Abstract
The various benefits of biofuels versus fossil fuels due to recent global challenges and issues are the best approach toward low-cost economic production of renewable energy. This study is trying to obtain economic catalysts with easy fabrication technology. The synthesized catalysts were obtained using calcium oxide/nanoclay catalysts by an initial ion-exchange reaction of calcium oxide and nanoclays (montmorillonite). These catalysts have been synthesized for the first time by being stirred for 5 h at a temperature of 80 °C, and the colloidal supernatant is obtained and kept in an ultrasonic bath for 20 min. The solution was filtered, washed several times, the residual mixture on filter paper was dried in the oven at 50 °C for few hours, and the powder was calcined for 8 h in a furnace at 600 °C. After identification and characterization, using XRD, BET, and SEM, the results approved the formation of a new nanostructure in synthesized catalysts, which were suitable to be used in biodiesel production from waste oils with high free fatty acids content. The results of this study indicate that the catalysts production process is not complicated, and methyl ester production rates in all biodiesel samples were more than 97% (97.1–98.8%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Rapid urbanization and energy consumption are the main challenges due to sustainable energy saving and clean production. Fossil fuels are the largest source of energy which are readily available and are considered as nonrenewable resources [1]. These resources are expensive and also cause environmental pollution which is not sustainable. To find renewable sources of energy with less environmental pollution is one of the most effective approaches to tackle the issues. Biodiesel is a biofuel that is more environmentally friendly than fossil fuels. Research results show that biofuels not only emit far less soot, but the soot from these fuels is oxidized much easier than the soot from fossil fuels [2].
Biodiesels are one of the biodegradable, nontoxic, and renewable fuels [3]. Biodiesel is a diesel fuel comprising fatty acid esters that are prepared from renewable sources such as edible oils like palm oil [4, 5] and castor oil [6], non-edible oils [7], waste oils [8], and algae [9, 10]. Biodiesel is typically produced through the transesterification reaction of oil. In this reaction, different ratios of oil and alcohol react to produce fatty acid esters. Although the reaction is carried out without a catalyst, the cost of production will be much higher and the reaction is not justified economically. Researchers are looking for economic ways to produce biodiesel using renewable materials [11]. Strategies of using the residual vegetable oil waste are of very low-cost and effective catalysts for the biodiesel production.
From the beginning of the laboratory-scale and industrial-scale biodiesel production, many catalysts such as acidic or basic homogeneous catalysts, heterogeneous catalysts, nanocatalysts, and biocatalysts have been used in this reaction [12], but in the industrial-scale, the homogeneous catalysts are generally used. Environmental pollution and high amount of waste water become the disadvantages of homogeneous catalyst, and the investigation for suitable heterogeneous catalysts which can be regenerated, reused, and operated in continuous process becomes the main concern of the recent studies [1]. Each of the aforementioned catalysts has advantages and disadvantages; most of the prepared catalysts do not have the ability to catalyze the oil with high free fatty acids; or if the catalyst has this ability, it has high cost and complex manufacturing process. For these reasons, these synthesized new catalysts remain in the laboratory scale.
In recent years, nanoclays due to their exceptional properties that it has rather less cost and time than older methods [3] are taken into consideration. The most common clay is montmorillonite, which is an aluminosilicate clay made up of plates compact together. Montmorillonite group, due to its nanometer dimension layered structure, and easy separation of these layers, has a very high chemical activity. Also, the isomorphous substitution effect in montmorillonite causes its cations to be readily replaced with other cations, even with different charges. Due to these unique properties, montmorillonite has many industrial applications, and extensive research on their application in various fields such as production of polymer nanocomposites has been carried out [13,14,15,16]. Montmorillonite as the catalyst was used in 1968 for the first time [17]. In recent decades, this clay is used as a catalyst in various reactions in both industrial and laboratory scales. This has been used for the dimerization of fatty acids to carboxylic acids and alkylation of phenols. Clays are also used in the production of biodiesel, such as the use of acidic silica for converting oils with high fatty acid contents [18], modification of bentonite [19] or montmorillonite [20] with acid and use in biodiesel production, or the use of bentonite with a base such as KOH for the catalytic production of biodiesel [21]. Clays such as K10 or KSF naturally occurring or modified with acid have also been used for biodiesel production. But in most of these studies, the percentage of methyl esters in obtained biodiesel is lower than 50% [22, 23]. In previous studies, the improvement in process of biodiesel production from castor oil using functionalized mesoporous silica HMS and MCM-41 with different catalysts such as amine and lysine was carried out [24]. To date, the usage of montmorillonite and alkaline property of CaO as catalyst has not been investigated.
In this study, using the isomorphous substitution property of montmorillonite and alkaline property of CaO, several catalysts were synthesized and investigated in order to facilitate the ease of synthesis and also to catalyze the oil with high fatty acids. The significant amount of Bentonite reservoir in country and the desirable usage of these clays as catalysts will be one of the best sustainable options for biodiesel production.
2 Experimental Section
2.1 Materials
Nanoclay Cloisite 15A was purchased from Southern Clay Products, K-10, Na-montmorillonite was provided by Sigma-Aldrich. CaO, methanol, and n-hexane were obtained from Merck. Waste oil was produced from the potato chips factory that was filtered to remove particles and solid impurities.
2.2 Synthesis of Catalysts
All of the catalysts, used in this study, were synthesized by a similar method; the ion-exchange reaction between calcium oxide and nanoclays has not been investigated as a catalysis in other studies. The favorable properties of these materials have a significant desire to have ester exchange with biodiesel. The conventional methods mentioned in the literature for the ion-exchange reaction of montmorillonite with organic and inorganic ions were used [25].
First, clay and calcium oxide in different weight proportions were added into a glass reactor equipped with a magnetic stirrer. After adding deionized water, it was dispersed via ultrasonication for a period of time of 20 min until adequate clay and calcium oxide in water were obtained. A mixture of clay and calcium oxide was stirred by a magnetic stirrer for 5 h at the temperature of 80 °C, and the colloidal supernatant was obtained and kept in an ultrasonic bath for 20 min. The solution was filtered, washed several times, the residual mixture on filter paper was dried in the oven at 50 °C for few hours, and the powder was calcined for 8 h in a furnace at 600 °C.
Nanocatalysts provided with different ratios of calcium oxide and nanoclays were used in the biodiesel production. With respect to the percentage of methyl esters in biodiesel production, the optimum ratio of calcium oxide to nanoclays for the synthesis of nanocatalyst was determined. The highest percentage of methyl esters in biodiesel, with an equal proportion of nanocatalyst and calcium oxide, was obtained (Table 1).
2.3 Transesterification Reaction
Synthesized nanocatalysts were used in biodiesel production with transesterification reaction method, using waste oil from potato chips factory with properties listed in Table 2. Waste oil and methanol (with oil/methanol weight ratio of 1:3) and 1% by weight nanocatalyst were added to a glass reactor, and the reaction was carried out using magnetic stirring under controlled conditions of temperature. The reaction temperature remained in the range of 65 °C (± 2 °C). After 3 h, the reaction was stopped. At several times after the start of the reaction, the biodiesel production was sampled. Samples were washed, and for the percentage of methyl esters detection, GC-MS tests were prepared.
3 Results and Discussion
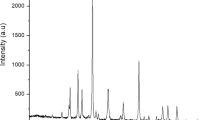
For structural evaluation of the synthesized nanocatalysts, all samples were analyzed using X-ray diffraction technique (XRD) Siemens D5000 instrument. Results of raw materials and synthesized nanocatalysts after calcination are compared in Fig. 1. As the figure shows, most of the samples have broad peaks, and raw materials and calcined catalysts have various phases. The results show that a new phase was composed into the synthesized nanocatalysts.
Synthesized nanocatalysts were identified by BET analysis using BELSORP-mini II (BEL. Japan, Inc.) analyzer used in order to determine the specific surface area before and after calcination. For BET test, after the exposure of cell sample in liquid nitrogen tank, with a gradual increase in nitrogen gas pressure at each stage, the amount of gas absorbed by the sample was calculated. Thereafter, the gradual decrease in gas pressure performed and desorption rate was measured. The diagrams of adsorbed and desorbed nitrogen volume by the sample at a constant temperature were plotted. Based on the BET theory, with measured values of gas absorption and desorption, the specific surface area was calculated. BET test results of the synthesized nanocatalysts are shown in Table 3.
The results confirmed that the nanostructure of the samples with a specific surface area of catalysts after calcination was much greater, and the catalytic sites of the samples were significantly increased. Results of catalysts specific surface area, with measuring the percentage of methyl esters in biodiesel production, were confirmed (97–98%).
The scanning electron microscope (SEM) images of the nanocatalysts before and after calcination are shown in Fig. 2. For SEM analysis using Cambridge S360 SEM instrument, a few amount of each sample was dispersed in acetone and was placed under the ultrasonic process. During ultrasonic, a glass slide was immersed in the solution, and then, the slide was dried, covered with gold and used for the SEM analysis.
Figure 2a shows nanocatalyst 1 (CaO/ Cloisite 15A) before calcination; the two phases are evident. Needle-shaped particles are observed along the clay layers, and the two phases are quite distinct. Figure 2b shows nanocatalyst 1 after calcination. According to the figure, it can be seen that after calcination, only one phase is observed and there are no longer two separate phases. Also, the catalyst mass contains high porosity compared to before.
Figure 2c shows nanocatalyst 2 before being calcined. There are two phases in this figure again. Discrete layers of clay are visible, and there are so many pores in the mass. The ion-exchange reaction between clay and calcium oxide in Fig. 2a, due to the low distance between the layers in Na MMT, is reduced. In Fig. 2d that shows catalyst 2 after calcination, distinct layers of clay are not observed and the mass is quite different from the mass before calcination. Instead of separate layers, spongy masses with many pores can be seen. According to this figure, it can be said that after calcination, catalytic sites are created in the catalyst.
The nanocatalyst synthesized with K10 clay, before calcination (Fig. 2e), is partly different from other clays. Two distinct phases are not clearly observed in the structure of this catalyst. Needle-shaped particles could not be seen, and mass has many pores. The SEM image of nanocatalyst 3 after calcination is shown in Fig. 2f. According to this figure, we can say that after calcination, the mass is homogeneous and quite spongy. Pores in this nanocatalyst are much more than in other catalysts, and pore sizes are in the nanometer range. By calcination, many catalytic sites in the catalyst are formed.
In Fig. 3 a, a comparison between the calcined nanocatalysts was done. Catalysts difference is evident in this image. It can be seen that the nanocatalyst 3 (synthesized with K10 MMT) has the highest porosity and is expected to show better catalytic behavior. This prediction with the GC-MS results of produced biodiesel was confirmed.
According to the results, it can be seen that in all biodiesel samples, the amount of methyl esters after up to 3 h of reaction is more than 95% (Table 4). That shows the efficiency of the synthesized nanocatalysts in producing biodiesel from heavy oils (such as waste oils) with high free fatty acids.
Synthesized nanocatalysts were used in the reaction of biodiesel production, using waste edible oil with high fatty acids. To ensure the production of biodiesel using synthesized nanocatalysts, the amount of methyl esters in biodiesel production was measured.
Biodiesel samples were identified using GC-MS, Agilent 6890 N and Mass: Agilent 5973 N, Agilent Company. To prepare biodiesel samples for GC-MS analysis, after the measurement of pH and neutralization, separation of glycerin and catalyst and removal of water content were conducted. The prepared samples were diluted with normal hexane.
According to the results shown in Table 4, all biodiesel samples contain more than 95% of methyl esters after up to 3 h period time of the reaction. This agreed that the method has the highest efficiency of the synthesized nanocatalysts in producing biodiesel from heavy oils (such as waste oils) with high free fatty acids [6].
4 Conclusion
The new nanocatalysts were synthesized using calcium oxide, and three types of montmorillonite nanoclay and after identification was used to produce biodiesel from potato chips factory’s waste oil. By comparing the XRD peak of catalysts with the peak of raw materials, it can be seen that the new phase is formed in catalysts during the calcination reaction. BET results also indicated the formation of nanostructure in all synthesized nanocatalysts. It was observed that among these nanocatalysts, the highest specific surface area was related to calcium oxide/ K10 montmorillonite, and the lowest was related to calcium oxide/ Cloisite 15A. The low specific surface area in calcium oxide/ Cloisite 15A may be due to the presence of large organic ions that had been used for the organic modification of Cloisite 15A nanoclay. By examining the SEM images of catalysts, it can be concluded that the synthesized nanocatalyst with K10 montmorillonite clay (CaO/ K10 MMT) has a sponge structure and its catalytic sites are higher than others.
Biodiesel produced from each of the synthesized nanocatalysts for studying the formation of methyl esters was identified by gas chromatography–mass analysis. The formation of methyl esters in all biodiesel samples was obtained over 95%, which indicates the efficiency of synthesized nanocatalysts. Also, according to the results of GC-MS, it was observed that the biodiesel produced with calcium oxide/ K10 montmorillonite nanocatalyst has the highest percentage of methyl esters.
After completion of the reaction, all the catalysts are easily separated from the product. The sedimentation rate in calcium oxide/K10 MMT nanocatalyst is more than that in the other catalysts. This study shows that the potassium clay is a very suitable material for the preparation of catalysts for biodiesel synthesis. The countries with a significant amount of Bentonite reservoirs and the desirable usage of these clays as catalysts will be one of the best sustainable options for biodiesel production from oil wastes as it is more cost-effective and it helps the country’s economy.
References
Vahid, B.R.; Haghighi, M.; Toghiani, J.; Alaei, S.: Hybrid-coprecipitation vs. combustion synthesis of Mg–Al spinel based nanocatalyst for efficient biodiesel production. Energy Convers. Manag. 160, 220–229 (2018). https://doi.org/10.1016/j.enconman.2018.01.030
Liati, A.; Spiteri, A.; Eggenschwiler, P.D.; Vogel-Schäuble, N.: Microscopic investigation of soot and ash particulate matter derived from biofuel and diesel: implications for the reactivity of soot. J. Nanopart. Res. 14(1224), 1–18 (2012)
Alaei, S.; Haghighi, M.; Toghiani, J.; Rahmani Vahid, B.: Magnetic and reusable MgO/MgFe2O4 nanocatalyst for biodiesel production from sunflower oil: influence of fuel ratio in combustion synthesis on catalytic properties and performance. Ind. Crops Prod. 117, 322–332 (2018). https://doi.org/10.1016/j.indcrop.2018.03.015
Taufiqurrahmi, N.; Mohamed, A.; Bhatia, S.: Nanocrystalline zeolite beta and zeolite Y as catalysts in used palm oil cracking for the production of biofuel. J. Nanopart. Res. 13(8), 3177–3189 (2011). https://doi.org/10.1007/s11051-010-0216-8
Shajaratun Nur, Z.A.; Taufiq-Yap, Y.H.; Rabiah Nizah, M.F.; Teo, S.H.; Syazwani, O.N.; Islam, A.: Production of biodiesel from palm oil using modified Malaysian natural dolomites. Energy Convers. Manag. 78, 738–744 (2014). https://doi.org/10.1016/j.enconman.2013.11.012
Halek, F.; Delavari, A.; Kavousi-rahim, A.: Production of biodiesel as a renewable energy source from castor oil. Clean Technol. Environ. Policy 15(6), 1063–1068 (2013). https://doi.org/10.1007/s10098-012-0570-6
Dawodu, F.A.; Ayodele, O.; Xin, J.; Zhang, S.; Yan, D.: Effective conversion of non-edible oil with high free fatty acid into biodiesel by sulphonated carbon catalyst. Appl. Energy 114, 819–826 (2014). https://doi.org/10.1016/j.apenergy.2013.10.004
Mata, T.; Martins, A.; Caetano, N.: Valorization of waste frying oils and animal fats for biodiesel production. In: Lee, J.W. (ed.) Advanced Biofuels and Bioproducts, pp. 671–693. Springer, New York (2013)
Zayadan, B.K.; Purton, S.; Sadvakasova, A.K.; Userbaeva, A.A.; Bolatkhan, K.: Isolation, mutagenesis, and optimization of cultivation conditions of microalgal strains for biodiesel production. Russ. J. Plant Physiol. 61(1), 124–130 (2014). https://doi.org/10.1134/S102144371401018X
Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G.: Production and characterization of biodiesel from algae. Fuel Process. Technol. 120, 79–88 (2014). https://doi.org/10.1016/j.fuproc.2013.12.003
Gude, V.; Grant, G.; Patil, P.; Deng, S.: Biodiesel production from low cost and renewable feedstock. Cent. Eur. J. Eng. 3(4), 595–605 (2013). https://doi.org/10.2478/s13531-013-0102-0
Dalai, A.; Issariyakul, T.; Baroi, C.: Biodiesel production using homogeneous and heterogeneous catalysts: a review. In: Guczi, L., Erdôhelyi, A. (eds.) Catalysis for Alternative Energy Generation, pp. 237–262. Springer, New York (2012)
Ianchis, R.; Corobea, M.C.; Donescu, D.; Rosca, I.D.; Cinteza, L.O.; Nistor, L.C.; Vasile, E.; Marin, A.; Preda, S.: Advanced functionalization of organoclay nanoparticles by silylation and their polystyrene nanocomposites obtained by miniemulsion polymerization. J. Nanopart. Res. 14(11), 1–12 (2012). https://doi.org/10.1007/s11051-012-1233-6
Wong, A.; Wijnands, S.L.; Kuboki, T.; Park, C.: Mechanisms of nanoclay-enhanced plastic foaming processes: effects of nanoclay intercalation and exfoliation. J. Nanopart. Res. 15(8), 1–15 (2013). https://doi.org/10.1007/s11051-013-1815-y
Tsai, C.J.; White, D.; Rodriguez, H.; Munoz, C.; Huang, C.-Y.; Tsai, C.-J.; Barry, C.; Ellenbecker, M.: Exposure assessment and engineering control strategies for airborne nanoparticles: an application to emissions from nanocomposite compounding processes. J. Nanopart. Res. 14(7), 1–14 (2012). https://doi.org/10.1007/s11051-012-0989-z
De Maria, A.; Aurora, A.; Montone, A.; Tapfer, L.; Pesce, E.; Balboni, R.; Schwarz, M.; Borriello, C.: Synthesis and characterization of PMMA/silylated MMTs. J. Nanopart. Res. 13(11), 6049–6058 (2011). https://doi.org/10.1007/s11051-011-0496-7
Adams, J.M.; Clapp, T.V.; Clement, D.E.: Catalysis by montmorillonites. Clay Miner. 18(4), 411–421 (1983). https://doi.org/10.1180/claymin.1983.018.4.06
Shah, K.; Parikh, J.; Dholakiya, B.; Maheria, K.: Fatty acid methyl ester production from acid oil using silica sulfuric acid: process optimization and reaction kinetics. Chem. Pap. 68(4), 472–483 (2014). https://doi.org/10.2478/s11696-013-0488-4
Jeenpadiphat, S.; Tungasmita, D.N.: Esterification of oleic acid and high acid content palm oil over an acid-activated bentonite catalyst. Appl. Clay Sci. 87, 272–277 (2014). https://doi.org/10.1016/j.clay.2013.11.025
Zatta, L.; Ramos, L.P.; Wypych, F.: Acid-activated montmorillonites as heterogeneous catalysts for the esterification of lauric acid acid with methanol. Appl. Clay Sci. 80–81, 236–244 (2013). https://doi.org/10.1016/j.clay.2013.04.009
Soetaredjo, F.E.; Ayucitra, A.; Ismadji, S.; Maukar, A.L.: KOH/bentonite catalysts for transesterification of palm oil to biodiesel. Appl. Clay Sci. 53(2), 341–346 (2011). https://doi.org/10.1016/j.clay.2010.12.018
Zanette, A.F.; Barella, R.A.; Pergher, S.B.C.; Treichel, H.; Oliveira, D.; Mazutti, M.A.; Silva, E.A.; Oliveira, J.V.: Screening, optimization and kinetics of Jatropha curcas oil transesterification with heterogeneous catalysts. Renew. Energy 36(2), 726–731 (2011). https://doi.org/10.1016/j.renene.2010.08.028
Kansedo, J.; Lee, K.T.; Bhatia, S.: Cerbera odollam (sea mango) oil as a promising non-edible feedstock for biodiesel production. Fuel 88(6), 1148–1150 (2009). https://doi.org/10.1016/j.fuel.2008.12.004
Mohamadpanah, T.; Kazemzad, M.; Halek, F.: Improvement of biodiesel production reaction using functionalized spherical mesoporous silica CM-41 and HMS. J. Middle East Appl. Sci. Technol 8, 366–370 (2014)
Ali, I.; AlGhamdi, K.; Al-Wadaani, F.T.: Advances in iridium nano catalyst preparation, characterization and applications. J. Mol. (2019). https://doi.org/10.1016/j.molliq.2019.02.050
Acknowledgements
The authors would like to express their sincere appreciation to Department of Energy and Environment, Materials, and Energy Research Center (MERC) of Iran, Department of Social and Preventive Medicine, Faculty of Medicine University of Malaya (Partnership Project RK003-2017), for providing the required dataset for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Halek, F., Aghamohammadi, N. & Mohamadi, F. Biodiesel Production from Waste Edible Oil with Heterogeneous Catalysts (Nanoclay-Based Nanocatalysts). Arab J Sci Eng 44, 9919–9924 (2019). https://doi.org/10.1007/s13369-019-03986-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-019-03986-5