Abstract
Varicella zoster virus (VZV) is a neurotropic alphaherpesvirus that causes neurological manifestations either as a complication of primary infection or reactivation. VZV induced neurological diseases have a good prognosis when confirmed early and treated with anti-viral therapy. Myelitis, encephalitis, ventriculitis or meningitis can occur without a telltale rash in immunocompetent and immunocompromised individuals making the diagnosis difficult. We analyzed CSF and serum samples from 30 unvaccinated study participants (17 male and 13 female) to determine the presence of VZV DNA by PCR in CSF and to estimate serum and CSF anti-VZV IgG and albumin levels in participants with neurological manifestations with/without rash. Anti-VZV IgG was detected in CSF (n = 22, [73%]) and serum (n = 29, [97%]) of pediatric and adult participants. Anti-VZV IgG were detected in CSF of participants with varied clinical presentation altered sensorium (n = 8, [36%]), meningitis (n = 4, [18%]), acute febrile illness (n = 3, [14%], encephalopathy/meningoencephalitis (n = 2, [9%]), irritability (n = 2, [9%]) and each patient from cerebrovascular stroke, demyelinating disorder and febrile seizure (n = 1, [4.5%]). VZV DNA was detected from one participant and CSF serum albumin levels were elevated in 53% of study participants. VZV DNA is present up to 1–2 weeks post onset of disease, after which anti-VZV antibody may be the only indicator of disease and therefore both VZV DNA and anti-VZV IgG need to be tested for in CSF. As VZV DNA and VZV IgG antibody are both good indicators of VZV reactivation, routine testing would result in reduced morbidity and mortality by early detection of disease and antiviral treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Varicella is a vaccine preventable and self-limiting disease. Central nervous system (CNS) complications of varicella zoster virus (VZV) following primary infection and reactivation are known to occur especially in unvaccinated individuals. Complications of primary varicella in the CNS include meningitis, encephalitis, cerebellar ataxia, seizure, myelitis and vasculopathies (Lenfant et al. 2022; Science et al. 2014). Reactivation of VZV in adults in the cranial nerve and trigeminal ganglia causes CNS manifestations that are life-threatening if not diagnosed early and treated promptly (Lenfant et al. 2022). Both immunocompetent and immunocompromised individuals are prone to VZV CNS infections, but in immunocompromised hosts it is well described (Science et al. 2014). There is a marked decreased incidence of varicella and associated CNS complications in children in countries which adopted routine universal immunization programs (UIP). Zoster vaccines are also known to reduce zoster and neurological complications in adults (Grahn and Studahl 2015).
The use of high sensitivity PCR in routine clinical practice enabled the rapid diagnosis of the viral infections of the CNS and assists in potential use of antiviral therapy (Ihekwaba et al. 2008). During acute phase of the disease, in the absence of rash, routine diagnosis is done for herpes simplex virus (HSV), but not for VZV. Detection of VZV DNA in CSF during acute phase of the disease strongly points to active infection. Productive virus particles are eliminated by host immune response within one to two weeks. In such instances, anti-VZV antibody in the CSF may be detected, which is not normally found in CSF of healthy individuals (Gilden et al. 1998). Unlike HSV-1 which usually presents as an acute CNS infection, neurological complications due to varicella or zoster may present as acute, subacute, or chronic infection. Thus in protracted VZV diseases like encephalitis or myelitis, diagnosis of VZV DNA in CSF has less utility compared to acute HSV encephalitis. (Gilden et al. 1998).
To detect the neurological diseases caused by VZV, anti-VZV IgG in CSF is a more reliable method (Nagel et al. 2007) and the role of anti-VZV IgG in vasculopathy is well established (Gilden et al. 2009; Nagel et al. 2007). The diagnosis of VZV vasculopathy is missed most of the time, due to the long duration between the appearance of rash and stroke, no rash, or the absence of VZV DNA and pleocytosis in CSF and hence antiviral treatment is not initiated (Nagel et al. 2007). Due to rapid viral clearance in the CNS for most of the viral infections, detection of viral DNA by PCR rarely confirms the diagnosis. Demonstration of intrathecal virus specific antibody confirms the diagnosis of infection, especially in PCR negative case (Shamier et al. 2021).
In immune privileged areas like the CNS, calculation of antibody index in CSF is helpful to confirm the clinical suspicion of infection. When no pathogen is detected by direct methods, the antibody index provides the evidence of infection by demonstrating pathogen specific intrathecal antibodies. The blood-brain barrier usually restricts the systemic antibodies to enter the brain. However, the function changes if there is inflammation (Shamier et al. 2021).
The CSF albumin to antibody ratio, also known as the CSF/serum albumin ratio, IgG index used to assess blood-brain barrier integrity elevated ratio indicates increased permeability of blood brain barrier, allowing antibodies to enter CNS thus acting as an indirect measure of blood-brain barrier dysfunction. Since the elevated ratio is associated with various neurological conditions, measuring the ratio aids in diagnosis and differentiation of the conditions in combination with other clinical and laboratory findings (Shamier et al. 2021).
In France, VZV is the commonest herpesvirus to cause human infections and second cause of viral encephalitis. The incidence of HSV and VZV associated encephalitis has increased over the last decade (Mirouse et al. 2022).
In this report we investigated the presence of VZV DNA and anti-VZV IgG in CSF and serum, and albumin levels in CSF and serum of 30 varicella unvaccinated individuals with acute, sub-acute and chronic neurological conditions.
Materials and methods
Study design and ethics approval
The study design was conducted adhering to the declaration of Helsinki, 1964 and was approved by the Institutional Ethics Committee of Sri Ramachandra Institute of Higher Education and Research (IEC-NI/17/JUN/60/64).
Participants and clinical samples
The study participants n = 30 was enrolled (0–75 years of age) based on the inclusion and exclusion criteria. Inclusion and exclusion criteria were listed in Table 1. A written informed consent/assent form was collected from all participants/immediate relative before enrolment. Clinical proforma was maintained for all the study participants with all relevant clinical details. The CSF and serum samples (n = 30) were processed, aliquoted and stored in -80℃.
Quantitative real time PCR
VZV DNA real-time quantitative PCR was performed using Artus R VZV RG PCR kit (Qiagen, Hilden, Germany) using Rotor-Gene Q 5 plex platform Real-time PCR. The analytical sensitivity of the assay is 0.136 copies/µl (p = 0.05).
>Quantitative anti-VZV IgG ELISA
Anti-VZV IgG antibodies were estimated by quantitative ELISA using a VZV IgG ELISA kit (Abnova, Taiwan). Absorbance was measured at 450 nm with reference reading at 670 nm using microplate reader (ThermoFischer Scientific, India). The concentrations of anti-VZV antibodies (mIU/mL) were calculated by antibody (mIU) in sample from calibration curve with the sample dilution of 101x for serum and 2x for CSF. The lower limit of quantification of the assay was 30 mIU/mL. A total of 30 samples were tested as paired samples with serum and CSF were analyzed along with other CSF samples in the same run with reference to the standard curve.
Albumin levels were estimated in CSF and serum samples. CSF Serum albumin index is the ratio of CSF albumin and serum albumin, that indicates the integrity of blood brain barrier. In clinical practice, the index value greater than 9 is considered abnormal and indicative of blood brain barrier dysfunction.
Statistical analysis: Statistical analysis was done using R software version 4.3.2 to calculate the p value using paired T test for anti-VZV IgG and albumin levels in serum and CSF samples.
Results
Combined clinical and laboratory analyses were completed for 30 patients including pediatric (aged 0–18 years) (n = 7) and adults (> 18 years) (n = 23). Among the 30 participants 17 (57%) were male and 13 (43%) were female.
Clinical features, CSF analysis and virological studies
Clinical diagnosis, CSF analysis and virological studies of all 30 patients are shown in Table 2. Of the 30 patients, CSF pleocytosis was seen in 15 (50%) and CSF protein level was elevated in 22 (73%). All the adult participants had a history of varicella infection in childhood and none of the pediatric participants had a history of chickenpox. None of our study participants were vaccinated for varicella.
Virological study
VZV DNA was detected in CSF from one patient (3%) (#VZVCS17) admitted with altered sensorium with viral load of 30,564 copies/mL, all others tested negative for VZV DNA. Anti-VZV IgG was detected in CSF from (n = 22) 73% and in serum from (n = 29) 97% from pediatric and adult patients.
CSF Versus serum antibody
Of the 30 CSF samples tested for anti-VZV IgG antibodies, 22 (73%) had > 150mIU/mL of anti-VZV antibodies with the median of 603.18 mIU/mL. The anti-VZV IgG antibodies were detected in 29 (97%) participants from serum samples with the median of 122607.4 mIU/mL. Anti-VZV IgG were detected in CSF of participants with varied clinical presentation altered sensorium (n = 8, [36%]), meningitis (n = 4, [18%]), acute febrile illness (n = 3, [14%], encephalopathy/meningoencephalitis (n = 2, [9%]), irritability (n = 2, [9%]) and each patient from cerebrovascular stroke, demyelinating disorder and febrile seizure (n = 1, [4.5%]). Anti-VZV IgG was detected in serum samples from all the patients except one (#VZVCS6).
CSF versus serum albumin
CSF and serum albumin levels were tested for all 30 patients. The mean value of CSF albumin was 407.89 mg/dL which is nearly nine times the normal upper range of healthy individuals (normal value of CSF albumin- 11-48 mg/dL) and serum albumin level was 3.363 g/dL, which is normal range (normal value of serum albumin– 3.5–5.2 g/dL). About n = 16 (53%) patients of the 30 study participants had blood brain barrier dysfunction.
Statistical analyses
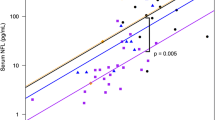
were conducted using R software using paired t test for anti-VZV IgG between serum and CSF samples, and it was found to be statistically significant (p < 0.001) (Fig. 1). Albumin ratio between CSF and serum samples were analyzed using R software and it was found to be statistically significant (p < 0.001) (Fig. 2A).
Treatment and outcome
All the patients that tested positive for VZV DNA or anti-VZV IgG antibodies were treated with intravenous acyclovir for 14 days duration and all of them showed clinical improvement.
Box and whisker plot shows anti-VZV IgG titer of serum and CSF from study participants with CNS manifestations. Box represents the interquartile range with median, Whisker extended to minimum and maximum values. Paired t test (p < 0.001) was done and found to be statistically significant. The study participants included are unvaccinated for varicella from pediatric (n = 8) and adult (n = 22) participants
A. Box and Whisker plot shows serum and CSF albumin levels from study participants with CNS manifestations. Box represents the interquartile range with median, Whisker extended to minimum and maximum values. Paired t test (p < 0.0001) was done and found to be statistically significant. B. Scatter plot shows the differences in the albumin levels against CSF and serum samples
Discussion
Globally, stroke is one of the common causes of high mortality and morbidity. The known risk factors for stroke include hypertension, obesity, and diabetes. Recently, viral etiology was also found to be associated as risks factor for stroke, which includes VZV, human immunodeficiency virus (HIV) and cytomegalovirus. VZV is the only virus that can directly invade the cerebral arteries and cause vasculopathy (Nagel et al. 2010).
The incidence and prevalence of VZV associated stroke is unknown, while in children it is estimated to cause 7 to 31% of arterial ischemic stroke and 44% of cases of transient cerebral ischemia (Nagel et al. 2010). In adults, VZV associated vasculopathy is was detected in 1.5 to 4.4% of immunocompromised adults (Nagel et al. 2010). In a review from China VZV associated ischemic stroke (74%) has been reported to be more common than other strokes in adults (Wu et al. 2022). The increased risk of stroke after zoster was reported to be 30% in Taiwan (Gershon et al. 2015; Kang et al. 2009), 17% in Denmark (Sreenivasan et al. 2013) and a 1.74-2.24-fold increase in the UK (Breuer et al. 2014). There is a reduced risk of stroke in about 55% of patients who received antiviral therapy compared with untreated zoster patients, proving the importance of antiviral treatment in zoster patients (Amlie-Lefond and Gilden 2016).
Diagnostic value of detection of anti-VZV IgG in vasculopathy and hemorrhagic stroke is well established (Gilden et al. 2009; Nagel and Bubak 2018; Wu et al. 2022) though well-established CSF antibody are not routinely determined. Previously we have documented the importance of detection of anti-VZV IgG from CSF samples of unvaccinated pediatric and adults’ participants with diverse neurological manifestations (Srikanth et al. 2024).
In our study, the anti-VZV IgG was detected in 73% of study participants with different neurological manifestations. The antibody titer was high in CSF samples among adults, which is statistically significant (p = 0.03). The antibody titer between serum and CSF samples is (p < 0.001). The anti-VZV IgG antibody was detected from those with altered sensorium (n = 8/8), irritability (n = 2/2), cerebrovascular stroke (n = 1/1), and demyelinating disorder (n = 1/1), 80% of meningitis (n = 4/5), 67% of encephalitis (n = 2/3) and 60% with acute febrile illness (n = 3/5) of study participants. A proportion 53% of the study participants were observed to have increased CSF serum albumin levels, which is indicative of blood brain dysfunction.
Normally, presence of IgG antibody in serum maybe due to current infection or vaccination. Since none of our study participant were vaccinated for VZV, the presence of anti-VZV IgG in CSF and serum indicates recent infection. The possibility of the anti-VZV IgG leaking into the CNS is to be understood in the context of lack of varicella vaccination. Most of varicella vaccinated individuals will have IgG antibodies in serum. In varicella unvaccinated individuals anti-VZV IgG will represent past or recent infection and its presence in the CNS is indicative of disease especially among those with CNS manifestations.
VZV vasculopathy can be acute or chronic manifestation due to VZV reactivation (Bubak et al. 2023); detection of VZV DNA in CSF using PCR in particular poses diagnostic challenge. The detection rate of VZV DNA in CSF in vasculopathies was 30%, whereas anti-VZV antibody were present in 93% (Gilden et al. 2009).
Neurological disease produced by VZV has a good prognosis when detected early and treated with anti-viral therapy. However, the challenge is in identifying the cases with VZV involvement. Atypical manifestations make clinical diagnosis and identification difficult. CNS diseases like myelitis, granulomatous arteritis (large vessel encephalitis), small vessel encephalitis, ventriculitis and meningitis can occur without a telltale rash in both immunocompetent and immunocompromised individuals. (Gilden et al. 2000) Thus, the methods of diagnosis need to be updated. PCR detection of VZV DNA and antibodies to VZV are strong indicators of disease. (Grahn and Studahl 2015). In VZV induced myelitis, evidence of VZV infection can be demonstrated by detection of VZV DNA and VZV antibody in the CSF (Gilden et al. 1994; Hung et al. 2012). The detection of VZV DNA in CSF sample from patient with large vessel encephalitis proves the direct invasion of VZV in cerebral arteries (Melanson et al. 1996). Detection of anti-VZV IgG and VZV DNA from four patients with encephalitis was proved the diagnosis of encephalitis in CNS manifestations of VZV disease (Gilden et al. 1998). Detection of antibodies to VZV in the sera is not a good indicator of ongoing infection, since most adults will have serological IgG antibodies that persisted from childhood infection, subclinical infection or following vaccination (Vafai et al. 1988; Zerboni et al. 1998). Therefore, there is a need for routine CSF analysis for VZV DNA and antiviral antibodies in CNS disease. (Grahn and Studahl 2015).
The detection of VZV DNA in stroke cases in other studies were found to be 28% and anti-VZV IgG 100% in USA (Nagel et al. 2007) and 20% PCR positive 60% anti-VZV IgG in South Africa (Marais et al. 2022). The detection of VZV DNA from CNS infection varied between 1.6 and 3.1%. In Finland 1.6% (Koskiniemi et al. 2001), in Switzerland 2.1% (Becerra et al. 2013) and in Sweden 3.1% (Bergström 1996a). Gilden et al. have documented several case reports of VZV reactivation disease in the absence of rash. There are mounting body of literature supports the routine use of VZV DNA and VZV antibody in patients with neurological manifestations of disease (Becerra et al. 2013; Bergström 1996b; Echevarria et al. 1997; Gilden et al. 1994; Koskiniemi et al. 2001) as mortality can be prevented by early detection and treatment with antiviral therapy.
In this present study, we have looked for both VZV DNA and VZV IgG antibodies that were tested in the CSF samples of patients with diverse clinical manifestations affecting the CNS. The focus on IgG antibody was deliberate as VZV IgM antibody, an indication of recent infection, has limited value and is present only up to a maximum of 10 weeks (Min et al. 2016; Nagel and Gilden 2013). VZV IgG antibody that can remain positive in the CSF sample for several years, is a better indicator of VZV CNS disease caused by reactivation and is more specific than VZV DNA (Nagel et al. 2007).
In 44 countries varicella vaccination is adopted in UIP either as partial or complete vaccination schedule. In 29 countries, two doses of a complete vaccination schedule was implemented with first dose administered at the age of 12 months and 4–6 years old for the second dose (Lee et al. 2022). In the context of the unvaccinated older Indian population, susceptibility to severe forms of CNS disease, despite having natural immunity from primary infection, remains high. Moreover, the natural seropositive protection against VZV has a delayed peak at 15–25 years of age in India (Venkitaraman et al. 1986). As VZV DNA and VZV IgG antibody are both good indicators of VZV reactivation, routine testing would result in reduced morbidity and mortality by early detection of disease. In 2006, the Advisory Committee on Immunization Practices (ACIP) of the Indian Academy of Pediatrics recommended a two dose vaccination schedule for children in India (IAP| ACVIP, n.d.). This recommendation only targets the younger population however and leaves a larger part of the population vulnerable.
Conclusion
As the increased risk of stroke in zoster patients is well established with certain neurological conditions following reactivation of VZV, early detection of either VZV DNA or anti-VZV IgG in CSF of patients with neurological manifestations is crucial. The respective antiviral treatment with acyclovir will reduce the incidence of VZV associated vasculopathy among zoster patients.
Data availability
All data (deidentified) generated data in this study will be made available. The comprehensive data of this study is available with the corresponding author in case of any clarification. Additional related documents including de-identified participants’ data as per ICMJE guidelines, study protocol, informed consent will be made available.
References
Abdalla DSP (2003) Clinical chemistry: theory, analysis, correlations. Revista Brasileira De Ciências Farmacêuticas 39(3). https://doi.org/10.1590/S1516-93322003000300018
Amlie-Lefond C, Gilden D (2016) Varicella Zoster Virus: A Common cause of stroke in children and adults. J Stroke Cerebrovasc Diseases: Official J Natl Stroke Association 25(7):1561–1569. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.03.052
Becerra JCL, Sieber R, Martinetti G, Costa ST, Meylan P, Bernasconi E (2013) Infection of the central nervous system caused by varicella zoster virus reactivation: a retrospective case series study. Int J Infect Dis 17(7):e529–e534. https://doi.org/10.1016/j.ijid.2013.01.031
Bergström T (1996a) Polymerase chain reaction for diagnosis of varicella zoster virus central nervous system infections without skin manifestations. Scand J Infect Dis Suppl 100:41–45
Bergström T (1996b) Polymerase chain reaction for diagnosis of varicella zoster virus central nervous system infections without skin manifestations. Scand J Infect Dis Suppl 100:41–45
Breuer J, Pacou M, Gauthier A, Brown MM (2014) Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology 82(3):206–212. https://doi.org/10.1212/WNL.0000000000000038
Bubak AN, Coughlan C, Posey J, Saviola AJ, Niemeyer CS, Lewis SWR, Lopez SB, Solano A, Tyring SK, Delaney C, Neeves KB, Mahalingam R, Hansen KC, Nagel MA (2023) Zoster-Associated Prothrombotic plasma exosomes and increased stroke risk. J Infect Dis 227(8):993–1001. https://doi.org/10.1093/infdis/jiac405
Echevarria JM, Casas I, Martinez-Martin P (1997) Infections of the nervous system caused by varicella-zoster virus: a review. Intervirology 40(2–3):72–84. https://doi.org/10.1159/000150535
Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PGE, Oxman MN, Seward JF, Yamanishi K (2015) Varicella Zoster virus infection. Nat Reviews Disease Primers 1(1):15016. https://doi.org/10.1038/nrdp.2015.16
Gilden DH, Beinlich BR, Rubinstien EM, Stommel E, Swenson R, Rubinstein D, Mahalingam R (1994) Varicella-Zoster virus myelitis: an expanding spectrum. Neurology 44(10):1818–1818. https://doi.org/10.1212/WNL.44.10.1818
Gilden DH, Bennett JL, Kleinschmidt-DeMasters BK, Song DD, Yee AS, Steiner I (1998) The value of cerebrospinal fluid antiviral antibody in the diagnosis of neurologic disease produced by varicella zoster virus. J Neurol Sci 159(2):140–144. https://doi.org/10.1016/s0022-510x(98)00153-1
Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ (2000) Neurologic complications of the reactivation of varicella–zoster virus. N Engl J Med 342(9):635–645. https://doi.org/10.1056/NEJM200003023420906
Gilden D, Cohrs RJ, Mahalingam R, Nagel MA (2009) Varicella Zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 8(8):731–740. https://doi.org/10.1016/S1474-4422(09)70134-6
Grahn A, Studahl M (2015) Varicella-Zoster virus infections of the central nervous system– prognosis, diagnostics and treatment. J Infect 71(3):281–293. https://doi.org/10.1016/j.jinf.2015.06.004
Hung C-H, Chang K-H, Kuo H-C, Huang C-C, Liao M-F, Tsai Y-T, Ro L-S (2012) Features of varicella zoster virus myelitis and dependence on immune status. J Neurol Sci 318(1–2):19–24. https://doi.org/10.1016/j.jns.2012.04.017
IAP| ACVIP. (n.d.). Retrieved November 1 (2023) from https://acvip.org/parents/columns/qa-varicella.php
Ihekwaba UK, Kudesia G, McKendrick MW (2008) Clinical features of viral meningitis in adults: significant differences in cerebrospinal fluid findings among herpes Simplex Virus, Varicella Zoster Virus, and Enterovirus infections. Clin Infect Dis 47(6):783–789. https://doi.org/10.1086/591129
Kang J-H, Ho J-D, Chen Y-H, Lin H-C (2009) Increased risk of Stroke after a herpes zoster attack: a Population-Based Follow-Up study. Stroke 40(11):3443–3448. https://doi.org/10.1161/STROKEAHA.109.562017
Koskiniemi M, Rantalaiho T, Piiparinen H, Von Bonsdorff C-H, Färkkilä M, Järvinen A, Kinnunen E, Koskiniemi S, Mannonen L, Muttilainen M, Linnavuori K, Porras J, Puolakkainen M, Räihä K, Salonen E-M, Ukkonen P, Vaheri A, Valtonen V (2001) Infections of the central nervous system of suspected viral origin: a collaborative study from Finland. J Neurovirol 7(5):400–408. https://doi.org/10.1080/135502801753170255
Lee YH, Choe YJ, Lee J, Kim E, Lee JY, Hong K, Yoon Y, Kim Y-K (2022) Global varicella vaccination programs. Clin Experimental Pediatr 65(12):555–562. https://doi.org/10.3345/cep.2021.01564
Lenfant T, L’Honneur A-S, Ranque B, Pilmis B, Charlier C, Zuber M, Pouchot J, Rozenberg F, Michon A (2022) Neurological complications of varicella zoster virus reactivation: prognosis, diagnosis, and treatment of 72 patients with positive PCR in the cerebrospinal fluid. Brain Behav 12(2):e2455. https://doi.org/10.1002/brb3.2455
Marais G, Naidoo M, McMullen K, Stanley A, Bryer A, Van Der Westhuizen D, Bateman K, Hardie DR (2022) Varicella-Zoster virus reactivation is frequently detected in HIV‐infected individuals presenting with stroke. J Med Virol 94(6):2675–2683. https://doi.org/10.1002/jmv.27651
Melanson M, Chalk C, Georgevich L, Fett K, Lapierre Y, Duong H, Richardson J, Marineau C, Rouleau GA (1996) Varicella-Zoster virus DNA in CSF and arteries in delayed contralateral hemiplegia: evidence for viral invasion of cerebral arteries. Neurology 47(2):569–570. https://doi.org/10.1212/wnl.47.2.569
Min S-W, Kim YS, Nahm FS, Yoo DH, Choi E, Lee P-B, Choo H, Park Z-Y, Yang CS (2016) The positive duration of varicella zoster immunoglobulin M antibody test in herpes zoster. Medicine 95(33):e4616. https://doi.org/10.1097/MD.0000000000004616
Mirouse A, Sonneville R, Razazi K, Merceron S, Argaud L, Bigé N, Faguer S, Perez P, Géri G, Guérin C, Moreau A-S, Papazian L, Robert R, Barbier F, Ganster F, Mayaux J, Azoulay E, Canet E (2022) Neurologic outcome of VZV encephalitis one year after ICU admission: a multicenter cohort study. Ann Intensiv Care 12(1):32. https://doi.org/10.1186/s13613-022-01002-y
Nagel MA, Bubak AN (2018) Varicella Zoster Virus Vasculopathy. J Infect Dis 218(suppl2):S107–S112. https://doi.org/10.1093/infdis/jiy425
Nagel MA, Gilden D (2013) Complications of Varicella Zoster Virus Reactivation. Curr Treat Options Neurol 15(4):439–453. https://doi.org/10.1007/s11940-013-0246-5
Nagel MA, Forghani B, Mahalingam R, Wellish MC, Cohrs RJ, Russman AN, Katzan I, Lin R, Gardner CJ, Gilden DH (2007) The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology 68(13):1069–1073. https://doi.org/10.1212/01.wnl.0000258549.13334.16
Nagel MA, Mahalingam R, Cohrs RJ, Gilden D (2010) Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect Disord Drug Targ 10(2):105–111. https://doi.org/10.2174/187152610790963537
Science M, MacGregor D, Richardson SE, Mahant S, Tran D, Bitnun A (2014) Central nervous system complications of varicella-zoster virus. J Pediatr 165(4):779–785. https://doi.org/10.1016/j.jpeds.2014.06.014
Shamier MC, Bogers S, Yusuf E, Van Splunter M, Berge T, Titulaer JCEM, Van Kampen M, J. J. A., GeurtsvanKessel CH (2021) The role of antibody indexes in clinical virology. Clin Microbiol Infect 27(9):1207–1211. https://doi.org/10.1016/j.cmi.2021.03.015
Sreenivasan N, Basit S, Wohlfahrt J, Pasternak B, Munch TN, Nielsen LP, Melbye M (2013) The short- and long-term risk of stroke after herpes zoster—A nationwide population-based cohort study. PLoS ONE 8(7):e69156. https://doi.org/10.1371/journal.pone.0069156
Srikanth P, Arumugam I, Jeganathan SN, Ramesh R, Ranganathan LN, Vijayaraghavan S (2024) Expanded spectrum of varicella disease and the need for vaccination in India. Hum Vaccines Immunotherapeutics 20(1):2328955. https://doi.org/10.1080/21645515.2024.2328955
Vafai A, Mahalingam R, Zerbe G, Wellish M, Gilden DH (1988) Detection of antibodies to varicella-zoster virus proteins in sera from the elderly. Gerontology 34(5–6):242–249. https://doi.org/10.1159/000212962
Venkitaraman AR, Seigneurin J-M, Lenoir GM, John TJ (1986) Infections due to the human herpes-viruses in Southern India: a Seroepidemiological Survey. Int J Epidemiol 15(4):561–566. https://doi.org/10.1093/ije/15.4.561
Wu H, Wang R, Li Y, Sun X, Li J, Bi X (2022) Cerebrovascular complications after adult-onset varicella-zoster Virus Encephalitis in the Central Nervous System: A literature review. Neuropsychiatr Dis Treat 18:449–462. https://doi.org/10.2147/NDT.S343846
Zerboni L, Nader S, Aoki K, Arvin AM (1998) Analysis of the persistence of humoral and cellular immunity in children and adults immunized with varicella vaccine. J Infect Dis 177(6):1701–1704. https://doi.org/10.1086/517426
Acknowledgements
We gratefully acknowledge the financial assistance provided by Scheme for Promotion of Academic and Research collaboration (SPARC) Graduate grant Program, Ministry of Human resource development, Government of India, IIT Kharagpur, India.
Funding
The Scheme for Promotion of Academic and Research collaboration (SPARC) Graduate grant Program, Ministry of Human resource development, Government of India, IIT Kharagpur, India. Its primary objective is to enhance the research environment and capabilities of higher educational institutions in India by fostering collaborations between Indian institutions and some of the world’s leading academic and research institutions.
Author information
Authors and Affiliations
Contributions
IA-Contributed to the study design, collection of clinical samples, processing, Realtime PCR assay, ELISA, CSF and serum albumin ratio, data interpretation and preparation of the manuscript. SSR data analysis and drafted the manuscript. GK contributed to laboratory work and drafting the manuscript. SG contributed to data analysis and drafted the manuscript. SNJ contributed to data analysis and drafted the manuscript. JA and RS contributed to sample collection, performance of assay. RR, SV, KK, LNR and UB contributed to the collection of clinical samples and clinical details. RM contributed to the conception and design of the study and preparation of the manuscript. JTB contributed to sequence analysis and interpretation of it and preparation of the manuscript. ANB contributed to data analysis and preparation of the manuscript. MAN contributed to the conception and design of the study and preparation of the manuscript. PS contributed to the conception and design of the study, preparation of manuscript and interpretation of data and critical revision of intellectual content and final approval of the version to be published.
Corresponding author
Ethics declarations
Institutional review board statement
Institutional Ethics Committee approval was obtained prior to start of the study, the IEC number- IEC-NI/17/JUN/60/64.
Informed consent
Informed consent/Assent form was obtained from all the participants/guardian.
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arumugam, I., Rajasekaran, S.S., Gopalakrishnan, K. et al. Diagnostic value of anti-VZV IgG in neurological diseases among varicella unvaccinated individuals. J. Neurovirol. 30, 327–335 (2024). https://doi.org/10.1007/s13365-024-01224-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-024-01224-9