Abstract
Understanding the progression of HIV-1-associated neurocognitive disorders (HAND) is a critical need as the prevalence of HIV-1 in older individuals (>50 years) is markedly increasing due to the great success of combination antiretroviral therapy (cART). Longitudinal experimental designs, in comparison to cross-sectional studies, provide an opportunity to establish age-related disease progression in HAND. The HIV-1 transgenic (Tg) rat, which has been promoted for investigating the effect of long-term HIV-1 viral protein exposure, was used to examine two interrelated goals. First, to establish the integrity of sensory and motor systems through the majority of the animal’s functional lifespan. Strong evidence for intact sensory and motor system function through advancing age in HIV-1 Tg and control animals was observed in cross-modal prepulse inhibition (PPI) and locomotor activity. The integrity of sensory and motor system function suggested the utility of the HIV-1 Tg rat in investigating the progression of HAND. Second, to assess the progression of neurocognitive impairment, including temporal processing and long-term episodic memory, in the HIV-1 Tg rat; the factor of biological sex was integral to the experimental design. Cross-modal PPI revealed significant alterations in the development of temporal processing in HIV-1 Tg animals relative to controls; alterations which were more pronounced in female HIV-1 Tg rats relative to male HIV-1 Tg rats. Locomotor activity revealed deficits in intrasession habituation, suggestive of a disruption in long-term episodic memory, in HIV-1 Tg animals. Understanding the progression of HAND heralds an opportunity for the development of an advantageous model of progressive neurocognitive deficits in HIV-1 and establishes fundamental groundwork for the development of neurorestorative treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 73% of individuals with human immunodeficiency virus type 1 (HIV-1) will be 50 years or older by 2030 (Smit et al. 2015) due to the marked increase in life expectancy following the great success of combination antiretroviral therapy (cART). The prevalence of the most severe forms of neurocognitive impairment, including HIV-1-associated dementia, dramatically decreased following the advent of cART. However, milder forms of neurocognitive impairment, including HIV-1-associated neurocognitive disorders (HAND), continue to afflict between 40 and 70% of HIV-1 seropositive individuals (Letendre et al. 2010; McArthur et al. 2010; Heaton et al. 2011). Cross-sectional studies have demonstrated that older HIV-1 seropositive individuals are at an elevated risk for developing neurological impairments compared to older HIV-1 seronegative individuals (Sheppard et al. 2015) and younger HIV-1 seropositive individuals (Valcour et al. 2004; Fazeli et al. 2014). Extrapolating findings from cross-sectional studies to age-related disease progression, however, is inferentially fraught (Kraemer et al. 2000; Coleman 2004). Longitudinal experimental designs, in sharp contrast, provide an opportunity to understand age-related disease progression using repeated measures across the lifespan (Kraemer et al. 2000; West et al. 2004).
In a seminal paper, Reid et al. (2001) reported the development of the HIV-1 transgenic (Tg) rat, which contains a gag-pol-deleted HIV-1 provirus regulated by the viral promotor. The highest levels of transgene (located on chromosomes 2 and 9) expression were observed in the lymph nodes, spleen, kidney, and thymus (Reid et al. 2001). Clinical manifestations, including neurological abnormalities (i.e., circling behavior, hind-limb paralysis), skin lesions, cataract formation, and relatively early wasting (i.e., between 5 to 9 months of age) were also observed in the original derivation of the HIV-1 Tg rat (Reid et al. 2001).
The current derivation of the HIV-1 Tg rat, used in the present studies, is a healthier derivation than those originally described, with the transgene now limited to chromosome 9, and in the F344/N, rather than Sprague-Dawley, background strain. Similar growth rates (Peng et al. 2010; Moran et al. 2012, 2013b; Roscoe et al. 2014) and a longer lifespan (i.e., 50% of HIV-1 Tg rats survive through 21 months of age; Peng et al. 2010) have been reported in the contemporary HIV-1 Tg phenotype. Viral proteins are expressed in the non-infectious HIV-1 Tg rat constitutively throughout development (Peng et al. 2010; Abbondanzo and Chang 2014), resembling HIV-1 seropositive individuals on cART. There is growing acceptance and confidence that the HIV-1 Tg rat is a valuable tool to model the neurocognitive deficits observed in HAND (e.g., Vigorito et al. 2007; Lashomb et al. 2009; Moran et al. 2013a, 2014a; Repunte-Canonigo et al. 2014) and may provide an opportunity to assess the effect of long-term HIV-1 viral protein exposure (Vigorito et al. 2015). However, there is a critical need to determine the integrity of sensory and motor system function, which will help establish the utility of the HIV-1 Tg rat in longitudinal studies on the progression of neurocognitive deficits through advancing age.
Prepulse inhibition (PPI) of the auditory startle response (ASR) has been promoted as a versatile experimental technique for the evaluation of sensory system function (Ison 1984; Wecker et al. 1985; Crofton and Sheets 1989) and temporal processing (e.g., Moran et al. 2013a; McLaurin et al. 2017a, b). The technique, popularized by Ison and Hammond (1971), introduces a punctate prestimulus (i.e., light, tone) prior to a startling stimulus. Introduction of the prestimulus 30 to 500 ms prior to the startling stimulus produces robust inhibition of the ASR (Hoffman and Ison 1980). The amplitude of the startle response can be used to determine whether the subject (i.e., rat, mouse, human) is able to detect the prestimulus (Wecker et al. 1985), thus assessing the integrity of sensory system function. Additionally, manipulation of the interstimulus interval (ISI; i.e., the time between the prestimulus and startle stimulus), as in the present studies, allows for assessment of temporal processing.
Characteristics of PPI, including brevity (i.e., rodents: approximately 20–30 min, Fitting et al. 2006; Moran et al. 2013a), repeatability (Braff et al. 1978; Schwarzkopf et al. 1993), and ease of administration (i.e., no prior training, no invasive procedures) make it an attractive method for the evaluation of sensory function (Ison 1984; Crofton and Sheets 1989). PPI has previously been used to assess the effect of toxicant exposure on sensory system function. For example, Young and Fechter (1983) assessed the effect of neomycin treatment on auditory system function. Results indicated not only that neomycin treatment resulted in selective hearing loss, but also the sensitivity of PPI to auditory system function (Young and Fechter 1983). Additionally, Wecker and Ison (1986) assessed the effect of inherited retinal degeneration on visual system function using PPI. Across age, animals with inherited retinal degeneration failed to exhibit robust inhibition to the visual prestimulus at any ISI, indicative of alterations in visual system function (Wecker and Ison 1986).
Temporal processing deficits, a potential elemental dimension of neurocognitive impairment in HIV-1, have been previously observed in the HIV-1 Tg rat using cross-modal PPI (Moran et al. 2013a; McLaurin et al. 2017a, b). Specifically, a within-subject assessment from 2 to 6 months of age revealed a relative insensitivity to the manipulation of ISI and a lack of perceptual sharpening with age in HIV-1 Tg animals relative to control animals (Moran et al. 2013a). Alterations in the development of perceptual sharpening have also been reported in preweanling HIV-1 Tg animals relative to controls (McLaurin et al. 2017a). Additionally, HIV-1 Tg and control animals assessed in a cross-sectional study between 9 and 10 months of age suggested the generality and relative permanence of temporal processing deficits (McLaurin et al. 2017b). Accordingly, the present study employed auditory and visual PPI to assess the integrity of the auditory and visual sensory systems and the progression of temporal processing deficits in the HIV-1 Tg rat through the majority of the animal’s functional lifespan.
Locomotor activity has been recognized as a state-of-the art technique used for the assessment of gross motor movement (Pierce and Kalivas 2007). The protocol employed in the present study used an automated monitoring system to measure the number of photocell interruptions within a test session (i.e., 60 min) as an index of motor behavior (Pierce and Kalivas 2007). Assessments of locomotor activity are commonly used as a preclinical screening tool for motoric impairment in pharmacological studies (Robbins 1977; Pierce and Kalivas 2007). For example, Booze and Mactutus (1990) used locomotor activity as an assessment of the long-term effects of neonatal exposure to triethyl lead. No significant differences in locomotor activity were observed at testing on postnatal day (PD) 90, suggesting that neonatal exposure to triethyl lead has no long-term effects on motor system function (Booze and Mactutus 1990). Additionally, locomotor activity has been used to examine the effects of prenatal cocaine exposure (1 or 3 mg/kg) on locomotor and stereotyped behavior following a cocaine challenge in adulthood (Peris et al. 1992). Animals prenatally exposed to 3 mg/kg cocaine exhibited the most profound increases in locomotor and stereotyped behavior following cocaine injections in adulthood, suggesting that cocaine in utero behaviorally sensitized animals to subsequent cocaine exposure (Peris et al. 1992).
Measures of novelty and habituation can be used to assess memory, including episodic memory, in animal models (Eacott et al. 2005; Barker et al. 2007; Moran et al. 2013b; Chao et al. 2016). Habituation, one of the simplest forms of learning characterized by a decrease in response following repeated stimulation (Harris 1943; Thompson and Spencer 1966; Rankin et al. 2009), allows for the assessment of learning and memory in locomotor activity, both within sessions (intrasession) and between sessions (intersession; review, Leussis and Bolivar 2006). Significant alterations in intrasession habituation have been reported in the HIV-1 Tg rat, assessed using three consecutive test sessions in locomotor activity at 6, 7, and 11 months of age (Moran et al. 2013b). Profound deficits in intrasession habituation were observed during the third test session at 7 and 11 months of age in HIV-1 Tg animals compared to controls, suggestive of a deficit in long-term episodic memory (Moran et al. 2013b). Alterations in intersession habituation have also been reported in neonatal Sprague-Dawley rats stereotaxically injected with Tat1–86, a HIV-1 viral protein, compared to vehicle-treated controls (Moran et al. 2014b). Therefore, the present study used locomotor activity to assess the integrity of the motoric system and the progression of long-term episodic memory in the HIV-1 Tg rat through advancing age.
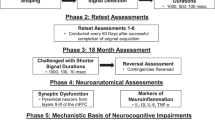
The aims of the present study were thus twofold. First, to assess the integrity of sensory and motor system function through advancing age in the HIV-1 Tg rat. Determining the integrity of sensory and motor systems in the HIV-1 Tg rat through the majority of the animal’s functional lifespan provides an opportunity to help establish the applicability of the HIV-1 Tg rat in longitudinal studies of the progression of HAND. Second, to establish the progression of neurocognitive deficits, including temporal processing and long-term episodic memory, in the HIV-1 Tg rat, through the majority of the animal’s functional lifespan. The factor of biological sex was an integral component of the experimental design. Understanding the progression of neurocognitive impairment, and the effect of biological sex, heralds an opportunity for the development of an advantageous model of progressive neurocognitive deficits in HIV-1 and establishes fundamental groundwork for the development of neurorestorative treatments.
Methods
Animals
Intact male and female Fischer (F344/N; Harlan Laboratories Inc., Indianapolis, IN) HIV-1 Tg (male, n = 37; female, n = 33) and control rats (male, n = 34; female, n = 33) were sampled from a total of 37 litters (HIV-1 Tg, N = 20 litters; control N = 17 litters). Cross-modal PPI and locomotor activity assessments were conducted every 30 days from PD 90 to PD 180 and every 60 days from PD 240 to PD 480.
Pups, housed with their biological dam, were delivered to the animal vivarium between PD 7 and PD 9 across 12 months. Following weaning, which occurred at PD 21, animals were pair- or group-housed with animals of the same sex. Beginning at approximately PD 60, animals were placed on food restriction (Pro-Lab Rat, Mouse, Hamster Chow #3000) to maintain 85% body weight due to their participation in a concurrently conducted signal detection task. Rodent food was again provided ad libitum for the duration of the study following the successful completion of signal detection (PD 100-PD 277). Water was available ad libitum throughout the duration of the study.
Due to health issues, including significant weight loss (i.e., approximately 20%; n = 6) or tumors (n = 4), some animals were euthanized prior to the completion of the study. Specifically, two HIV-1 Tg animals (male n = 1; female n = 1) and one control animal (male, n = 1) were euthanized prior to PD 420 and an additional five HIV-1 Tg animals (male n = 3; female n = 2) and two control animals (male n = 1; female n = 1) were euthanized prior to PD 480.
Guidelines established by the National Institute of Health (NIH) were used for the maintenance of animals in AAALAC-accredited facilities. The targeted environmental conditions for the animal vivarium were 21° ± 2 °C, 50% ± 10% relative humidity and a 12-h light:12-h dark cycle with lights on at 0700 h (EST). The project protocol, under federal assurance (# A3049–01), was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina.
Cross-modal Prepulse inhibition
Apparatus
An isolation cabinet (external dimensions 10 cm-thick, double-walled, 81 × 81 × 116-cm; Industrial Acoustic Company, INC., Bronx, NY), which provided 30 db(A) of sound attenuation relative to the external environment, enclosed the startle platform (SR-Lab Startle Reflex System, San Diego Instruments, Inc., San Diego, CA), instead of the 1.9-cm-thick ABS plastic or laminate cabinets offered with this system. Background noise of 70 dB(A) was continuously delivered. All auditory prepulse and startle stimuli were presented using a high-frequency loudspeaker of the SR-Lab system (model#40-1278B, Radio Shack, Fort Worth, TX). A sound level meter (model #2203, Bruël & Kjaer, Norcross, GA) was used to measure and calibrate the sound levels, with the microphone placed inside the Plexiglas test cylinder. Visual prepulse stimuli were presented using a white LED light (22 lx; Light meter model #840006, Sper Scientific, Ltd., Scottsdale, AZ). The high-frequency loudspeaker of the SR-Lab system was mounted 30 cm above the Plexiglas animal test cylinder, and the white LED light was affixed on the wall in front of the test cylinder. A piezoelectric accelerometer integral to the bottom of the cylinder converted the deflection of the test cylinder, resulting from the animal’s ballistic response to the auditory stimulus, into analog signals. The response signals were digitized (12 bit A to D, recorded at a rate of 2000 samples/s) and saved to a hard disk. A SR-LAB Startle Calibration System was used to calibrate the response sensitivities.
Procedure
Cross-modal PPI, used to assess auditory and visual sensory system function and the progression of temporal processing, was conducted similar to our prior publications (e.g., Moran et al. 2013a). In brief, a 30-min test session, beginning with a 5-min adjustment period, was conducted in the dark every 30 days from PD 90 to PD 180 and every 60 days from PD 240 to PD 480. Six pulse-only ASR trials, with a fixed 10-s intertrial interval (ITI), were used for habituation at the beginning of the test session. An equal number of auditory (85 dB(A) white noise stimulus) and visual (22 lx) prepulse trials, totaling 72 trials, were presented using a counterbalanced sequence (i.e., ABBA) to control for order-effects. ISIs (0, 30, 50, 100, 200, 4000 ms) were presented in six-trial blocks according to a Latin-square design with a variable ITI (15–25 s). All prepulse stimuli and the auditory startle stimulus (100 db(A)) had a 20 ms duration. The peak ASR amplitude values were collected for analysis.
Locomotor activity
Apparatus
Square (40 × 40 cm) activity monitors (Hamilton Kinder, San Diego Instruments, San Diego, CA) were converted into round (~40 cm diameter) compartments using perspex inserts. Infrared photocell (32 emitter/detector pairs) interruptions were used to detect free movement; the sensitivity of the photocells was tuned by the manufacturer to maintain their sensitivity with the additional layer of perspex.
Procedure
Motor system function and long-term episodic memory impairments were assessed using locomotor activity every 30 days from PD 90 to PD 180 and every 60 days from PD 240 to PD 480. A 60-min test session was conducted between 700 and 1200 h (EST) under dim light conditions (<10 lx) in an isolated room. The number of photocell interruptions within the 60-min test session was collected for analysis.
Statistical analysis
Analysis of variance (ANOVA) statistical techniques (SPSS Statistics 24, IBM Corp., Somer, NY) were used for the analysis of all data. Individual observations were analyzed using litter means and standard errors, dependent upon biological sex, to account for the nested design (Denenberg 1984; Wears 2002).The mean series imputation method was used for all censored data, either due to euthanization or an equipment malfunction, which occurred in locomotor activity at PD 90 (control: n = 1) and PD 150 (HIV-1 Tg: n = 7; control: n = 4). Potential violations of compound symmetry were precluded by the use of orthogonal decomposition of the repeated-measures factors or addressed, post hoc, via the conservative Greenhouse-Geisser df correction factor (Greenhouse and Geisser 1959). Age-dependent effects of the HIV-1 transgene were evaluated using tests of simple main effects and specific linear contrasts (Winer 1971). Partial eta squared (η p 2) was used as a measure of effect size. GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA) was used for all regression analyses and to create all graphs. For all statistical tests, significance was set at an alpha level of p ≤ 0.05.
Specifically, for free-feeding body weight, a mixed-factor ANOVA was conducted independently for each sex. Genotype (HIV-1 Tg vs. control) served as the between-subject’s factor, while age served as the within-subjects factor. The peak ASR amplitude values, collected in cross-modal PPI, were analyzed for the assessment of the integrity of auditory and visual system function. The progression of temporal processing was examined using the area of inflection of the ASR amplitude response curve (McLaurin et al. 2016). Locomotor activity was assessed using the total number of photocell interruptions in a 60-min period, to establish the integrity of motoric system function, and the number of photocell interruptions in 5-min bins, to assess intrasession habituation, as an index of long-term episodic memory. For cross-modal PPI and locomotor activity, a mixed-factor ANOVA was performed, with genotype (HIV-1 Tg vs. control) and sex (male vs. female) as the between-subject’s factors. As appropriate, age, time, ISI, and trial served as the within-subjects factors.
Results
Establishing the integrity of sensory and motor system function
Body weight: somatic growth
Somatic growth, assessed using body weight, was examined by litter dependent upon food access (i.e., food restriction vs. ad libitum) and sex. Regardless of sex and/or food access, HIV-1 Tg animals weighed significantly less than control animals throughout the duration of the experiment.
Male HIV-1 Tg and control animals exhibited a linear increase in growth (Fig. 1a), with no significant differences between genotype in the rate of growth (F(1,60) < 1.0, p ≥ 0.05), while on food restriction due to a concurrently conducted signal detection task. All male HIV-1 Tg and control animals completed the concurrently conducted signal detection task by 6 months of age (PD 180), at which point food was subsequently available ad libitum. During free-feeding, a one-phase association was the best fit for both HIV-1 Tg and control animals (R 2s ≥ 0.97); however, significant differences were observed in the rate of growth (F(3,357) = 1230, p ≤ 0.001). The overall ANOVA conducted on somatic growth during free-feeding confirmed these observations, revealing a significant age × genotype interaction (F(10,310) = 70.0, p GG ≤ 0.001, η p 2 = 0.693) with a prominent linear component (F(1,31) = 126.4, p ≤ 0.001, η p 2 = 0.803). Significant main effects of age (F(10,310) = 607.1, p GG ≤ 0.001, η p 2 = 0.951) and genotype (F(1,31) = 381.7, p ≤ 0.001, η p 2 = 0.925) were also observed.
Mean body weight is illustrated for both males (a) and females (b) as a function of genotype (HIV-1 Tg vs. control) and age (±95% CI). HIV-1 Tg animals, regardless of sex, weighed significantly less than control animals, but exhibited steady growth through approximately 16 months of age. Notably, after a prior history of food restriction, both male and female HIV-1 Tg animals grew at a significantly slower rate relative to controls. The x-axis break at 6 months for males and 9 months for females indicates the point at which all animals were again receiving food ad libitum
Female HIV-1 Tg animals displayed an increase in weight according to a one-phase association, while female control animals exhibited an increase in weight according to a second-order polynomial, during food restriction (Fig. 1b). All female HIV-1 Tg and control animals completed the concurrently conducted signal detection task by approximately 9 months of age (PD 277), at which point food was subsequently available ad libitum. During free-feeding, a first-order polynomial was the best fit for both HIV-1 Tg and control animals (R 2s ≥ 0.98); however, significant differences were observed in the rate of growth (F(1,252) = 13.2, p ≤ 0.001). The rate of increase in body weight was significantly slower in HIV-1 Tg animals (β 1 = 3.97 ± 1.07(95% CI)) relative to control animals (β 1 = 7.16 ± 1.32(95% CI)). The overall ANOVA confirmed these observations, revealing a significant age × genotype interaction (F(7,210) = 22.8, p GG ≤ 0.001, η p 2 = 0.432) with a prominent linear component (F(1,30) = 34.4, p ≤ 0.001, η p 2 = 0.534). Significant main effects of age (F(7,210) = 224.6, p GG ≤ 0.001, η p 2 = 0.882) and genotype (F(1,30) = 59.9, p ≤ 0.001, η p 2 = 0.666) were also observed.
Cross-modal prepulse inhibition: auditory system function
Auditory PPI (Fig. 2) provided strong evidence for the integrity of auditory system function in both HIV-1 Tg and control animals through the majority of the animal’s functional lifespan. Control animals exhibited maximal inhibition at the 100 ms ISI at all test sessions. In sharp contrast, maximal inhibition was observed at multiple ISIs in HIV-1 Tg animals dependent upon age (i.e., PD 90, 30 ms; PD 120–PD 240, 100 ms; PD 300, 50 ms; PD 360–PD 420, 200 ms; PD 480, 50 ms). A significant increase in ASR as a function of age was observed in control animals, but not in HIV-1 Tg animals. However, both HIV-1 Tg and control animals exhibited robust inhibition to the auditory prepulse during all test sessions, suggesting the integrity of auditory system function.
Mean peak ASR amplitude (±SEM) for auditory prepulse inhibition is illustrated for all test sessions as a function of genotype (HIV-1 Tg vs. control). Both HIV-1 Tg and control animals exhibited robust inhibition to the auditory prepulse at all ages, albeit at different ISIs, suggesting the integrity of auditory system function through the majority of the animal’s functional lifespan
The overall ANOVA conducted on the peak ASR amplitude values confirmed these observations, revealing a significant age x ISI x genotype interaction (F(40,2440) = 11.3, p GG ≤ 0.001, η p 2 = 0.157) with a prominent linear-quadratic component (F(1,61) = 52.2, p ≤ 0.001, η p 2 = 0.461), and a significant age × ISI × sex interaction (F(40,2440) = 7.8, p GG ≤ 0.001, η p 2 = 0.113) with a prominent quadratic-quadratic component (F(1,61) = 34.1, p ≤ 0.001, η p 2 = 0.359). Significant two-way interactions included an age × sex interaction (F(8,488) = 10.3, p GG ≤ 0.001, η p 2 = 0.144) with a prominent quadratic component (F(1,61) = 31.6, p ≤ 0.001, η p 2 = 0.341), a significant age × genotype interaction (F(8,488) = 14.2, p GG ≤ 0.001, η p 2 = 0.188) with a prominent linear component (F(1,61) = 42.9, p ≤ 0.001, η p 2 = 0.413), a significant ISI × sex interaction (F(5,305) = 77.9, p GG ≤ 0.001, η p 2 = 0.561) with a prominent quadratic component (F(1,61) = 85.7, p ≤ 0.001, η p 2 = 0.584), a significant ISI × genotype interaction (F(5,305) = 127.2, p GG ≤ 0.001, η p 2 = 0.676) with a prominent quadratic component (F(1,61) = 139.1, p ≤ 0.001, η p 2 = 0.695), and a significant age x ISI interaction (F(40,2440) = 29.3, p GG ≤ 0.001, η p 2 = 0.324) with a prominent linear-quadratic component (F(1,61) = 113.5, p ≤ 0.001, η p 2 = 0.651). Significant main effects of age (F(8,488) = 42.7, p GG ≤ 0.001, η p 2 = 0.412), ISI (F(5,305) = 764.7, p GG ≤ 0.001, η p 2 = 0.926), sex (F(1,61) = 91.4, p ≤ 0.001, η p 2 = 0.600) and genotype (F(1,61) = 124.9, p ≤ 0.001, η p 2 = 0.672) were also observed. Further analyses were conducted to examine the locus of these interactions.
The ASR at the point of maximal inhibition in auditory PPI was used to further examine the significant age × ISI × genotype interaction (Supplementary Fig. 1a). A linear increase in the ASR at the point of maximal inhibition, well-described using a global first-order polynomial, was observed across age in both HIV-1 Tg and control animals (R 2 = 0.70). No significant differences in the rate of increase in the ASR at the point of maximal inhibition were observed between genotypes (F(2,14) = 3.2, p ≥ 0.05). The ASR at the point of maximal inhibition increased at the same rate in HIV-1 Tg (β 1 = 0.028 ± 0.01 (95% CI)) and control animals (β 1 = 0.017 ± 0.01 (95% CI)), suggesting the integrity of the auditory system. Thus, both control and HIV-1 Tg animals displayed robust inhibition to the auditory prepulse, albeit at different ISIs, at all test sessions, suggesting the integrity of auditory system function through advancing age.
Cross-modal prepulse inhibition: visual system function
Visual PPI revealed the integrity of visual system function in HIV-1 Tg and control animals through the majority of the animal’s functional lifespan, illustrated in Fig. 3. Control animals, but not HIV-1 Tg animals, exhibited a significant increase in ASR as a function of age. However, both HIV-1 Tg and control animals exhibited robust inhibition to the visual prepulse at the 50-ms ISI at all test sessions, suggesting the integrity of visual system function.
Mean peak ASR amplitude (±SEM) for visual prepulse inhibition is illustrated for all test sessions as a function of genotype (HIV-1 Tg vs. control). Maximal inhibition was observed at the 50 ms ISI for both HIV-1 Tg and control animals at all test sessions. Robust inhibition to the visual prepulse was observed in both HIV-1 Tg and control animals at all assessments, suggesting the integrity of visual system function through the majority of the animal’s functional lifespan
The overall ANOVA conducted on the peak ASR amplitude values confirmed these observations, revealing significant higher-order interactions, including an age × ISI × genotype × sex interaction with a prominent quadratic-quadratic component (F(1,61) = 5.0, p ≤ 0.029, η p 2 = 0.076), an age × genotype × sex interaction with a prominent quadratic component (F(1,61) = 4.9, p ≤ 0.031, η p 2 = 0.074), an age × ISI × genotype interaction (F(40,2440) = 9.0, p GG ≤ 0.001, η p 2 = 0.129) with a prominent linear-quadratic component (F(1,61) = 78.1, p ≤ 0.001, η p 2 = 0.561), an age × ISI × sex interaction (F(40,2440) = 4.3, p GG ≤ 0.001, η p 2 = 0.066) with a prominent quadratic-quadratic component (F(1,61) = 38.6, p ≤ 0.001, η p 2 = 0.387), and an ISI × sex × genotype interaction (F(5,305) = 4.4, p GG ≤ 0.013, η p 2 = 0.068) with a prominent quadratic component (F(1,61) = 6.5, p ≤ 0.013, η p 2 = 0.096). Significant two-way interactions included, an age × sex interaction (F(8,488) = 10.8, p GG ≤ 0.001, η p 2 = 0.151) with a prominent quadratic component (F(1,61) = 37.0, p ≤ 0.001, η p 2 = 0.377), an age × genotype interaction (F(8,488) = 13.4, p GG ≤ 0.001, η p 2 = 0.180) with a prominent linear component (F(1,61) = 35.1, p ≤ 0.001, η p 2 = 0.365), an ISI × sex interaction (F(5,305) = 60.9, p GG ≤ 0.001, η p 2 = 0.500) with a prominent quadratic component (F(1,61) = 79.2, p ≤ 0.001, η p 2 = 0.565), an ISI × genotype interaction (F(5,305) = 149.5, p GG ≤ 0.001, η p 2 = 0.710) with a prominent quadratic component (F(1,61) = 190.6, p ≤ 0.001, η p 2 = 0.758), and an age × ISI interaction (F(40,2440) = 15.6, p GG ≤ 0.001, η p 2 = 0.204) with a prominent quadratic-quadratic component (F(1,61) = 113.8, p ≤ 0.001, η p 2 = 0.651). Significant main effects of age (F(8,488) = 46.9, p GG ≤ 0.001, η p 2 = 0.435), ISI (F(5,305) = 658.6, p GG ≤ 0.001, η p 2 = 0.915), sex (F(1,61) = 71.7, p ≤ 0.001, η p 2 = 0.540), and genotype (F(1,61) = 108.2, p ≤ 0.001, η p 2 = 0.640) were also observed.
Complementary analyses of the ASR at the point of maximal inhibition (i.e., 50 ms) in visual PPI were conducted to examine the significant age × ISI × genotype interaction (Supplementary Fig. 1b). A linear increase in the ASR at the point of maximal inhibition was observed as a function of age in both HIV-1 Tg and control animals, well-described using a first-order polynomial (R 2s ≥ 0.64). Significant differences in the rate of increase between genotypes were observed (F(1,14) = 14.5, p ≤ 0.002). The ASR at the point of maximal inhibition increased faster in HIV-1 Tg animals (β 1 = 0.14 ± 0.03 (95% CI)) relative to control animals (β 1 = 0.07 ± 0.03 (95% CI)); however, both HIV-1 Tg and control animals exhibited robust inhibition (i.e., ≥55% inhibition) to the visual prestimulus at all test sessions. Thus, visual PPI provides strong evidence for the integrity of visual system function through advancing age in both HIV-1 Tg and control animals.
Locomotor activity: motor system function
The mean number of photocell interruptions in a 60-min locomotor activity test session was used to assess motoric system function through advancing age in HIV-1 Tg and control animals (Fig. 4). A one-phase decay was the best fit for both HIV-1 Tg and control animals (R 2s ≥ 0.91); however, a significant difference was observed between the best fit functions (F(3,12) = 41.1, p ≤ 0.001). Specifically, control animals, relative to HIV-1 Tg animals, exhibited a significantly greater mean number of photocell interruptions from PD 90 to PD 180. No significant differences in the mean number of photocell interruptions were observed between HIV-1 Tg and control animals at PD 240 or PD 300. At PD 360 and all subsequent test sessions, HIV-1 Tg animals displayed a significantly greater mean number of photocell interruptions in comparison to controls. The repeated-measures ANOVA confirmed these observations, revealing a significant age × genotype interaction (F(8,448) = 20.5, p GG ≤ 0.001, η p 2 = 0.251) with a prominent linear component (F(1,61) = 96.9, p ≤ 0.001, η p 2 = 0.614), and a significant age × sex interaction (F(8,448) = 2.2, p GG ≤ 0.039, η p 2 = 0.035) with a prominent quadratic component (F(1,61) = 9.8, p ≤ 0.003, η p 2 = 0.139). Significant main effects of age (F(8,448) = 102.4, p GG ≤ 0.001, η p 2 = 0.627), and sex (F(1,61) = 10.5, p ≤ 0.002, η p 2 = 0.147) were also observed. Therefore, neither HIV-1 Tg nor control animals exhibited any gross-motoric system impairments, evidenced using the mean number of photocell interruptions, suggesting the integrity of motor system function though advancing age.
Mean number of photocell interruptions in a 60-min locomotor activity test session are illustrated as a function of genotype (HIV-1 Tg vs. control) and age (±95% CI). A one-phase decay was the best fit for HIV-1 Tg and control animals; however, significant differences in the fit of the function were observed (F(3,12) = 41.1, p ≤ 0.001). From PD 90 to PD 180, control animals exhibited a significantly greater number of mean photocell interruptions relative to HIV-1 Tg animals. However, beginning at PD 360 and all subsequent test sessions, HIV-1 Tg animals, compared to controls, displayed a significantly greater number of mean photocell interruptions. Results, therefore, suggest no gross-motoric impairments in either HIV-1 Tg or control animals through advancing age
Understanding the progression of HIV-1 associated neurocognitive disorders
Cross-modal prepulse inhibition (auditory): temporal processing
In auditory PPI, area of the inflection of the ASR response curve, a measure of prepulse inhibition, was used to establish the progression of temporal processing, illustrated in Fig. 5. HIV-1 Tg animals exhibited a profound alteration in the progression of temporal processing relative to control animals. A segmental linear regression was the best fit for both HIV-1 Tg and control animals (R 2s ≥ 0.91). However, a leftward shift in maximal prepulse inhibition was observed in HIV-1 Tg animals relative to controls. Specifically, control animals exhibited a linear increase in prepulse inhibition through approximately PD 300, followed by a subsequent decrease. HIV-1 Tg animals displayed a leftward shift, with maximal peak inhibition observed at approximately PD 270, followed by a subsequent decrease. The overall ANOVA confirmed these observations, revealing a significant age × genotype interaction (F(8,488) = 11.8, p GG ≤ 0.001, η p 2 = 0.162) with a prominent linear component (F(1,61) = 35.2, p ≤ 0.001, η p 2 = 0.366) and a significant age × sex interaction (F(8,488) = 8.5, p GG ≤ 0.001, η p 2 = 0.123) with a prominent quadratic component (F(1,61) = 31.9, p ≤ 0.001, η p 2 = 0.343). Significant main effects of genotype (F(1,61) = 123.3, p ≤ 0.001, η p 2 = 0.669), age (F(8,488) = 35.4, p GG ≤ 0.001, η p 2 = 0.367), and sex (F(1,61) = 85.1, p ≤ 0.001, η p 2 = 0.583) were also observed.
Auditory prepulse inhibition, assessed using mean area of the peak inflection curve, is illustrated as a function of genotype (HIV-1 Tg vs. control) and age (±95% CI). a A segmental linear regression was the best fit for HIV-1 Tg and control animals (R 2s ≥ 0.91). Temporal processing in control animals was well-described using a segmental linear regression, with maximal prepulse inhibition observed at PD 300. In contrast, HIV-1 Tg animals displayed a prominent leftward shift, with maximal inhibition observed at approximately PD 270. b A segmental linear regression was the best fit for both male and female (R 2s ≥ 0.90) control animals, with maximal prepulse inhibition occurring at approximately PD 300. However, female control animals exhibited a significantly slower rate of temporal processing development from PD 90 to PD 300 compared to male control animals. c A segmental linear regression, with maximal prepulse inhibition at approximately PD 270, provided a well-described fit for male HIV-1 Tg animals (R 2 = 0.88). Female HIV-1 Tg animals, however, failed to exhibit any significant temporal processing development as a function of age, best fit using a horizontal line. Thus, regardless of sex, HIV-1 Tg animals displayed significant alterations in the progression of temporal processing, assessed using auditory prepulse inhibition, compared to control animals
Separate analyses of each genotype were conducted to examine the locus of these interactions (control: Fig. 5b; HIV-1 Tg: Fig. 5c). A segmental linear regression was the best fit for both male and female control animals (R 2s ≥ 0.90). Both male and female control animals exhibited maximal prepulse inhibition at approximately PD 300, followed by a subsequent decrease. However, a significant difference in the rate of temporal processing development from PD 90 to PD 300 was observed (F(1,12) = 12.4, p ≤ 0.004). Specifically, temporal processing development was significantly greater in male control animals (β 1 = 4815 ± 1645) relative to female control animals (β 1 = 2387 ± 367.5); no significant differences between males and females were observed in the rate of temporal processing decline (F(1,12) = 2.7, p ≥ 0.05). The overall ANOVA for control animals confirmed these observations, revealing a significant age × sex interaction (F(8,256) = 4.5, p GG ≤ 0.001, η p 2 = 0.122) with a prominent quadratic component (F(1,32) = 16.3, p ≤ 0.001, η p 2 = 0.337). Significant main effects of age (F(8,256) = 34.6, p GG ≤ 0.001, η p 2 = 0.519) and sex (F(1,32) = 80.3, p ≤ 0.001, η p 2 = 0.715) were also observed. For HIV-1 Tg animals, a segmental linear regression, with maximal prepulse inhibition at approximately PD 270, was the best fit for male HIV-1 Tg animals (R 2 = 0.88). In sharp contrast, female HIV-1 Tg animals failed to exhibit any significant development of temporal processing, best fit using a horizontal line. The overall ANOVA for HIV-1 Tg animals confirmed these observations, revealing a significant age × sex interaction (F(8,232) = 5.5, p GG ≤ 0.001, η p 2 = 0.160) with a prominent quadratic component (F(1,29) = 16.7, p ≤ 0.001, η p 2 = 0.366). Significant main effects of age (F(8,232) = 5.3, p GG ≤ 0.001, η p 2 = 0.154) and sex (F(1,29) = 24.1, p ≤ 0.001, η p 2 = 0.454) were also observed. Thus, regardless of biological sex, HIV-1 Tg animals exhibited significant alterations in the development of temporal processing. However, examination of the factor of biological sex revealed alterations which were more profound in female HIV-1 Tg animals.
Cross-modal prepulse inhibition (visual): temporal processing
In visual PPI (Fig. 6), HIV-1 Tg rats exhibited significant alterations in the development of temporal processing, indexed using prepulse inhibition, relative to controls. A segmental linear regression was the best fit for both HIV-1 Tg and control animals (R 2s ≥ 0.70). HIV-1 Tg animals exhibited a significant leftward shift in maximal prepulse inhibition relative to control animals. Specifically, control animals exhibited a linear increase in prepulse inhibition through PD 360, followed by a subsequent decrease. In contrast, HIV-1 Tg animals displayed maximal prepulse inhibition at PD 300, followed by a subsequent decrease. The overall ANOVA on mean area of the amplitude inflection curve confirmed these observations, revealing a significant age × genotype interaction with a prominent linear component (F(1,61) = 17.5, p ≤ 0.001, η p 2 = 0.223), a significant age × sex interaction (F(8,488) = 4.4, p GG ≤ 0.001, η p 2 = 0.068) with a prominent linear component (F(1,61) = 16.4, p ≤ 0.001, η p 2 = 0.211), and a significant genotype × sex interaction (F(1,61) = 4.8, p ≤ 0.032, η p 2 = 0.073). Significant main effects of genotype (F(1,61) = 23.6, p ≤ 0.001, η p 2 = 0.279), age (F(8,488) = 10.3, p GG ≤ 0.001, η p 2 = 0.145), and sex (F(1,61) = 69.9, p ≤ 0.001, η p 2 = 0.534) were also observed.
Visual prepulse inhibition, assessed using mean area of the peak inflection curve, is illustrated as a function of genotype (HIV-1 Tg vs. control) and age (±95% CI). a A segmental linear regression provided the best fit function for both HIV-1 Tg and control animals (R 2s ≥ 0.70). A significant leftward shift in the point of maximal inhibition was observed in HIV-1 Tg animals (PD 300) relative to control animals (PD 360). b Significant alterations in the development of temporal processing were observed in control animals dependent upon biological sex. Specifically, both male and female control animals were best fit using a segmental linear regression (R 2s ≥ 0.82). Male control animals exhibited a significant increase in prepulse inhibition through PD 360, followed by a subsequent decrease. In contrast, a rightward shift, with maximal prepulse inhibition observed at PD 420, was observed in female control animals. c Biological sex moderated the progression of temporal processing deficits in HIV-1 Tg animals. A segmental linear regression provided a well-described fit for both male and female HIV-1 Tg animals, with maximal prepulse inhibition observed at PD 300 and PD 420, respectively (R 2s ≥ 0.62). The factor of biological sex, however, altered the rate of temporal processing development in HIV-1 Tg rats, with slower development observed in female HIV-1 Tg rats relative to male HIV-1 Tg rats. HIV-1 Tg animals, regardless of sex, exhibited profound alterations in the progression of temporal processing, assessed using visual prepulse inhibition, relative to control animals
Complementary analyses were conducted for each genotype to determine the locus of these interactions (control: Fig. 6b; HIV-1 Tg: Fig. 6c). A segmental linear regression provided a well-described fit for both male and female control animals (R 2s ≥ 0.82). Male control animals exhibited a significant increase in prepulse inhibition through PD 360, followed by a subsequent decrease. Female control animals, however, exhibited a significant rightward shift, with maximal prepulse inhibition observed at PD 420, followed by a subsequent decrease. Differences in the rate of temporal processing development were also observed, with female control animals exhibiting slower development (β 1 = 626.4 ± 201.2) relative to male animals (β 1 = 1852 ± 874.8). The overall ANOVA confirmed these observations, revealing a significant age × sex interaction (F(8,256) = 3.6, p GG ≤ 0.003, η p 2 = 0.102) with a prominent linear component (F(1,32) = 16.3, p ≤ 0.001, η p 2 = 0.338). Significant main effects of age (F(8,256) = 11.5, p GG ≤ 0.001, η p 2 = 0.264) and sex (F(1,32) = 103.0, p ≤ 0.001, η p 2 = 0.763) were also observed. Male and female HIV-1 Tg animals were also best fit using a segmental linear regression (R 2s ≥ 0.62). Male HIV-1 Tg animals exhibited maximal prepulse inhibition at approximately PD 300, while a rightward shift, with maximal prepulse inhibition at PD 420, was observed in female HIV-1 Tg rats. Biological sex altered the rate of temporal processing development in HIV-1 Tg rats, with slower development observed in female HIV-1 Tg rats (β 1 = 201.8 ± 127.1) relative to male HIV-1 Tg rats (β 1 = 1400 ± 1097.5). The overall ANOVA confirmed these observations, revealing a significant main effect of age with a prominent linear component (F(1,29) = 13.9, p ≤ 0.001, η p 2 = 0.324) and sex (F(1,29) = 12.3, p ≤ 0.002, η p 2 = 0.297).
Locomotor activity: intrasession habituation
The progression of intrasession habituation, used to assess long-term episodic memory, is illustrated in Fig. 7. A one-phase decay was the best fit for HIV-1 Tg and control animals at all test sessions; however, significant differences between the best fit line were observed. Specifically, at PD 90 and PD 120, control animals, compared to HIV-1 Tg animals, exhibited a greater number of mean photocell interruptions, during the latter half of the test session. In contrast, at PD 300 and all subsequent test sessions, HIV-1 Tg animals displayed a greater number of mean photocell interruptions in the latter half of the session relative to controls.
Mean number of photocell interruptions during locomotor habituation trials across 5-min trial blocks are illustrated at all test sessions as a function of genotype (HIV-1 Tg vs. control; ±95% CI). Significant alterations in intrasession habituation in the HIV-1 Tg rat were observed at PD 300 and all subsequent test sessions, evidenced by a higher level of activity during the latter half of the session. Alterations in intrasession habituation suggest an impairment in long-term episodic memory in HIV-1 Tg animals
The overall ANOVA confirmed these observations, revealing a significant age × time × genotype interaction (F(88,5368) = 1.6, p GG ≤ 0.025, ηp 2 = 0.0.25) with a prominent linear-quadratic component (F(1,61) = 20.5, p ≤ 0.001, η p 2 = 0.252). Significant two-way interactions included an age × genotype interaction (F(8,488) = 20.5, p GG ≤ 0.001, η p 2 = 0.252) with a prominent linear component (F(1,61) = 96.9, p ≤ 0.001, η p 2 = 0.614), an age × sex interaction (F(8,488) = 2.2, p GG ≤ 0.039, η p 2 = 0.035) with a prominent quadratic component (F(1,61) = 9.8, p ≤ 0.003, η p 2 = 0.139), a time × genotype interaction (F(11,671) = 18.3, p GG ≤ 0.001, η p 2 = 0.231) with a prominent linear component (F(1,61) = 38.4, p ≤ 0.001, η p 2 = 0.386), a time × sex interaction (F(11,671) = 5.0, p GG ≤ 0.002, η p 2 = 0.076) with a prominent linear component (F(1,61) = 10.6, p ≤ 0.002, η p 2 = 0.148), and an age × time interaction (F(88,5368) = 8.6, p GG ≤ 0.001, η p 2 = 0.124) with a prominent linear-linear component (F(1,61) = 221.5, p ≤ 0.001, η p 2 = 0.784). Significant main effects of age (F(8,488) = 102.4, p GG ≤ 0.001, η p 2 = 0.627), time (F(11,671) = 1408.1, p GG ≤ 0.001, η p 2 = 0.958), and sex (F(1,61) = 10.5, p ≤ 0.002, η p 2 = 0.147) were also observed. Thus, HIV-1 Tg animals failed to exhibit intrasession habituation, evidenced by significantly greater activity in the latter half of the test session, through advancing age, suggesting an impairment in long-term episodic memory.
Discussion
Two interrelated goals were pursued to authenticate the utility of the HIV-1 Tg rat for the study of progressive neurocognitive deficits, a sequelae of long-term HIV-1 viral protein exposure (Vigorito et al. 2015). First, the integrity of auditory and visual sensory system function, assessed using cross-modal PPI, and motoric system function, examined using locomotor activity, was established. The integrity of sensory and motor systems in the HIV-1 Tg rat confirms the utility of the HIV-1 Tg rat in longitudinal experimental designs to determine the progression of HAND. Second, the progression of neurocognitive impairments, including temporal processing and long-term episodic memory, in the HIV-1 Tg rat were evaluated; the factor of biological sex was integral to the experimental design. Regardless of sex, HIV-1 Tg animals, relative to controls, displayed significant alterations in the development of auditory and visual PPI, suggesting a deficit in temporal processing. Notably, significant sex differences in the progression of temporal processing were also observed, with female HIV-1 Tg animals exhibiting more pronounced deficits relative to male HIV-1 Tg animals. An impairment in long-term episodic memory, evidenced by significant alterations in intrasession habituation, was also observed in HIV-1 Tg animals relative to controls. Understanding the progression of HAND heralds an opportunity for the development of an advantageous model of neurocognitive deficits in HIV-1 and establishes critical groundwork for the development of neurorestorative and/or preventative treatments.
The integrity of sensory system function through advancing age in HIV-1 Tg and control animals was assessed using cross-modal PPI. Robust inhibition to the presence of auditory and visual prepulses was observed at all test sessions, confirming the integrity of sensory system function in both HIV-1 Tg and control animals. Notably, the phenotypic cataracts observed in the HIV-1 Tg rat do not prevent the animal from detecting brightness; visual acuity, however, has not yet been systematically investigated in the HIV-1 Tg rat. Robust inhibition to auditory, visual, and tactile prepulses have been previously observed in HIV-1 Tg and control animals, albeit at younger ages (e.g., 2–6 months of age, Moran et al. 2013a; 8–10 months of age, McLaurin et al. 2017b, c). The present study provides a longitudinal assessment through the majority of the animal’s functional lifespan, providing strong evidence for the integrity of sensory system function in the HIV-1 Tg rat.
Motoric system function was assessed using locomotor activity in HIV-1 Tg and control animals through advancing age. From PD 90 to PD 180, control animals exhibited a significantly greater number of mean photocell interruptions during the 60-min test session, consistent with previous reports of decreased motor movement in HIV-1 Tg animals early (i.e., between 3 and 6 months) in development (June et al. 2009). No significant differences in the number of photocell interruptions between HIV-1 Tg and control animals were observed at PD 240 or PD 300. However, beginning at PD 360, and at all subsequent test sessions, HIV-1 Tg animals exhibited a significantly greater number of mean photocell interruptions relative to control animals. Thus, neither HIV-1 Tg nor control animals displayed gross-motoric system impairments through advancing age, indicative of the integrity of motor system function.
The overall health of HIV-1 Tg and control animals through advancing age was also assessed using growth rate. HIV-1 Tg animals, regardless of sex, weighed significantly less than control animals, but exhibited steady growth through PD 480 (approximately 16 months of age). The rate of growth, however, was significantly slower in male and female HIV-1 Tg animals relative to their control counterparts. Previous observations have reported no significant differences in the rate of growth; however, observations were restricted to younger animals (e.g., Peng et al. 2010; Moran et al. 2012, 2013b, 2014a; Roscoe et al. 2014). On the other hand, early adulthood food restriction, as in the present study, may have played a role in altering the growth trajectory of HIV-1 Tg animals.
It is noteworthy that the observations in the present study (i.e., growth rates, integrity of sensory system function, intact motoric system function) differ from those reported in the original derivation of the HIV-1 Tg (Sprague-Dawley) rat, with transgene expression on chromosomes 2 and 9 (Reid et al. 2001). Specifically, Reid et al. (2001) reported relatively early wasting (i.e., 5–9 months of age), hind-limb paralysis, and AIDS-related organ pathologies. However, the current derivation of the HIV-1 Tg (F344/N) rat, used in the present study, is a healthier derivation of those originally described, with transgene expression limited to chromosome 9. In the present study, HIV-1 Tg rats exhibited no alterations in sensory system function (i.e., auditory or visual) or motor system function through advancing age. The integrity of gustatory system function has also been established using both one-bottle (Peng et al. 2010) and five-bottle (Bertrand et al. 2013) sucrose taste preference tests, which revealed no significant differences between HIV-1 Tg and control animals in sucrose consumption at any concentration. Thus, the present studies provide strong evidence for the functional health of the HIV-1 Tg rat through the majority of the animal’s functional lifespan, suggesting the utility of the HIV-1 Tg rat in studies of the progression of neurocognitive deficits, including temporal processing and long-term episodic memory, commonly observed in HAND.
Prominent alterations in the progression of temporal processing, indexed using the area of inflection of the ASR amplitude response curve (McLaurin et al. 2016), were observed in HIV-1 Tg animals, regardless of sex, relative to controls using cross-modal PPI. HIV-1 Tg animals displayed a significant leftward shift in the age at which maximal prepulse inhibition was observed in both auditory and visual PPI. Specifically, in auditory PPI, control animals exhibited a significant increase in prepulse inhibition as a function of age through approximately PD 300, followed by a subsequent decrease. HIV-1 Tg animals, however, displayed maximal prepulse inhibition at approximately PD 270, followed by a subsequent decrease. In visual PPI, control animals exhibited an increase in prepulse inhibition as a function of age through PD 360, followed by a subsequent decrease. In contrast, HIV-1 Tg animals displayed a significant leftward shift, with maximal prepulse inhibition observed at PD 300. The present study establishes, via longitudinal assessment, alterations in the progression of temporal processing through the majority of the functional lifespan of the HIV-1 Tg rat.
Temporal processing deficits in the HIV-1 Tg rat may result from dopaminergic system dysfunction, which has been implicated as a key target of HIV-1 infection. Alterations in dopamine system function have previously been reported using multiple techniques in the HIV-1 Tg rat, including immunofluorescence and Western blots (Webb et al. 2010; Moran et al. 2012; Reid et al. 2016a), as well as (18F) fallypride positron emission tomography (PET; Lee et al. 2014). The serial circuitry involved in PPI, established using lesioning (e.g., Fendt et al. 1994; Leitner and Cohen 1985) and electrical stimulation studies (Li et al. 1998; Li and Yeomans 2000), includes the brainstem and pedunculuopontine pathways. Alterations in the dopaminergic inputs at multiple points within the serial circuitry mediating PPI have downstream effects, ultimately altering startle response (Koch 1999). The nucleus accumbens (Nac), which receives dopaminergic inputs from the ventral tegmental area (VTA), may be a critical loci for the regulation of PPI (Swerdlow et al. 1992). Specifically, the medial prefrontal cortex, which exhibits thinning in HIV-1 seropositive humans (Thompson et al. 2005), may mediate dopamine (DA) release from the Nac via glutamateric projections (Koch 1999). Decreased dopaminergic activity in the prefrontal cortex increases DA release in the Nac (Koch 1999), an effect which may be mediated by the glutamateric projection from the prefrontal cortex to the VTA (Taber and Fibiger 1995).
Pharmacological and behavioral studies have also been used to establish the role of dopamine (DA) in PPI (review, Geyer et al. 2001). Administration of DA agonists, including apomorphine (e.g., Fitting et al. 2006), SCH 39166 (e.g., Ellenbroek et al. 1996), sulpiride (e.g., Ellenbroek et al. 1996), and amphetamine (e.g., Mansbach et al. 1998; Zhang et al. 2000), produced a marked decrease in PPI. Administration of apomorphine subcutaneously in Sprague-Dawley rats stereotaxically injected with gp120, a HIV-1 viral protein, produced significant alterations in prepulse inhibition, evidenced by a decrease in startle response amplitude and an insensitivity to the manipulation of ISI (Fitting et al. 2006), results which are comparable to temporal processing deficits observed in the HIV-1 Tg rat. Significant reductions in PPI were also observed following local injections of either SCH39166 or sulpiride into the medial prefrontal cortex (Ellenbroek et al. 1996). Examination of the neurotransmitter adenosine, which may interact with DA in the central nervous system (Ferré et al. 1992), provides additional evidence for the role of DA in the regulation of PPI (e.g., Hauber and Koch 1997). Thus, DA system dysfunction in the HIV-1 Tg rat may underlie alterations in the progression of temporal processing deficits observed in the present study.
Profound alterations in the progression of intrasession habituation were observed in the HIV-1 Tg rat in comparison to control animals, suggesting an impairment in long-term episodic memory. Beginning at PD 300 and at all subsequent test sessions, HIV-1 Tg animals displayed significant deficits in intrasession habituation, evidenced by a higher level of activity during the latter half of the session, a pattern compatible with impairment in long-term episodic memory. Multiple experimental paradigms (e.g., locomotor activity, rotarod, wheel running) have revealed alterations in both intrasession habituation (Moran et al. 2013b) and intersession habituation (Chang and Vigorito 2006; Reid et al. 2016b) in the HIV-1 Tg rat. The present study, using a longitudinal experimental design, confirmed robust alterations in intrasession habituation through the majority of the functional lifespan of the HIV-1 Tg rat.
Alterations in intrasession habituation may also result from DA system dysfunction. Multiple pharmacological studies have provided evidence for the role of DA in intrasession habituation (Carlsson 1972; Giros et al. 1996; Wong et al. 2003). Specifically, administration of apomorphine, a DA agonist, in rats produced decreased intrasession habituation (Carlsson 1972). DA transporter (Giros et al. 1996), and D1 receptor knockout mice (Wong et al. 2003) also exhibited decreased intrasession habituation in locomotor activity. Additional studies are needed, however, to determine whether alterations in intrasession habituation result from the effects of increased DA on the motor system (Leussis and Bolivar 2006). Notably, DA release may also be critical for the formation of long-term episodic memories, commonly assessed in animal models using intrasession habituation. Lisman and Grace (2005) proposed the hippocampus-VTA loop and suggested that DA release from the hippocampus may be vital for long-term episodic memory encoding and retrieval. A pharmacological study found that infusion of a DA antagonist into the rat hippocampus prior to encoding impairs long-term memory (Bethus et al. 2010).
The assessment of sex differences was an integral component of the experimental design, addressing the need for direct comparisons of neurocognitive deficits in males and females (Maki and Martin-Thormeyer 2009; Maki et al. 2015). The factor of biological sex may moderate the progression of neurocognitive deficits, including temporal processing, in the HIV-1 Tg rat. Female HIV-1 Tg animals, compared to male HIV-1 Tg animals, exhibited more prominent deficits in temporal processing. Specifically, in auditory PPI, female HIV-1 Tg animals failed to display any significant development in temporal processing as a function of age. In visual PPI, female HIV-1 Tg animals exhibited a slower development of prepulse inhibition, relative to male HIV-1 Tg animals. Sex differences in the progression of temporal processing extend those reported in auditory gap prepulse inhibition, examined using an early, time-limited longitudinal experimental design (McLaurin et al. 2016), suggesting the generality and relative permanence of these deficits. In sharp contrast, neither compelling nor consistent sex differences were observed in the progression of intrasession habituation. Thus, biological sex may selectively moderate the influence of the HIV-1 transgene on the progression of neurocognitive deficits.
The present study provides a critical foundation for future studies examining the progression of executive function and attention deficits, which are commonly altered in HIV-1 seropositive individuals (Heaton et al. 2010, 2011). For example, signal detection tasks (McGaughy and Sarter 1995), which rely on intact sensory and motoric system function, can be used to assess sustained attention, as well as more complex executive functions, including inhibition and flexibility. In signal detection, the presence or absence of a stimulus (i.e., houselight, auditory tone) indicates the response an animal must make (i.e., which lever to press) to receive a reinforcer. We have previously used the signal detection task to assess cognitive functions in the HIV-1 Tg rat, reporting significant deficits in sustained attention, flexibility, and inhibition (Moran et al. 2014a). However, a systematic evaluation of the progression of sustained attention deficits, and the effect of biological sex, in the HIV-1 Tg rat is needed.
Establishing the integrity of auditory and visual system function, as well as motoric system function, suggests the utility of the HIV-1 Tg rat for examining the progression of HAND. Further, results of the present study substantiated significant alterations in the progression of temporal processing, which may underlie executive function and attentional deficits commonly observed in the post-cART era (Heaton et al. 2010, 2011). Additionally, alterations in the progression of intrasession habituation, suggestive of an impairment in long-term episodic memory, were observed. Results provide a strong foundation for future studies assessing the progression of executive function deficits, and the effect of biological sex, in the HIV-1 Tg rat using a longitudinal experimental design. Understanding the progression of HAND heralds an opportunity for the development of an advantageous model of neurocognitive deficits in HIV-1 and establishes critical groundwork for the development of neurorestorative treatments.
References
Abbondanzo SJ, Chang SL (2014) HIV-1 transgenic rats display alterations in immunophenotype and cellular responses associated with aging. PLoS One 9:e105256. doi:10.1371/journal.pone.0105256
Barker GRI, Bird F, Alexander V, Warburton EC (2007) Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 27:2948–2957. doi:10.1523/JNEUROSCI.5289-06.2007
Bertrand SJ, Mactutus CF, Harrod SB, Morgan AJ, Booze RM (2013) HIV-1 transgenic rat: altered internal motivational state for natural rewards [abstract]. J Neurovirol 19:S12
Bethus I, Tse D, Morris RG (2010) Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J Neurosci 30:1610–1618. doi:10.1523/JNEUROSCI.2721-09.2010
Booze RM, Mactutus CF (1990) Developmental exposure to organic lead causes permanent hippocampal damage in Fischer-344 rats. Experientia 46:292–297
Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L (1978) Prestimulus effects on human startle reflex in normal and schizophrenics. Psychophysiology 15:339–343. doi:10.1111/j.1469-8986.1978.tb01390.x
Carlsson SG (1972) Effects of apomorphine on exploration. Physiol Behav 9:127–129
Chang SL, Vigorito M (2006) Role of HIV-1 infection in addictive behavior: a study of the HIV-1 transgenic rat model. Am J Infect Diseases 2:98–106
Chao OY, Huston JP, Li JS, Wang AL, de Souza Silva MA (2016) The medial prefrontal cortex—lateral entorhinal cortex circuit is essential for episodic-like memory and associative object-recognition. Hippocampus 26:633–645. doi:10.1002/hipo.22547
Coleman PD (2004) How old is old? Editorial Neurobiol Aging 25:1 Original work published 1989
Crofton KM, Sheets LP (1989) Evaluation of sensory system function using reflex modification of the startle response. J Am Coll Toxicol 8:199–211
Denenberg VH (1984) Some statistical and experimental considerations in the use of the analysis-of-variance procedure. Am J Physiol-Reg I 246:R403–R408
Eacott MJ, Easton A, Zinkivskay A (2005) Recollection in an episodic-like memory task in the rat. Learn Mem 12:221–223. doi:10.1101/lm.92505
Ellenbroek BA, Budde S, Cools AR (1996) Prepulse inhibition and latent inhibition: the role of dopamine in the medial prefrontal cortex. Neuroscience 75:535–542
Fazeli PL, Crowe M, Ross LA, Wadley V, Ball K, Vance DE (2014) Cognitive functioning in adults aging with HIV: a cross-sectional analysis of cognitive subtypes and influential factors. J Clin Res HIV AIDS Prev 1:155–169. doi:10.14302/issn.2324-7339.jcrhap-13-191
Fendt M, Koch M, Schnitzler HU (1994) Sensorimotor gating deficit after lesions of the superior colliculus. Neuroreport 5:1725–1738
Ferré S, Fuxe K, von Euler G, Johansson B, Fredholm BB (1992) Adenosine-dopamine interactions in the brain. Neuroscience 51:501–512
Fitting S, Booze RM, Mactutus CF (2006) Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alterations in prepulse inhibition in adult rats. J Pharmacol Exp Ther 318:1352–1358. doi:10.1124/jpet.106.105742
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156:117–154
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379:606–612. doi:10.1038/379606a0
Greenhouse SW, Geisser S (1959) On methods in the analysis of profile data. Psychometrika 24:95–112
Harris JD (1943) Habituatory response decrement in the intact organism. Psychol Bull 40:385–422
Hauber W, Koch M (1997) Adenosine A2a receptors in the nucleus accumbens modulate prepulse inhibition of the startle response. Neuroreport 8:1515–1518
Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, JC MA, Morgello S, Simpson DM, JA MC, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER study. Neurology 75:2087–2096. doi:10.1212/WNL.0b013e318200d727
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group, HNRC Group (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16. doi:10.1007/s13365-010-0006-1
Hoffman HS, Ison JR (1980) Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev 87:175–189
Ison JR, Hammond GR (1971) Modification of startle reflex in rat by changes in auditory and visual environments. J Comp Physiol Psychol 75:435–452
Ison JR (1984) Reflex modification as an objective test for sensory processing following toxicant exposure. Neurobehav Toxicol Teratol 6:437–445
June HL, Yang ARST, Bryant JL, Jones O, Royal W (2009) Vitamin A deficiency and behavioral and motor deficits in the HIV-1 transgenic rat. J Neurovirol 15(5–6):380–389. doi:10.3109/13550280903350200
Koch M (1999) The neurobiology of startle. Prog Neurobiol 59:107–128
Kraemer HC, Yesavage JA, Taylor JL, Kupfer D (2000) How can be learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry 157:163–171. doi:10.1176/appi.ajp.157.2.163
Lashomb AL, Vigorito M, Chang SL (2009) Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol 15(1):14–24. doi:10.1080/13550280802232996
Lee DE, Reid WC, Ibrahim WG, Peterson KL, Lentz MR, Maric D, Choyke PL, Jagoda EM, Hammoud DA (2014) Imaging dopaminergic dysfunction as a surrogate marker of neuropathology in a small-animal model of HIV. Mol Imaging 13:1–10. doi:10.2310/7290.2014.00031
Leitner DS, Cohen ME (1985) Role of the interior colliculus in the inhibition of acoustic startle in the rat. Physiol Behav 34:65–70
Letendre SL, Ellis RJ, Ances BM, McCutchan JA (2010) Neurologic complications of HIV disease and their treatment. Top HIV Med 18:45–55
Leussis MP, Bolivar VJ (2006) Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev 30:1045–1064. doi:10.1016/j.neubiorev.2006.03.006
Li L, Priebe PM, Yeomans JS (1998) Prepulse inhibition of acoustic or trigeminal startle of rats by unilateral electrical stimulation of the inferior colliculus. Behav Neurosci 112:1187–1198
Li L, Yeomans JS (2000) Using intracranial electrical stimulation to study the timing of prepulse inhibition of the startle reflex. Brain Res Protocol 5:67–74
Lisman JE, Grace AA (2005) The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46:703–713. doi:10.1016/j.neuron.2005.05.002
Maki PM, Martin-Thormeyer E (2009) HIV, cognition and women. Neuropsychol Rev 19:204–214. doi:10.1007/s11065-009-9093-2
Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Weber KM, Manly J, Richardson J, Alden C, Anastos K (2015) Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology 84:231–240. doi:10.1212/WNL.0000000000001151
Mansbach RS, Geyer MA, Braff DL (1998) Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology 94:507–514
McArthur JC, Steiner J, Sacktor N, Nath A (2010) Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol 67:699–714. doi:10.1002/ana.22053
McGaughy J, Sarter M (1995) Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology 117:340–357
McLaurin KA, Booze RM, Mactutus CF (2016) Progression of temporal processing deficits in the HIV-1 transgenic rat. Sci Rep 6:32831. doi:10.1038/srep32831
McLaurin KA, Booze RM, Mactutus CF (2017a) Selective developmental alterations in the HIV-1 transgenic rat: opportunities for diagnosis of pediatric HIV-1. J Neurovirol 23(1):87–98. doi:10.1007/s13365-016-0476-x
McLaurin KA, Moran LM, Li H, Booze RM, Mactutus CF (2017b) A gap in time: extending our knowledge of temporal processing deficits in the HIV-1 transgenic rat. J NeuroImmune Pharmacol 12(1):171–179. doi:10.1007/s11481-016-9711-8
McLaurin KA, Booze RM, Mactutus CF (2017c) Temporal processing demands in the HIV-1 transgenic rat: amodal gating and implications for diagnostics. Int J Dev Neurosci 57:12–20. doi:10.1016/j.ijdevneu.2016.11.004
Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF (2012) Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV-1 Res 10:415–424
Moran LM, Booze RM, Mactutus CF (2013a) Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J NeuroImmune Pharmacol 8:988–997. doi:10.1007/s11481-013-9472-6
Moran LM, Booze RM, Webb KM, Mactutus CF (2013b) Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol 239:139–147. doi:10.1016/j.expneurol.2012.10.008
Moran LM, Booze RM, Mactutus CF (2014a) Modeling deficits in attention, inhibition, and flexibility in HAND. J NeuroImmune Pharmacol 9:508–521. doi:10.1007/s11481-014-9539-z
Moran LM, Fitting S, Booze RM, Webb KM, Mactutus CF (2014b) Neonatal intrahippocampal HIV-1 protein Tat(1-86) injection: neurobehavioral alterations in the absence of increase inflammatory cytokine activation. Int J Dev Neurosci 38:195–203. doi:10.1016/j.ijdevneu.2014.09.004
Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL (2010) The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol 218:94–101. doi:10.1016/j.jneuroim.2009.09.014
Peris J, Coleman-Hardee M, Millard WJ (1992) Cocaine in utero enhances the behavioral response to cocaine in adult rats. Pharmacol Biochem Behav 42(3):509–515
Pierce RC, Kalivas PW (2007) Locomotor behavior. Curr Protoc Neurosci:8.1.1–8.1.9. doi:10.1002/0471142301.ns0801s40
Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton D, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney F, Wilson DA, Wu C-F, Thompson RF (2009) Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 92:135–138. doi:10.1016/j.nlm.2008.09.012
Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J (2001) An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A 98:9271–9276. doi:10.1073/pnas.161290298
Reid WC, Ibrahim WG, Kim SJ, Denaro F, Casas R, Lee DE, Maric D, Hammoud DA (2016a) Characterization of neuropathology in the HIV-1 transgenic rat at different ages. J Neuroimmunol 292:116–125. doi:10.1016/j.jneuroim.2016.01.022
Reid WC, Casas R, Papadakis GZ, Muthusamy S, Lee DE, Ibrahim WG, Nair A, Koziol D, Maric D, Hammoud DA (2016b) Neurobehavioral abnormalities in the HIV-1 transgenic rat do not correspond to neuronal hypometabolism on 18F-FDG-PET. PLoS One 11:e0152265. doi:10.1371/journal.pone.0152265
Repunte-Canonigo V, Lefebvre C, George O, Kawamura T, Morales M, Koob GF, Califano A, Masliah E, Sanna PP (2014) Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol Neurodegener 9:26. doi:10.1186/1750-1326-9-26
Robbins TW (1977) A critique of the methods available for the measurement of spontaneous motor activity. In: Iversen LL, Iversen SD, Snyder SH (Eds) Handbook of psychopharmacology, Volume 7. Plenum Press, NY, pp 37–82.
Roscoe RF Jr, Mactutus CF, Booze RM (2014) HIV-1 transgenic female rat: synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J NeuroImmune Pharmacol 9:642–653. doi:10.1007/s11481-014-9555-z
Schwarzkopf SB, McCoy L, Smith DA, Boutros NN (1993) Test-retest reliability of prepulse inhibition of the acoustic startle response. Biol Psychiatry 34:896–900
Sheppard DP, Iudicello JE, Bondi MW, Doyle KL, Morgan EE, Massman PJ, Gilbert PE, Woods SP (2015) Elevated rates of mild cognitive impairment in HIV disease. J Neurovirol 21:576–584. doi:10.1007/s13365-015-0366-7
Smit M, Binkman K, Geerlings S, Smit C, Thyagarajan K, Sighem AV, de Wolf F, Hallett TB, ATHENA observational cohort (2015) Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 15:810–818. doi:10.1016/S1473-3099(15)00056-0
Swerdlow NR, Caine SB, Braff DL, Geyer MA (1992) The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. J Psychopharmacol 6:176–190. doi:10.1177/026988119200600210
Taber MT, Fibiger HC (1995) Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci 15:3896–3904
Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT (2005) Thinning of the cerebral cortex visualized in HIV/AIDS reflect CD4(+) T lymphocyte decline. Proc Natl Acad Sci U S A 102:15647–15652. doi:10.1073/pnas.0502548102
Thompson RF, Spencer WA (1966) Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73:16–43
Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Holck P, Grove J, Sacktor N (2004) Higher frequency of dementia in older HIV-1 individuals: the Hawaii aging with HIV-1 cohort. Neurology 63:822–827
Vigorito M, LaShomb AL, Chang SL (2007) Spatial learning and memory in HIV-1 transgenic rats. J NeuroImmune Pharmacol 2(4):319–328. doi:10.1007/s11481-007-9078-y
Vigorito M, Connaghan KP, Chang SL (2015) The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun 48:336–349. doi:10.1016/j.bbi.2015.02.020
Wears RL (2002) Advanced statistics: statistical methods for analyzing cluster and cluster-randomized data. Acad Emerg Med 9:330–341
Webb KM, Aksenov MY, Mactutus CF, Booze RM (2010) Evidence for developmental dopaminergic alterations in the human immunodeficiency virus-1 transgenic rat. J Neurovirol 16(2):168–173. doi:10.3109/13550281003690177
Wecker JR, Ison JR, Foss JA (1985) Reflex modification as a test for sensory function. Neurobehav Toxicol Teratol 7:733–738
Wecker JR, Ison JR (1986) Visual function measured by reflex modification in rats with inherited retinal dystrophy. Behav Neurosci 100:679–684
West SG, Biesanz JC, Kwok O-M (2004) Within-subject and longitudinal experiments: design and analysis issues. In: Sansone C, Morf C, Panter AT (eds) The SAGE handbook of methods in social psychology. Sage Publications, Thousand Oaks, pp 287–312
Winer BJ (1971) Statistical principles in experimental design, 2nd ed. New York.
Wong JYF, Clifford JJ, Massalas JS, Kinsella A, Waddington JL, Drago J (2003) Essential conservation of D1 mutant phenotype at the level of individual topographies of behaviour in mice lacking both D1 and D3 dopamine receptors. Psychopharmacology 167:167–173. doi:10.1007/s00213-003-1415-0
Young JS, Fechter LD (1983) Reflex inhibition procedures for animal audiometry: a technique for assessing ototoxicity. J Acoust Soc Am 73:1686–1693
Zhang J, Forkstam C, Engel JA, Svensson L (2000) Role of dopamine in prepulse inhibition of acoustic startle. Psychopharmacology 149:181–188
Acknowledgements
This work was supported in part by grants from NIH (National Institute on Drug Abuse, DA013137; National Institute of Child Health and Human Development, HD043680; National Institute of Mental Health, MH106392; National Institute of Neurological Diseases and Stroke, NS100624) and the interdisciplinary research training program supported by the University of South Carolina Behavioral-Biomedical Interface Program. We thank Elizabeth M. Balog, Iris K. Dayton, Madison R. Gassmann, and Abigail L. Lafond for assistance with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The project protocol, under federal assurance (# A3049–01), was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Figure 1
The ASR at the point of maximal inhibition is illustrated for auditory (a) and visual (b) prepulse inhibition (PPI) as a function of genotype (HIV-1 Tg, control) and age (±95% CI). (a) In auditory PPI, a linear increase in ASR at the point of maximal inhibition was observed in both HIV-1 Tg and control animals as a function of age. A global first-order polynomial was the best fit for both HIV-1 Tg and control animals; no significant differences in the rate of increase in ASR at the point of maximal inhibition was observed between genotypes [F(2,14) = 3.2, p ≥ 0.05]. (b) In visual PPI, both HIV-1 Tg and control animals displayed a linear increase in the ASR at the point of maximal inhibition as a function of age. Although the ASR at the point of maximal inhibition increased significantly faster in HIV-1 Tg animals relative to controls [F(1,14) = 14.5, p ≤ 0.002], robust inhibition to the visual prestimulus was observed at all test sessions. (GIF 57.7 kb)
Rights and permissions
About this article
Cite this article
McLaurin, K.A., Booze, R.M. & Mactutus, C.F. Evolution of the HIV-1 transgenic rat: utility in assessing the progression of HIV-1-associated neurocognitive disorders. J. Neurovirol. 24, 229–245 (2018). https://doi.org/10.1007/s13365-017-0544-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-017-0544-x