Abstract
Hypertrophic pachymeningitis is a rare inflammatory condition characterized by the thickening of the dura mater. We describe a patient who presented with intractable headache and complex cranial nerve palsy. Hypertrophy of the frontal dura was accompanied by pleocytosis and detection of Epstein-Barr virus (EBV) by PCR in cerebrospinal fluid. Clinical symptoms gradually improved after acyclovir and corticosteroid treatment, whereas dural pathology remained unchanged on neuroimaging. This case points at an expansion of the spectrum of neurological manifestations for EBV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertrophic pachymeningitis (HPM) is a rare inflammatory disorder, which is characterized by a localized or diffuse thickening of spinal or intracranial dura mater. The etiology of HPM is heterogenous and includes infection, autoimmune disease, and paraneoplastic condition (Hahn et al. 2016). The condition is termed idiopathic in the absence of an identifiable cause. Epstein-Barr virus (EBV) is a widely disseminated human DNA virus and the primary cause of infectious mononucleosis (Dunmire et al. 2015). In the acute form of infection, up to 5% of patients develop neurological symptoms (Ascenção et al. 2016). In contrast, neurological complications are rare for reactivation of latent RBV infection. Here, we describe a patient with hypertrophic pachymeningitis associated with EBV reactivation.
Case report
A 64-year-old man with unremarkable medical history was admitted for frontal headache unresponsive to analgesic medication. He had suffered from recurrent episodes of double vision, which had started 4 days earlier. He was afebrile, he did not exhibit clinical signs of mononucleosis and there were no abnormalities in his routine laboratory examination. The neurological examination revealed a right-sided third to sixth cranial nerve palsy together as well ptosis and miotic pupil. Antibody testing for antinuclear antibody, anti-DNA antibody, anti-cardiolipin antibody, P- and C-anti-neutrophil cytoplasmic antibody (ANCA), β-D-glucan were negative. Serum electrophoresis and analysis of IgG1-4 was unremarkable. His thoracic CT scan did not show any abnormalities. Examination of the cerebrospinal fluid (CSF) revealed a lymphocytic pleocytosis (83 cells/μl), slightly elevated protein (47 mg/dl) and normal glucose levels (52 mg/dl). Bacterial (including mycobacterial) and fungal cultures and the anti-Borrelia antibody index were negative, and CSF-specific oligoclonal bands were absent. EBV-DNA was detected by polymerase chain reaction (PCR) in CSF (3.600 copies/ml). Serum antibody titers for EBV were as follows: VCA-IgM < 1:10, VCA-IgG 1:320, and EBNA-1-IgG 1:80. CMV and HIV serology were negative. PCR analysis for herpes simplex, varicella-zoster virus, cytomegalovirus, and enterovirus were negative. Cytological examination of CSF did not disclose atypical cells.
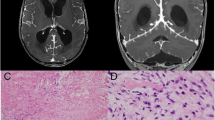
Cranial magnetic resonance imaging (MRI) showed a thickening right frontal dura and the leptomeninges (Fig. 1A). Diffuse contrast enhancement can be found within these pathologies (Fig. 1B). Treatment with intravenous acyclovir (10 mg/kg body weight three times daily) was started and maintained for 14 days. Intravenous methylprednisolone (1 g/day) was administered for 3 days, followed by an oral tapering regimen over 4 weeks. By the time the dosage of prednisone reached 40 mg/day, the patient’s headache and ocular symptoms had improved. The MRI 4 weeks later revealed a slight increase of the dural and leptomeningeal hypertrophy towards the parietal hemisphere whereas extent of contrast-enhancement was unchanged. Six months later, he continued to have recurrent episodes of headache and required analgetics.
Discussion
A recent nationwide epidemiological survey of hypertrophic pachymeningitis in Japan shed further light on this rare condition. The authors identified 159 cases (44% idiopathic) and determined a prevalence of 0.95/10000 (Yonekawa et al. 2014). The most frequent etiologies were ANCA (34%) and IgG4-related disease (6%), whereas only tuberculosis and aspergillosis (2 each) were among the infectious causes. There were no infectious causes among 22 patients from the USA(Hahn et al. 2016). Regarding EBV as potential causative agent, Okuma and coworkers reported a case of hypertrophic pachymeningitis based on serology but normal CSF parameters (cell count, protein, no PCR performed) and lacking symptoms of infectious mononucleosis (Okuma et al. 2007). Thus, our case is the first to detect EBV virus copies in CSF indicative of reactivation from latency in a patient with hypertrophic pachymeningitis. EBV detection by PCR can be an unspecific finding in CSF (Cocuzza et al. 2014). The presence of EBV copies in cell-free CSF together with inflammatory CSF findings and rapid clinical response to antiviral and anti-inflammatory therapy imply a potential involvement of this virus in the pathogenesis of dural and leptomeningeal hypertrophy in our patient. Notably, EBV is often found in CSF of patients with AIDS who develop primary CNS lymphoma (Tselis 2014), and both conditions were excluded. Leptomeningeal enhancement can be observed in some cases of EBV encephalitis but parenchymal involvement was lacking in our patient.
Headache and cranial nerve dysfunction are the most common clinical manifestations of HPM (Hahn et al. 2016). Dural inflammation and increase of intracranial pressure is the presumed cause of headache, whereas dural compression and CSF inflammation is seen as the pathogenesis of cranial nerve palsy (Huang et al. 2017). Recurrence or progression after tapering of steroids is common, and therefore requiring continued use of analgesics and consideration of immunosuppressive therapy. Dural biopsy needs to be considered in case of progressive symptoms despite conservative therapy. Management of recurrence is challenging and may even require surgical decompression to prevent irreversible neurological damage, notably if the craniocervical region or the optic nerve are involved (Ranasinghe et al. 2015). We conclude that further studies should elucidate the role of viruses in the pathogenesis of HPM.
References
Ascenção BB, Gonçalves AC, Luís N, Sá J, Brito AP, Poças JM (2016) Epstein-Barr virus hemorrhagic meningoencephalitis: case report and review of the literature. J Neuro-Oncol 22(5):695–698
Cocuzza CE, Piazza F, Musumeci R, Oggioni D, Andreoni S, Gardinetti M, Fusco L, Frigo M, Banfi P, Rottoli MR, Confalonieri P, Rezzonico M, Ferrò MT, Cavaletti G, EBV-MS Italian Study Group (2014, 2014) Quantitative detection of Epstein-Barr virus DNA in cerebrospinal fluid and blood samples of patients with relapsing-remitting multiple sclerosis. PLoS One, 4 9:e94497
Dunmire SK, Hogquist KA, Balfour HH (2015) Infectious mononucleosis. Curr Top Microbiol Immunol 390(Pt 1):211–240
Hahn LD, Fulbright R, Baehring JM (2016) Hypertrophic pachymeningitis. J Neurol Sci 367:278–283
Huang Y, Chen J, Gui L (2017) A case of idiopathic hypertrophic pachymeningitis presenting with chronic headache and multiple cranial nerve palsies: a case report. Medicine (Baltimore) 96(29):e7549
Yonekawa T, Murai H, Utsuki S, Matsushita T, Masaki K, Isobe N, Yamasaki R, Yoshida M, Kusunoki S, Sakata K, Fujii K, Kira J (2014) A nationwide survey of hypertrophic pachymeningitis in Japan. J Neurol Neurosurg Psychiatry 85:732–739
Okuma H, Kobori S, Shinohara Y, Takagi S (2007) A case of hypertrophic pachymeningitis with prolonged headache, attributable to Epstein–Barr virus. Headache 47:620–622
Ranasinghe MG, Zalatimo O, Rizk E, Specht CS, Reiter GT, Harbaugh RE, Sheehan J (2015) Idiopathic hypertrophic spinal pachymeningitis. J Neurosurg Spine 15(2):195–201
Tselis AC (2014) Epstein-Barr virus infections of the nervous system. Handb Clin Neurol 123:285–305
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nardone, R., Brigo, F., Covi, M. et al. Hypertrophic chronic pachymeningitis associated with Epstein-Barr virus reactivation: a case report. J. Neurovirol. 25, 426–428 (2019). https://doi.org/10.1007/s13365-019-00731-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-019-00731-4