mix proportions. However, after mixing both C1 and C3, it was noticed that both mixes were very dry, and hence, additional water was added, which changed the H2O to Na2O ratio from 12.53 (as in B5) to 14.54, and 13.98, respectively. Similarly, C2 and C4 mixes were designed to have molar ratios close to the mix A3 from Group 1 but with lower MK content (350 kg/m3, and 400 kg/m3). Mix C5 was the same as mix B5 from Group 2, but the coarse aggregate used was in an SSD condition instead of oven-dried condition. In all mixes of Group 3, aggregate content of different sizes remained identical to the amounts used in the reference mixes A3 and B5, but the coarse aggregate of 20 mm was excluded and replaced by 10 mm aggregate. This was done to address the issues of workability and size effect on small test cylinders.
Mix design variables namely percentage of aggregate (from the total wet weight of the mix), sodium silicate to sodium hydroxide ratio, water to solids ratio, and alkaline solids to metakaolin ratio, along with associated molar ratios (Na2O/SiO2, SiO2/Al2O3, H2O/Na2O, and Na2O/Al2O3) are summarized in Table 4 for each mix.
2.3 Casting and curing procedure
All concrete components, including aggregates and metakaolin, were dry mixed for two minutes. Alkaline solutions and additional water were then added and mixed for a few minutes until a homogenous mixture was obtained. The slump test was conducted as per ASTM C143 [25] to assess the concrete workability. Concrete was then cast in 100 × 200 mm standard cylinders to assess the mix compressive strength, as per ASTM C39 [26].
Specimens were left in the ambient conditions for 24 h then demolded. For Group 1 specimens, three curing schemes were adopted, namely (1) laboratory temperature 24 ± 2 °C, and 20% ± 2% relative humidity, (2) temperature control room at 40 °C and 10–12% relative humidity, and (3) temperature and humidity control chamber at 35 °C temperature, and 70% relative humidity. Concrete cylinders were cured for 28 days before testing. However, Group 2, and Group 3 specimens were cured at the laboratory room temperature of 24 ± 2 °C and 20% ± 2% relative humidity for 7 and 28 days.
3 Test Results and Discussion
3.1 Group 1 mixes
3.1.1 Workability
Workability of the geopolymer concrete mixes varied across the mixes A1-A5. Poor workability was observed for mix A1 with nearly zero slump. However, better workability was obtained for the remaining mixes with about 150 mm for A2, 250 mm for the mixes A3, and A5, and 90 mm for mix A4. It is well noted that the workability of conventional cement concrete is primarily affected by the water to cement ratio, which is equivalent to the water to solids ratio in geopolymer concrete. Generally, water to solids ratio might not be the only controlling parameter for geopolymer concrete workability; however, aggregate content in the mix can significantly affect the workability, e.g., mixes A1 and A2. Although both mixes have similar water to solids ratio (0.34 and 0.36, respectively), a huge difference is observed in the workability properties primarily due to the different amounts of aggregate in the two mixes, which are 71.4%, and 63.1%, respectively.
3.1.2 Compressive strength
Compressive strength results for the five mixes A1–A5 ranged from 16.5 MPa to 48.3 MPa, as shown in Fig. 2. Under laboratory curing conditions, both mixes A2 and A3 attained high compressive strength compared to other mixes of about 44.3 MPa, and 48.3 MPa, respectively. However, lower strength values of 25.3 MPa, 16.5 MPa, and 22 MPa were obtained for mixes A1, A4, and A5, respectively. In fact, the better performance of mixes A2 and A3 is attributed to the high amounts of alkaline solutions that directly enhanced Na2O/SiO2 and Na2O/Al2O3 ratios compared to other mixes. Furthermore, both mixes had a lower water content than the remaining mixes (i.e., lower H2O/ Na2O).
3.1.2.1 Influence of Curing Conditions
Curing conditions of the specimens under (1) laboratory temperature of 24 ± 2 °C, and 20% ± 2% relative humidity, (2) temperature control room at 40 °C, and 10–12% relative humidity, and (3) 35 °C temperature, and 70% relative humidity, had an insignificant effect on the compressive strength of various geopolymer concrete mixes. Changing the curing environment from laboratory temperature of 24 ± 2 °C and relative humidity of 20% ± 2% to a temperature of 40 °C and 10% to 12% relative humidity resulted in an increase of 1.8% and 11.5% in compressive strength for mixes A1, and A4, respectively. However, the compressive strength for other mixes reduced from 3.3% to 6.3%. Similarly, no clear trend was observed when the curing conditions changed from laboratory conditions to 35 °C temperature and 70% relative humidity, as the compressive strength increased from 3.4% to 12.2% for all mixes except A2 and A3. Thus, it was decided to consider only curing at laboratory temperature for Group 2 and Group 3 mixes since insignificant variation in the compressive strength values was observed among different curing schemes, which is also an energy-efficient option.
3.1.2.2 Influence of NaOH Molarity
The effect of sodium hydroxide molarity on the compressive strength of geopolymer concrete can be inferred from the comparison of the compressive strength of mixes A3, and A2 in Fig. 2. Two values of molarity were initially selected as 14 M and 20 M for mixes A3, and A2, respectively. The former has been commonly adopted by researchers [27,28,29], while the latter represents the highest molarity level for NaOH solution. Although both mixes achieved high compressive strength values of more than 41 MPa, increasing molarity from 14 to 20 M resulted in a drop in the compressive strength of 8.2%, 9.9%, and 14.1% for specimens cured at laboratory temperature of 24 ± 2 °C and 20% ± 2% relative humidity, the temperature control room at 40 °C and 10–12% relative humidity, and 35 °C temperature and 70% relative humidity, respectively. Such a trend between the molarity and compressive strength is matching with research conducted elsewhere [10, 30]; however, an opposite trend has also been reported [28, 31, 32]. Generally, a higher concentration of NaOH solution is desirable as it aids in the detachment of silica and alumina from metakaolin, and hence promotes the monomer bond and improves the polymerization process. However, the compressive strength reduction when the molarity of NaOH solution was increased to 20 M might be attributed to the presence of a high alkaline environment, which could weaken silicate anions connectivity, leading to a limited polymerization and a lower compressive strength [33]. This justification is further evidenced by the fact that molar ratios in terms of sodium oxide to silicon oxide and sodium oxide to aluminum oxide are higher in the A2 mix than in A3. Based on the observations from mixes A2 and A3, it was decided to optimize the alkaline solutions for Group 2, and Group 3 mixes based on NaOH molarity not exceeding 14 M.
3.1.2.3 Influence of Sodium Silicate Content
It is shown from Fig. 2 that doubling the amount of sodium silicate solution (mix A4 vs. A5) resulted in a compressive strength enhancement of 33.5%, 15.8%, and 25.4% for specimens cured at laboratory temperature of 24 ± 2 °C and 20% ± 2% relative humidity, the temperature control room at 40 °C and 10–12% relative humidity, and 35 °C temperature and 70% relative humidity, respectively. In fact, this trend is consistent with the outcomes of previous research on fly ash-based geopolymer [28, 34, 35]. Increasing the amount of sodium silicate solution results in more silica gel from metakaolin, which contributes to the formation of denser Si–O–Si bonds in the geopolymer. Furthermore, the Si–O–Si bond is stronger than Si–O–Al and Al–O–Al bonds, and hence resulting in a higher compressive strength [21]. This is also consistent with silica content in mix A5 as compared to mix A4 since the amount of silicon oxide to aluminum oxide is higher in mix A5 than in mix A4 (2.81 vs. 2.42).
3.2 Group 2 mixes
3.2.1 Workability
Workability was a controlling parameter for Group 2 mixes. Based on the observations made on the Group 1 mixes, a combination of good concrete strength and workability was obtained, especially for mixes A2 and A3. However, the amount of alkaline solutions for those mixes was very high and not generally practical from an economic point of view. Therefore, Group 2 were designed to optimize the amount of alkaline solutions and hence the mixes were tried with NaOH not exceeding 45 kg/m3 and sodium silicate varying from 90 to 135 kg/m3 without additional water, as these proportions were generally adopted by other researchers, e.g., [21]. The trial mixes were very dry. Thus, adjustments in the mix liquids were made to fix the quantities of materials and reach to the just moldable conditions (nearly zero slump).
3.2.2 Compressive strength
3.2.2.1 Influence of NaOH Molarity
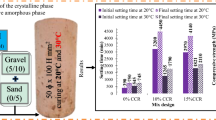
The 28-days compressive strength of mixes B1-B3 was low, which ranged from 11.8 MPa to 16.0 MPa (Fig. 3). However, the mixes B4-B6 had a better compressive strength between 20 MPa to 30.1 MPa. The enhancement in the compressive strength for the mixes B4-B6 compared with the mixes B1-B3 is attributed to the higher molarity of NaOH solution (8 M vs. 14 M).
3.2.2.2 Influence of Curing Age
Compressive strength development for the samples was assessed based on testing at 7 and 28 days for mixes B1–B6, as presented in Fig. 4. At 7-days of age, concrete specimens developed 48%, 73%, and 64% of the compressive strength at 28 days for mixes B1, B2, and B3, respectively. A similar trend was also observed but with a higher strength development rate for mixes B4, B5, and B6, which, respectively, accounted for 87%, 95%, and 80% of the 28-days compressive strength. Better early strength development was achieved for B4-B6 primarily due to the higher molarity of NaOH solution (as reflected in higher Na2O/SiO2, higher Na2O/ Al2O3, and lower H2O/ Na2O) than mixes B1-B3. The lowest early strength development rate was observed for mix B1, among other mixes since the Na2O/ SiO2 was very low at about 0.17; however, the opposite is true for mix B5. Generally, high early strength gain is dependent on the speed of the geopolymerization process, and the amount of reaction products.
3.3 Group 3 mixes
3.3.1 Workability
Mixes of this group had better workability than Group 2 mixes, slump results were 135 mm, 120 mm, 60 mm, 80 mm, and zero, respectively, for mixes C1, C2, C3, C4, and C5. A comparison of the test results of C2 and C3 shows that though there is an increase in the water to solids ratio from 0.44 for C2 to 0.46 for C3, the slump got reduced by half, i.e., from 120 to 60 mm, which might be due to the increase in the percentage of aggregates from 69.8% to 73.2%. Thus, the slump is affected by the water to solids ratio as well as the total aggregate percentage. Furthermore, using aggregate under saturated surface conditions in mix C5 had an insignificant influence on the workability properties as the slump was almost the same (i.e., zero) for mixes B5 (oven dried conditions) and C5.
3.3.2 Compressive strength
The compressive strength tests of the mixes C1–C5 were conducted after 7 and 28 days of curing, as shown in Fig. 5. Compressive strengths after 28 days for mixes C2 and C4 were 45.4 MPa, and 46.4 MPa, respectively, which were the highest in Group 3 mixes. However, low compressive strength was obtained for mixes C1, C3, and C5, which was 15.5 MPa, 20.2 MPa, and 21.2 MPa, respectively, for the three mixes. Compressive strength developed after 7 days accounted for 82% to 89% of the compressive strength achieved after 28 days due to the relatively high Na2O/SiO2 and Na2O/Al2O3 ratios, which is generally consistent with trend explained for mixes B1-B3 vs. B4-B6.
3.3.2.1 Influence of Molar Ratios and Aggregate Water Absorption
It is shown in Fig. 6a that the 28-days compressive strength for mixes A3, C2, and C4 are almost the same since these were designed to have similar molar ratios. However, this observation might not always hold since mixes of similar molar ratios may face issues with workability, e.g., mix B5 vs. C1 vs. C3. Both mixes C1 and C3 due to workability issues needed additional water (to be moldable), which resulted in increasing the H2O/Na2O ratio and consequently reduced the compressive strength, as shown in Fig. 6b. It can be concluded from Fig. 6a, b that the compressive strength is affected by both the molar ratios of the binder as well as the relative amounts of the aggregate and the binder. Furthermore, using coarse aggregate with saturated surface dry conditions instead of oven-dried conditions (mix C5 vs. B5) resulted in a significant strength reduction from 30.1 MPa to 21.2 MPa, as shown in Fig. 6c. In fact, the additional water absorbed by the aggregate pores increased the amount of H2O/Na2O ratio, which affected the compressive strength.
4 Model for Predicting Workability
The above discussion indicates that besides the water to solids ratio, the slump of the geopolymer mixes is also affected by the percentage of aggregates. In order to study the effect of both the variables (i.e., total aggregate percentage, and water to solids ratio) simultaneously on the slump, a bubble chart is plotted for slump values of different mixes of the three groups, as shown in Fig. 7. The bubble area is proportional to the slump value in mm. The geopolymer mixes of zero slump have a high percentage of aggregate and low water to solids ratio. It is observed from the figure that the increase in the quantity of aggregate raises the requirement of the water to solids ratio for achieving the desired workability. The regression analysis of the data for achieving the slump in the range of 100 ± 25 mm gives the following relationship:
where, \(w/s\) is the water to solids ratio for workable geopolymer mixes with the slump in the range of 100 ± 25 mm, and \({p}_{c}\) is the total aggregate percentage taken as a fraction from the total wet weight of the mix. The range of \({p}_{c}\) for the applicability of the above model, mentioned in Eq. (1), is based on the experimental data used in the derivation of the model.
5 Correlation between the Compressive Strength and the Molar Ratios
Concrete compressive strength is generally dictated by the proportions of its components. Unlike cement concrete, the compressive strength of geopolymer concrete is governed by both the binder chemical composition as well as the aggregate content in the mix. The chemical composition of the mix is often described by molar ratios, namely: Na2O/SiO2, Na2O/Al2O3 H2O/Na2O, and SiO2/Al2O3. Relationships in the form of contours were suggested in the literature for MK-based geopolymer [36, 37]. It is important to note that those suggested relationships were developed based on a limited number of samples for geopolymer paste /slurry; in other words, the implication of aggregate was not incorporated into those relationships.
Although this paper presents a limited number of mixes that might not be exhaustive enough to define ternary plots or contours, it will present initial recommendations on molar ratio thresholds to obtain certain compressive strength class. Compressive strength of all mixes were plotted against sodium oxide to silicon oxide ratio, sodium oxide to aluminum oxide ratio, and water to sodium oxide ratio in Figs. 8, 9, and 10, respectively. The data were divided into two classes of compressive strength, one less than or equal to 25 MPa, and another above 25 MPa. It can be seen from Fig. 8 that when the sodium oxide to silicon oxide ratio was higher than or equal to 0.3, all specimens achieved a compressive strength of at least 45 MPa. Specimens with less than 0.3 had a strength of less than 30 MPa. Similarly, the mixes with the sodium oxide to aluminum oxide ratio of 0.8 or higher had a compressive strength of 45 MPa or more, while lower sodium oxide to aluminum oxide ratio had a compressive strength of 25 MPa or less, as shown in Fig. 9. Moreover, water to sodium oxide ratio of more than 12 resulted in compressive strength of 25 MPa or less, as shown in Fig. 10.
In conclusion, MK geopolymer concrete with an aggregate percentage from 63 to 77% can be produced with a compressive strength greater than 25 MPa given that the sodium oxide to silicon oxide ratio is higher than or equal to 0.3, sodium oxide to aluminum oxide ratio of 0.8 or higher, and water to sodium oxide ratio not exceeding 12.
6 Conclusions
In this research, sixteen concrete mixes of MK-based geopolymer concrete were prepared under three groups to study the sensitivity of concrete compressive strength to the variations in curing conditions, curing age, NaOH molarity, sodium silicate content, molar ratios, and aggregate water absorption. Main findings from this study can be summarized as follows:
-
There was an insignificant change in the compressive strength for different curing conditions adopted involving variation in temperature from 24 °C to 40 °C and relative humidity from 10 to 70%.
-
Increasing NaOH molarity from 14 to 20 M resulted in a drop in the compressive strength varying from 8.2% to 14.1%; however, increasing molarity from 8 to 14 M resulted in a significant strength improvement. Doubling the amount of sodium silicate resulted in a compressive strength enhancement from 15.8% to 33.5%.
-
Higher early strength development rate was obtained for mixes with higher NaOH molarity as reflected in higher Na2O/SiO2, higher Na2O/ Al2O3, and lower H2O/Na2O. The 7 days compressive strength for the mixes with 14 M NaOH solution ranged from 80 to 95% of the 28 days strength.
-
Using coarse aggregate with saturated surface dry conditions instead of oven-dried conditions resulted in a significant strength reduction from 30.1 MPa to 21.2 MPa as water absorbed by the aggregate pores increased the H2O/Na2O ratio.
-
Besides the water to solids ratio, the workability is also affected by the aggregate content. A linear relationship is obtained between the water to solids ratio and the total aggregate percentage for workable geopolymer mixes with a slump in the range of 100 ± 25 mm.
-
The present study indicates that for producing MK-based structural geopolymer concrete with a compressive strength greater than 25 MPa, the sodium oxide to silicon oxide and sodium oxide to aluminum oxide ratios should be higher than or equal to 0.3 and 0.8, respectively, and water to sodium oxide ratio should not exceed 12.
References
Kumar, P.; Pankar, C.; Manish, D.; Santhi, A.S.: Study of mechanical and microstructural properties of geopolymer concrete with GGBS and Metakaolin. Mater. Today Proc. 5(14), 28127–28135 (2018)
Heah, C.Y.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Binhussain, M.; Luqman, M.; Khairul Nizar, I.; Ruzaidi, C.M.; Liew, Y.M.: Effect of curing profile on kaolin-based geopolymers. Phys. Proc. 22, 305–311 (2011)
Okoye, F.N.; Durgaprasad, J.; Singh, N.B.: Mechanical properties of alkali activated flyash/Kaolin based geopolymer concrete. Constr. Build Mater. 98, 685–691 (2015)
Aliabdo, A.A.; Abd Elmoaty, A.M.; Salem, H.A.: Effect of cement addition, solution resting time and curing characteristics on fly ash based geopolymer concrete performance. Constr. Build. Mater. 123, 581–593 (2016)
Nazari, A.; Bagheri, A.; Sanjayan, J.G.; Dao, M.; Mallawa, C.; Zannis, P.; Zumbo, S.: Thermal shock reactions of Ordinary Portland cement and geopolymer concrete: microstructural and mechanical investigation. Constr. Build. Mater. 196, 492–498 (2019)
Kathirvel, P.; Kaliyaperumal, S.R.M.: Influence of recycled concrete aggregates on the flexural properties of reinforced alkali activated slag concrete. Constr. Build. Mater. 102, 51–58 (2016)
He, J.; Jie, Y.; Zhang, J.; Yu, Y.; Zhang, G.: Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 37, 108–118 (2013)
Zabihi, S.M.; Tavakoli, M.; Mohseni, E.: Engineering and microstructural properties of fiber-reinforced rice husk–ash based geopolymer concrete. J Mater. Civ. Eng. 30(8), 1–10 (2018)
Bondar, D.; Lynsdale, C.J.; Milestone, N.B.; Hassani, N.; Ramezanianpour, A.A.: Engineering properties of alkali-activated natural pozzolan concrete. ACI Mater. J. 108, 1–9 (2011)
Kantarcı, F.; Türkmen, İ.; Ekinci, E.: Optimization of production parameters of geopolymer mortar and concrete: a comprehensive experimental study. Constr. Build. Mater. 228, 1–17 (2019)
Sagoe-Crentsil, K.; Weng, L.: Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: part II. High Si/Al ratio systems. J. Mater. Sci. 42, 3007–3014 (2007)
Weng, L.; Sagoe-Crentsil, K.: Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: part I—Low Si/Al ratio systems. J. Mater. Sci. 42, 2997–3006 (2007)
Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y.: Geopolymerization process of alkali–metakaolinite characterized by isothermal calorimetry. Thermochim Acta 493(1–2), 49–54 (2009)
Zhang, Y.; Sun, W.: Semi-empirical AM1 calculations on 6-memebered alumino-silicate rings model: implications for dissolution process of metakaoline in alkaline solutions. J Mater Sci 42(9), 3015–3023 (2007)
Yunsheng, Z.; Wei, S.; Zongjin, L.; Yantao, J.: Study of polycondensation process of metakaolin-based geopolymeric cement using semi-empirical AM1 calculations. Adv. Cem. Res. 21(2), 67–73 (2009)
Bing-hui, M.; Zhu, H.; Xue-min, C.; Yan, H.; Si-yu, G.: Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 99, 144–148 (2014)
Muñiz-Villarreal, M.S.; Manzano-Ramírez, A.; Sampieri-Bulbarela, S.; Gasca-Tirado, J.R.; Reyes-Araiza, J.L.; Rubio-Ávalos, J.C.; Pérez-Bueno, J.J.; Apatiga, L.M.; Zaldivar-Cadena, A.; Amigó-Borrás, V.: The effect of temperature on the geopolymerization process of a metakaolin-based geopolymer. Mater. Lett. 65(6), 995–998 (2011)
Perera, D.S.; Uchida, O.; Vance, E.R.; Finnie, K.S.: Influence of curing schedule on the integrity of geopolymers. J. Mater. Sci. 42, 3099–3106 (2007)
Rovnaník, P.: Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build Mater. 24(7), 1176–1183 (2010)
Pires, E.F.C.; Azevedo, C.M.C.; Pimenta, A.R.; Silva, F.J.; Darwish, F.A.I.: Fracture properties of geopolymer concrete based on metakaolin, fly ash and rice rusk ash. Mater. Res. 20, 630–636 (2017)
Mohseni, E.: Assessment of Na2SiO3 to NaOH ratio impact on the performance of polypropylene fiber-reinforced geopolymer composites. Constr. Build Mater. 186, 904–911 (2018)
Alanazi, H.; Yang, M.; Zhang, D.; Gao, Z.: Early strength and durability of metakaolin-based geopolymer concrete. Magn. Concr. Res. 69(1), 46–54 (2017)
Pouhet, R.; Cyr, M.: Formulation and performance of flash metakaolin geopolymer concretes. Constr. Build. Mater. 120, 150–160 (2016)
Xie, J.; Chen, W.; Wang, J.; Fang, C.; Zhang, B.; Liu, F.: Coupling effects of recycled aggregate and GGBS/metakaolin on physicochemical properties of geopolymer concrete. Constr. Build. Mater. 226, 345–359 (2019)
Standard, A. S. T. M.: ASTM C-143 Standard test method for slump of Portland cement concrete. ASTM International (1990)
Standard, A. S. T. M.: ASTM C39 Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International (2015)
Albitar, M.; Visintin, P.; Ali, M.S.M.; Drechsler, M.: Assessing behaviour of fresh and hardened geopolymer concrete mixed with class-F fly ash. KSCE J. Civ. Eng. 19, 1445–1455 (2015)
Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.J.; Rangan, B.V.: On the development of fly ash-based geopolymer concrete. Mater. J. 101, 467–472 (2004)
Nath, P.; Sarker, P.K.: Flexural strength and elastic modulus of ambient-cured blended low-calcium fly ash geopolymer concrete. Constr. Build Mater. 130, 22–31 (2017)
Aliabdo, A.A.; Abd Elmoaty, A.M.; Salem, H.A.: Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build Mater. 121, 694–703 (2016)
Parveen, S.D.; Junaid, M.T.; Jindal, B.B.; Mehta, A.: Mechanical and microstructural properties of fly ash based geopolymer concrete incorporating alccofine at ambient curing. Constr. Build Mater. 180, 298–307 (2018)
Wang, H.; Li, H.; Yan, F.: Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surf. A Physicochem. Eng. Asp. 268(1–3), 1–6 (2005)
Sperberga, I.; Rundans, M.; Cimmers, A.; Krage, L.; Sidraba, I.: Mechanical properties of materials obtained via alkaline activation of illite-based clays of Latvia. J. Phys. Conf. Ser. 602, 1–4 (2015)
Monita, O.; Nikraz, H.: Properties of fly ash geopolymer concrete designed by Taguchi method. Mater. Des. 36, 191–198 (2012)
Pan, Z.; Sanjayan, J.G.; Rangan, B.V.: Fracture properties of geopolymer paste and concrete. Magn. Concr. Res. 63(10), 763–771 (2011)
Rowles, M.R.; O'connor B, : Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 13, 1161–1165 (2003)
Lahoti, M.; Narang, P.; Tan, K.H.; Yang, E.: Mix design factors and strength prediction of metakaolin-based geopolymer. Ceram. Int. 43(14), 11433–11441 (2017)
Acknowledgements
he authors are grateful to the Deanship of Scientific Research, King Saud University, for funding through Vice Deanship of Scientific Research Chairs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Rights and permissions
About this article
Cite this article
Alghannam, M., Albidah, A., Abbas, H. et al. Influence of Critical Parameters of Mix Proportions on Properties of MK-Based Geopolymer Concrete. Arab J Sci Eng 46, 4399–4408 (2021). https://doi.org/10.1007/s13369-020-04970-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04970-0