Abstract
The purpose of the present study is to examine the integrity of white matter microstructure among individuals coinfected with HIV and HCV using diffusion tensor imaging (DTI). Twenty-five HIV+ patients, 21 HIV+/HCV+ patients, and 25 HIV− controls were included in this study. All HIV+ individuals were stable on combination antiretroviral therapy (cART; ≥3 months). All participants completed MRI and neuropsychological measures. Clinical variables including liver function, HIV-viral load, and CD4 count were collected from the patient groups. DTI metrics including mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD), and fractional anisotropy (FA) from five subregions of the corpus callosum were compared across groups. The HIV+/HCV+ group and HIV+ group were similar in terms of HIV clinical variables. None of the participants met criteria for cirrhosis or fibrosis. Within the anterior corpus callosum, significant differences were observed between both HIV+ groups compared to HIV− controls on DTI measures. HIV+ and HIV+/HCV+ groups had significantly lower FA values and higher MD and RD values compared to HIV− controls; however, no differences were present between the HIV+ and HIV+/HCV+ groups. Duration of HIV infection was significantly related to DTI metrics in total corpus callosum FA only, but not other markers of HIV disease burden or neurocognitive function. Both HIV+ and HIV+/HCV+ individuals had significant alterations in white matter integrity within the corpus callosum; however, there was no evidence for an additive effect of HCV coinfection. The association between DTI metrics and duration of HIV infection suggests that HIV may continue to negatively impact white matter integrity even in well-controlled disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Both HCV and HIV are independently associated with cognitive deficits (Forton, et al. 2004; Hilsabeck et al. 2003; McAndrews et al. 2005). Studies have shown that HIV+/HCV+ patients are more likely to exhibit greater global impairment (Hilsabeck et al. 2003; McAndrews et al.; Cherner et al. 2005; Ciccarelli et al. 2013; Letendre et al. 2005), slower reaction times (Martin et al. 2004; Perry et al. 2005; von Giesen et al. 2004), abstraction deficits (Cherner et al. 2005), and attention and concentration difficulties (Perry et al. 2005; Murray et al. 2008) compared to HIV+ individuals. However, controversy exists in the field, as recent studies observed no significant differences in cognitive function between HIV+ and HIV+/HCV+ individuals after controlling for confounding variables (Clifford et al. 2009, 2015) such as drug use and liver function, or when HCV viral load (VL) is undetectable (Crystal et al. 2012). None of these studies incorporated neuroimaging to identify potential CNS differences associated with coinfection with or without cognitive impairment.
Diffusion tensor imaging (DTI) is ideally suited to measure the microstructural integrity of cortical white matter, which is often impacted in HIV and HCV (for review see Masters and Ances 2014). DTI abnormalities are observed in HIV+ individuals, typically as reductions in fractional anisotropy (FA) or increases in mean diffusivity (MD) in the corpus callosum (Wu et al. 2006) and whole brain (Ragin et al. 2004; Ragin et al. 2005; Stebbins et al. 2007; Tate et al. 2010). These DTI metrics can be influenced by age (Chang et al. 2008; Togwood et al. 2012; Gongvatana et al. 2011), gender (Smith et al. 2008), initiation of combination antiretroviral therapy (cART) (Gongvatana et al. 2011; Wright et al. 2012), and substance use history (Thames 2011; Pfefferbaum et al. 2007). A few studies have examined the effects of HIV+/HCV+ individuals using DTI (Stebbins et al. 2007; Gongvatana et al. 2011; Jernigan et al. 2011) with some showing greater changes in FA and MD in HIV+/HCV+ compared to HIV+ alone. However, these studies did not have well-matched groups regarding HCV medications, liver status, or drug use histories all of which may impact white matter integrity (Niciu and Mason 2014; Zhang et al. 2014).
It is important to determine if the combined effects of HIV and HCV on the integrity of white matter in the brain are different from HIV alone, particularly given the high prevalence of coinfection and the potential additive/synergistic impact on health outcomes. The primary purpose of this study is to use DTI to determine if structural neuroimaging signatures of coinfection indicate a greater impact on white matter integrity than HIV alone in a group of well-matched HIV+ and HIV+/HCV+ individuals. Additionally, we examined how DTI metrics in these HIV+ groups compared to healthy controls.
Methods
Participants
A total of 71 patients (25 HIV+, 21 HIV+/HCV+, 25 HIV− controls) were included in the study (see Table 1). HIV+ and HIV+/HCV+ individuals were recruited from patients followed at the Infectious Disease clinic at Washington University in Saint Louis (WUSTL). HIV− controls were selected from archival data collected at WUSTL using similar study protocols for inclusion as well as identical imaging protocols. HIV− controls were selected according to age and education of the HIV+/HCV+ population. The WUSTL Human Research Protection Office approved this study. All participants provided informed consent for this study.
Inclusion/exclusion
Participants ranged from 21–61 years of age. All HIV+ individuals were required to be on a stable cART regimen for at least 3 months prior to scanning. All HIV+/HCV+ individuals were naïve to interferon and other HCV treatments or had discontinued therapy early due to negative side effects (n = 3). All participants had a previous history of drug use, including some intravenous drug use (IDU) but were not actively using at the time of testing (see drug testing below). HIV+, HIV+/HCV+ and HIV− individuals were excluded if they reported the following: drug or alcohol abuse (as defined by DSM-IV criteria) in the last 6 months, a history of mental disorders (e.g., schizophrenia), learning disability, confounding neurological disorders (e.g., multiple sclerosis, CNS infections), active diabetes, history of ascites, hepatic encephalopathy, esophageal varices, hepatorenal syndrome, non-HCV liver disease, or were pregnant.
Disease burden
For the HIV+ groups (both HIV+ and HIV+/HCV+), serostatus was determined using an HIV antibody test and confirmed by Western blot. HCV status was confirmed using HCV antibody testing. Current and nadir CD4 levels, plasma viral load (VL), and self-reported date of HIV diagnosis or confirmed diagnosis of seroconversion were recorded and used to assess group differences. In addition, laboratory markers of liver function (including AST, ALT, platelets, and albumin) were obtained within 30 days of study participation. Based on laboratory data, the aspartate to platelet ratio index (APRI) was calculated as a non-invasive marker of liver function to determine the likelihood of cirrhosis and fibrosis (Carvalho-Filho et al. 2008); Shaheen and Myers 2007; Tural et al. 2009). APRI was calculated using the following formula: [(AST/ upper limit for normal AST)/ Platelets (109 /L)]×100. This ratio was used as the outcome measure to compare HIV+ groups on liver function status.
Neuropsychological/psychiatric evaluation
Neuropsychological measures were performed for all participants (supplementary table). However, due to changes in the study design over the data collection period not all neuropsychological measures were completed by every participant. A z-score for four neuropsychological tests (total list learning, digit symbol modalities, trails A, trails B) was calculated based on current normative scores adjusted for age, education, ethnicity, and gender when applicable (Heaton et al. 1991; Gladsjo et al. 1999; Norman et al. 2011). A composite z-score (NPZ-4) was calculated from the average z-scores from tests as a primary measure to compare cognition between groups. The NPZ-4 has been used in a number of studies of neuroHIV (for review see McArthur and Smith 2013) and markers of brain integrity. Additional neuropsychological measures were also completed and are detailed in supplemental Table 1. Quality of life, fatigue, and depression were assessed in the HIV+/HCV+ group only using the Short-Form 36 (SF-36; Ware and Sherbourne 1992), Fatigue Severity Scale (FSS; Krupp et al. 1989), and Center for Epidemiological Studies Scale for Depression (CESD; Radloff 1977), respectively.
Drug testing
At the time of testing and imaging all participants, including HIV− controls, completed a rapid urine screen for drugs. The drug screen was primarily used to validate self-reported drug use and to ensure participants were not under the influence of substances at the time of MRI and testing. A structured clinical interview (SCID, Spitzer, 1987) was administered to assess alcohol/drug use and dependence and to determine if participants met DSM-IV criteria for abuse or dependence at the time of testing. Additional information about lifetime frequency, duration, and amount of each drug used was assessed using the Kreek-McHugh-Schluger-Kellogg Scale (KMSK; Kellogg et al. 2003). The KMSK assigns a score for frequency of use, duration use, and amount of drug used. The maximum score for each substance is 16. The total score for each substance was used to compare group differences in lifetime drug use.
DTI
Scanning was performed using a 3 T Siemens Tim TRIO whole-body magnetic resonance scanner (Siemens AG, Erlangen, Germany) with a product transmit/receive head coil. A T1-weighted three-dimensional magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence (time of repetition (TR)/inversion time (TI)/echo time (TE) = 2400/1000/3.16 ms, flip angle = 8°, and voxel size = 1 × 1 × 1 mm3) was obtained. A T2-weighted fast spin echo (T2W FSE) scan (TR = 4380 ms, TE = 94 ms, 1 × 1× 3 mm) was also acquired. Images were visually inspected at the time of scanning and an additional scan was performed if significant motion artifacts were observed. Two acquisitions of diffusion-weighted images were collected and subsequently averaged for the assessment of white matter microstructural integrity (2 × 2 × 2 mm voxels, TR = 9900 ms, TE = 102 ms, flip angle =90°, 23 directions with b values ranging between 0 and 1400 s/mm2).
DTI processing and ROI analysis
Preprocessing methods for registration and motion correction have been previously described (Shimony et al. 2006). Using standard methods, FA and MD for each brain voxel was calculated using the diffusion tensor (Shimony et al. 2009). In brief, in each voxel log-linear regression was used to calculate the three eigenvalues and diffusion tensor using a monoexponential decay diffusion model (Basser, 1994). FA measures the amount of diffusion that is anisotropic, or directional, compared to the diffusion that is isotropic or random. MD is a measure of total diffusion occurring in a given voxel (Basser and Pierpaoli 1996). Axial diffusivity (AD) and radial diffusivity (RD) may provide specific information about the integrity of either the axon itself, or the myelin surrounding the axons in white matter (Song et al., 2002). Specifically, AD is the diffusion along the longitudinal plane, i.e., along the primary eigenvector of the diagonalized diffusion tensor, while RD is the average of the diffusion values along the shorter, transverse axis, perpendicular to the primary eigenvector. Pathological conditions often impact the integrity and organization of white matter fibers. When the microstructure of these white matter fibers is disrupted, the pattern of direct diffusion along their lengths is altered, which is expressed as a reduction in FA, and typically an increase in MD and potential changes to AD or RD.
The corpus callosum (CC) was chosen as the primary region of interest (ROI) as white matter fibers in this structure are highly organized such that the voxels contain fibers that are primarily oriented in a single direction (Chanraud et al. 2010). Additionally, the CC connects cortical regions in an organized manner that can be related to specific cognitive functions (Hofer and Frahm 2006; Rosas et al. 2010). Previous studies of HIV have consistently reported abnormalities in the CC of HIV+ individuals (Filippi et al. 2001; Müller-Oehring et al. 2010; Wu et al. 2006; Tate et al. 2010; Wright et al. 2012 & 2015). As such, the corpus callosum is an ideal target to investigate the potential of an additive effect of HIV+/HCV+ coinfection on white matter integrity. Analyze 11.0 (Analyze Direct; Overland Park, KS) was used to outline the CC semi-automatically on five mid-sagittal slices from the FA map of each participant. A threshold of FA > 0.3 was used to minimize the potential for partial volume effects. Trained raters (JH and PW) visually inspected each of the images and performed manual edits as necessary to ensure the selected voxels met the threshold value and were white matter. As previously defined, the CC was further divided into five segments as seen in Fig. 1 (Hofer and Frahm 2006; Wright et al. 2015). These CC sections correspond with cortical connections to the prefrontal (CC1-anterior 1/6), premotor, and supplementary motor (CC2-anterior ½ minus 1/6), primary motor (CC3-posterior ½ minus 1/3), primary sensory (CC4-posterior 1/3 minus 1/4), and parietal, temporal, and occipital (CC5-posterior 1/4) areas.
a–d. Boxplots demonstrate the regions with statistically significant group differences for the CC regions. 1a shows the CCMD differences for regions 1 and 2, respectively. 1b shows the CC RD differences for regions 1–3, respectively. 1c shows the differences for CC FA for region 3. 1d provides a visual of the CC regions described 1, 2, and 3. Asterisks indicate p value for significant group difference compared to controls, after correction for multiple comparisons. There were no significant differences between the HIV+ and HIV+/HCV+ groups
Statistical analysis
Independent measures t tests were completed to compare the HIV+ and HIV+/HCV+ groups on current CD4 count, nadir CD4 count, plasma VL, duration of HIV+ infection, and APRI score. All variables were examined for normality. Plasma viral load (VL) was natural log transformed and both current CD4 and nadir CD4 were square root transformed to achieve a normal distribution for subsequent analyses. Descriptive analyses to characterize the three groups (HIV− controls, HIV+, and HIV+/HCV+) in terms of age, education, race, and gender were performed using an analysis of variance (ANOVA). No significant differences existed among groups on the demographic measures; therefore, we did not include any of these variables as covariates in analyses. Using separate ANOVAs for each of the dependent DTI measures (FA, MD, AD, and RD), a main effect of group status (HIV−, HIV+, HIV+/HCV+) on total CC FA, MD, RD, and AD was examined. A post hoc test was used to determine if group differences were present. Separate MANOVAs using each DTI metric for the five CC subregions as dependent variables and group status as the independent variable were completed to determine if differences existed between the groups in the subsections sections of the CC. A Holm-Sidak correction was used for multiple comparisons (Glanz 2005). A one-way ANOVA was used to compare the HIV+, HIV+/HCV+, and HIV− groups on the NPZ-4 composite score. Finally, Pearson’s correlations were used to determine the relationship between total CC DTI metrics and neuropsychological performance (both NPZ-4 and individual measures).
Results
Demographic variables
Demographic and drug use information are presented (Tables 1 and 2). There were no significant group differences in age or education between the three groups. The HIV− control group scored significantly higher on the WRAT-3, a proxy measure of premorbid intelligence quotient (IQ), compared to either the HIV+ or HIV+/HCV+ groups. There was a significant difference between the two HIV+ groups in terms of duration of infection with the HIV+/HCV+ group infected longer (m = 13.8 years, sd = 7.7) than the HIV+ group (m = 9.1 years, sd = 6.6, p = 0.01). The HIV+ and HIV+/HCV+ groups were similar in terms of current CD4, nadir CD4, and plasma VL, including the number of individuals who had undetectable viral loads (70 and 71 %, respectively). Although the HIV+/HCV+ group had a significantly higher APRI score (m = 0.73, sd = 0.61) compared to the HIV+ group (m = 0.29, sd = 0.13), none of the participants in the HIV+ group had scores that exceeded a standard cutoff (APRI ≥1.5) indicating cirrhosis or fibrosis (El Rihim et al. 2013).
There were no significant differences in terms of self-reported history of alcohol and tobacco use among the three groups. Both HIV+ groups reported current tobacco use (n = 29), whereas many of the HIV− controls (n = 10) reported a past history of tobacco use, with only four individuals in the HIV− group reporting current tobacco use. Marijuana and cocaine use were significantly less for the HIV− group compared to all other groups (Table 2). Correlational analyses did not reveal any significant relationships between the individual substance use scores (KMSK), liver status (APRI), and the DTI metrics or neuropsychological measures for the two HIV+ groups. As such, these variables were not used as covariates in subsequent analyses.
Effects of HIV and HCV on DTI metrics in the CC
A significant effect of group status on FA was observed for the entire CC (F (3,67) = 8.43, p < 0.01 η2 = 0.2), with HIV− individuals having significantly higher FA (m = 0.62, sd = 0.02) than either HIV+ (m = 0.60, sd = 0.03 p = 0.005) or HIV+/HCV+ groups (m = 0.59, sd = 0.03 p = 0.001). No significant differences in FA were observed between the two HIV+ groups. A significant effect of group status was also observed for MD (F (3,67) = 6.99, p < 0.01, η2 = 0.17) and RD (F (3,67) = 11.2, p < 0.01, η2 = 0.25) within the entire CC. HIV− controls had lower MD (m = 0.84, sd = 0.05) and RD (m = 0.50, sd = 0.04) in the entire CC compared to either HIV+ (p < 0.01 for MD m = 0.90, sd = 0.08 and RD m = 0.56 sd = 0.06) or HIV+/HCV+ (p < 0.01 for MD m = 0.90 sd = 0.08 and RD m = 0.57, sd = 0.07) participants. No significant difference existed between the two HIV+ groups with regard to RD or MD. AD values did not differ across the three groups.
Since FA, MD, and RD were significantly different between the HIV− control and the two HIV+ groups, MANOVAs were conducted by DTI metric for CC1-5 in order to identify differences between controls and the HIV+ groups within specific areas of the CC. Multivariate results indicated a significant effect of group on FA (Wilks' Lambda = 0.755, F = 1.93, p = 0.04) and RD (Wilks' Lambda = 0.720, F = 2.29, p = 0.017) results in Table 3 see also Fig. 1)). There was not an overall multivariate effect of MD between groups (Wilks’ Lambda = 0.783 F = 1.663, p = 0.097) After controlling for multiple comparisons, post hoc univariate analyses revealed that HIV+ and HIV+/HCV+ groups were significantly different than the HIV− controls on RD metrics in CC1-CC3 and MD metrics in CC1 and CC2. The HIV+/HCV+ group also had significantly lower FA compared to the HIV− group in CC3. Additionally, the HIV+ group had significantly lower RD compared to HIV− controls in the CC5 region also (see Table 3).
Relationship between DTI metrics and HIV disease burden
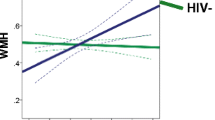
The regression model looking at the relationship between markers of disease burden (coinfection status, nadir CD4, log VL, duration of HIV infection) and the DTI indices (FA, MD, RD) in the total CC identified duration of infection as the only marker associated with any of the DTI indices. The overall model was not significantly associated with any of the DTI metrics. Duration of infection was inversely associated with total CC FA (B = −0.41 = p = 0.020) (Fig. 2).
Relationship between DTI metrics and neurocognitive function
There were no significant differences between the groups on the NPZ-4 measure. An additional linear regression model that included age and DTI indices within the CC was completed to determine the individual and combined associations of these variables with neurocognitive function. DTI indices included in the analysis were FA, MD, and RD in the total CC. The overall regression model did not identify significant associations between DTI metrics and NPZ-4 scores. We conducted exploratory correlational analyses to examine the relationship between individual neuropsychological measures and total CC DTI metrics. One measure of working memory/executive function, letter-number sequencing, was associated with all DTI metrics (total CC FA r = 0.39 p = 0.008; total CC RD r = −0.38 p = 0.009; total CCMD r = −0.41 p = 0.004); verbal learning and story memory were also associated with all of the DTI metrics (verbal learning: total CC FA r = 0.36 p = 0.01; total CC RD r = −0.31 p = 0.04; total CCMD r = −0.39 p = 0.008; story memory: total CC FA r = 0.36 p = 0.01; total CC RD r = −0.31 p = 0.04; total CCMD r = −0.37 p = 0.01). Digit symbol was associated with total CC FA r = 0.36 p = 0.02 and total CC RD r = −0.33 p = 0.03, and finally verbal fluency was associated with total CC FA (r = 0.29 p = 0.02. None of these correlations withstood correction for multiple comparisons. (Supplemental Table 2)
Discussion
This study identifies an impact of HIV on white matter integrity; however, no significant additive effect was observed in the HIV+/ HCV+ group compared to the HIV+ group. These results demonstrating DTI differences associated with HIV status are similar to previous DTI studies that indicated significantly lower FA in the frontal lobe and genu of the CC for HIV+ patients compared to healthy controls (Filippi et al. 2001; Thurnher et al. 2005; Pfefferbaum et al. 2007). In the present study, the observed differences in DTI metrics were only correlated with self-reported duration of infection in total CC FA, but not other markers of HIV disease burden such as current or nadir CD4, VL or coinfection status.
There were no significant differences in neurocognitive performance between the groups using the NPZ-4 measure. Although the NPZ-4 contains measures that are sensitive to detecting HIV and HCV-associated neuropsychological dysfunction, an extensive battery with multiple measures would provide greater confidence about the potential cognitive impact of HIV/HCV coinfection. There were no significant associations between the NPZ4 and the DTI metrics. Exploratory correlational analyses did indicate relationships between select neuropsychological measures and total CC DTI metrics for FA, MD, and RD. Because not all participants completed every neuropsychological measure, it is possible the lack of additional associations between DTI metrics and other neuropsychological measures is due to a relatively small sample within each of the groups.
Coinfection of HIV and HCV has been associated with lower FA and MD and increased MD in a preliminary whole brain analysis (Stebbins et al. 2007) compared to HIV+ individuals. The findings of this study are in contrast to previous studies (Stebbins et al. 2007; Gongvatana et al. 2011; Jernigan et al. 2011). However, potential differences exist between our cohort and sample populations previously studied. The two HIV+ groups in the present study exhibited similar proportions of individuals with undetectable HIV-VL (70 vs. 71 %) and current CD4 counts (430 vs. 488), suggesting a homogenous group in terms of HIV disease burden, which has not been the case in previous studies. Additionally, we specifically selected HIV+ and HIV+/HCV+ patients who had similar substance use histories to minimize group differences in substance abuse on brain integrity. HIV+/HCV+ individuals naïve to HCV treatment were included, but HIV+/HCV+ individuals who had completed HCV treatment were not included in this study. Previous studies of HIV+/HCV+ coinfection have not typically reported HCV treatment histories. While HCV treatments have been associated with neurocognitive dysfunction (Gess and Forton 2012), the effects of HCV therapy on neuroimaging indices has not been well described. The results from this study suggest that HIV and HCV do not have additive effects on white matter structural integrity in the CC in HIV+/HCV+ individuals who have similar levels of disease burden, drug use history, and in the absence of liver disease.
HCV has been found in brain tissue of autopsy patients (Vargas et al. 2002), leading to the speculation that the presence of HCV in the brain leads to an upregulation of the immune system and subsequent neuronal damage. However, there are no studies reporting any direct infection of neurons by HCV and no current reports have discussed potential exacerbation of the central inflammatory response in the brains of individuals with HIV/HCV coinfection compared to HIV monoinfection. Unfortunately, we did not have cerebrospinal HCV viral loads for this sample, and therefore could not quantify HCV viral presence in the CSF. Nevertheless, the observation of no significant differences in DTI metrics between HIV+/HCV+ individuals and HIV+ individuals despite a longer estimated duration of infection suggests that HCV presence in the brain may not significantly add to neuropathogenic processes of HIV as evidenced by either imaging or neuropsychological testing.
Abnormalities in DTI metrics have been associated with recreational drug use (Thames 2011; Pfefferbaum et al. 2007; Paul et al. 2007) in HIV+ individuals. In the present study both the HIV+ and HIV+/HCV+ groups had significantly higher self-reported use of recreational drugs, especially cocaine and marijuana compared to the HIV− controls. However, there was no difference between the HIV+ group and the HIV/HCV+ group in terms of DTI metrics or drug use, and the correlational analysis did not reveal associations between the scores on the drug use measure (KMSK) and any of the DTI metrics in the HIV+ groups. Drug use histories were recorded from self-report measures and required participants to recall information about drug use that primarily occurred in the past. It is possible that the participants did not accurately recall their drug use, although this would likely occur for all groups. Of note, the KMSK did not account for all possible types of drug use, and therefore, previous drug use, or use of drugs that were not accounted for (e.g., methamphetamines), cannot be ruled out as a potential mechanism of the alterations in white matter integrity that were evident in the HIV+ groups compared to HIV− controls.
Both HIV+ and HIV+/HCV+ groups were similar in terms of their current clinical profiles, with similar current CD4 levels and low or undetectable plasma VL for the majority of HIV+ individuals. Regression analyses revealed a relationship between duration of infection and total CC FA only. In this study, HIV-VL and nadir CD4 were not associated with DTI metrics, corresponding with other studies (Gongvatana et al. 2011); however, some reports (Tate et al. 2010; Li et al. 2014) did find lower nadir CD4 counts were associated with abnormal DTI metrics. Most of the HIV+ and HIV+/HCV+ individuals in the study had very low, or undetectable VL and had nadir CD4 levels below 200 cells. Other markers of HIV burden, such as proviral DNA, have been associated with significant cortical brain changes (Kallianpur et al. 2012) and may be a better marker for HIV-associated brain changes when HIV-VL is undetectable.
Our analysis is limited in that we focused on the CC and did not measure DTI metrics in other white matter fiber tracts. Because we limited our analysis to this region, it is possible that the limited selection might explain the lack of differences between the HIV+ groups that have been observed in other studies. The primary reason for the selection of the CC is the high directionality of fibers, previous literature showing an effect in HIV (Filippi et al. 2001; Müller-Oehring et al. 2010; Wu et al. 2006; Tate et al. 2010; Wright et al. 2012; 2015 and the anatomical relevance of the connections between the CC and specific cognitive functions (Hofer and Frahm 2006; Rosas, et al. 2010). Although it is possible other areas of the brain are differentially affected, as was observed across the CC ROIs, our relatively small sample does limit our power to detect statistically significant differences across a large number of comparisons. Future studies that look at changes throughout the brain will be beneficial.
In summary, this study identifies a significant impact of HIV on white matter microstructural integrity for HIV monoinfection and HIV+/HCV+ coinfection. However, there was no indication of an additive effect of HCV coinfection on DTI metrics. Our HIV+ and HIV+/HCV+ groups were very similar in terms of disease burden, substance use history and were naïve to HCV+ treatments, such as interferon, and all of these factors might impact brain integrity. The HIV+ and HIV+/HCV+ groups were matched on key variables that have not been described in great detail in the previous studies. The lack of significant differences on the DTI and neuropsychological measures between the HIV+ and HIV+/HCV+ group maybe due to the limited differences between the groups. Lack of differences between these groups has also been observed in other studies of mono- versus coinfection where there are little to no differences in brain integrity when the groups are well-matched on key demographic variables and markers of disease burden (see Clifford et al. 2015). The changes in DTI indices FA, MD, and RD (not AD) we observed, when taken together, suggest there may be compromise to myelin sheaths, resulting in increased diffusion perpendicular to axon fibers primarily in the anterior regions of the CC (Wang, 2011). These changes were related to duration of infection, indicating that despite stable HIV treatment and a lack of cirrhosis in this population, the brain is still vulnerable. These findings emphasize the importance of early and sustained treatment of both HIV and HIV-HCV coinfection to improve outcomes associated with chronic disease.
References
Analyze imaging software, version 11.0. AnalyzeDirect
Basser PJ, & Pierpaoli C (1996) Microstructural features measured using diffusion tensor imaging. J Magn Reson B 111(3):209–219
Carvalho-Filho RJ, Schiavon LL, Narciso-Schiavon JL (2008) Optimized cutoffs improve performance of the aspartate aminotransferase to platelet ratio index for predicting significant liver fibrosis in human immunodeficiency virus/hepatitis C virus co-infection. Liver Int 28(4):486–493. doi:10.1111/j.1478-3231.2008.01675.x
Chang L, Wong V, Nakama H et al (2008) Greater than age-related changes in brain diffusion of HIV patients after 1 year. J of Neuroimmune Pharm 3:265–274. doi:10.1007/s11481-008-9120-8
Chanraud S, Zahr N, Sullivan E,P, Fefferbausm A (2010) MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev 20(2):209–255
Cherner M, Letendre S, Heaton RK (2005) Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology 64(8)):1343–347. doi:10.1212/01.WNL.0000158328.26897.0D
Ciccarelli N, Fabbiani M, Grima P et al (2013) Comparison of cognitive performance in HIV or HCV mono-infected and HIV–HCV co-infected patients. Infection 41(6):1103–1109
Clifford DB, Smurzynski M, Park LS et al (2009) Effects of active HCV replication on neurologic status in HIV RNA virally suppressed patients. Neurology 73(4):309–314. doi:10.1212/WNL.0b013e3181af7a10
Clifford DB, Vaida F, Kao YT, Franklin DR, Letendre SL, Collier AC, Teshome M (2015) Absence of neurocognitive effect of hepatitis C infection in HIV-coinfected people. Neurology 84(3):241–250
Crystal H, Kleyman I, Anastos K et al (2012) Effects of hepatitis C and HIV on cognition in women: data from the women's interagency HIV study. J of Acquir Immune Defic Syndr 59(2):149–154
El Rihim AYA, Omar RF, Fathalah W, El Attar I, Hafez HA, Ibrahim W (2013) Role of fibroscan and APRI in detection of liver fibrosis: a systematic review and meta-analysis. Arab Journal of Gastroenterology 14(2):44–50
Filippi CG, Ulug AM, Ryan E et al (2001) Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. AJNR Am J Neuroradiol 22(2):277–283
Forton DM, Thomas HC, Taylor-Robinson SD (2004) Central nervous system involvement in hepatitis C virus infection. Metab Brain Dis 19(3–4):383–391
Gess, M., & Forton, D. (2012). Effect of hepatitis C virus on the central nervous system of HIV-infected individuals. Virus Adaptation & Treatment, 4
Gladsjo JA, Schuman CC, Evans JD et al (1999) Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment 6(2):147–178
SA Glantz, Primer of Biostatistics, 2005, ISBN = 978-0071435093
Gongvatana A, Cohen RA, Correia S et al (2011) Clinical contributors to cerebral white matter integrity in HIV-infected individuals. J of neurovirology 17(5):477–486
Heaton, R. K., Grant, I., & Matthews, C. G. (1991). Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic corrections, research findings, and clinical applications. Odessa, FL: Psychological Assessment Resources
Hilsabeck RC, Hassanein TI, Carlson MD et al (2003) Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J Int Neuropsychol Soc 9(6):847–854. doi:10.1017/S1355617703960048S1355617703960048
Hofer S, Frahm J (2006) Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32(3):989–994
Jernigan TL, Archibald SL, Fennema-Notestine C et al (2011) Clinical factors related to brain structure in HIV: the CHARTER study. J of Neurovirology 17:248–257. doi:10.1007/s13365-011-0032-7
Kallianpur KJ, Kirk GR, Sailasuta N et al (2012) Regional cortical thinning associated with detectable levels of HIV DNA. Cereb Cortex 22(9):2065–2075
Kellogg SH, McHugh PF, Bell K (2003) The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend 69(2):137–150
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46(10):1121–1123
Letendre SL, Cherner M, Ellis RJ (2005) The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS 19(Suppl 3):S72–78
Li R, Mi H, Zhao J, Yuan D, Ding J, Li H (2014) White matter damage and effects of nadir CD4+ count on patients with asymptomatic HIV associated dementia complex–a DTI study. Radiology of Infectious Diseases 1(1):11–16
Martin EM, Novak RM, Fendrich M et al (2004) Stroop performance in drug users classified by HIV and hepatitis C virus serostatus. J Int Neuropsychol Soc 10(2):298–300. doi:10.1017/S135561770410218XS135561770410218X
Masters, M. C., & Ances, B. M. (2014, February). Role of neuroimaging in HIV associated neurocognitive disorders (HAND). In Seminars in neurology (Vol. 34, No. 1, p. 89). NIH Public Access
McArthur J, Smith B (2013) Neurologic complications and considerations in HIV-infected persons. Current infectious disease reports 15(1):61–66
McAndrews MP, Farcnik K, Carlen P et al (2005) Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology 41(4):801–808. doi:10.1002/hep.20635
Murray J, Fishman SL, Ryan E et al (2008) Clinicopathologic correlates of hepatitis C virus in brain: a pilot study. J of neuroviro 14(1):17–27
Müller-Oehring EM, Schulte T, Rosenbloom MJ et al (2010) Callosal degradation in HIV-1 infection predicts hierarchical perception, a DTI study. Neuropsychologia 48(4):1133–1143
Niciu MJ, Mason GF (2014) Neuroimaging in alcohol and drug dependence. Current behavioral neuroscience reports 1(1):45–54
Norman MA, Moore DJ, Taylor M et al (2011) Demographically corrected norms for African Americans and Caucasians on the Hopkins verbal learning test–revised, brief visuospatial memory test–revised, Stroop color and word test, and Wisconsin card sorting test 64-card version. J Clin Exp Neuropsychol 33(7):793–804
Paul RH, Letendre S, Dearborn J (2007) Cognitive function in patients co-infected with hepatitis C and human immunodeficiency virus. Current Hepatitis Reports 6:145–152
Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV (2007) Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain 130(1):48–64
Perry W, Carlson MD, Barakat F (2005) Neuropsychological test performance in patients co-infected with hepatitis C virus and HIV. AIDS 19(Suppl 3):S79–84
Radloff LS (1977) The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement 1(3):385–401
Ragin AB, Storey P, Cohen BA et al (2004) Whole brain diffusion tensor imaging in HIV-associated cognitive impairment. AJNR Am J Neuroradiol 25(2):195–200
Ragin AB, Wu Y, Storey P et al (2005) Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol 11(3):292–298. doi:10.1080/13550280590953799
Rosas HD, Lee SY, Bender AC et al (2010) Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical “disconnection”. Neuroimage 49(4):2995–3004
Shaheen AA, Myers RP (2007) Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology 46(3):912–921. doi:10.1002/hep.21835
Shimony JS, Epstein AA, Kroenke CD et al (2006) Diffusion modeling using Bayesian probability theory applied to imaging of multiple sclerosis. In Proc Intl Soc Mag Reson Med 14:2114
Shimony JS, Sheline YI, D'Angelo G (2009) Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry 66:45–252
Smith CA, Stebbins GT, Bartt RE et al (2008) Gender effects on HIV-associated white matter alterations: a voxel-wise DTI study. Brain Imaging Behav 2(3):177–191
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17(3):1429–1436.
Spitzer RL, Williams JBW, Gibbon M, First MB (1987) Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute. Biometrics Research, 11
Stebbins GT, Smith A, Bartt A (2007) HIV-associated alterations in normal-appearing white matter: a voxel-wise diffusion tensor imaging study. J Acquir Immune Defic Syndr 46(5):564–573
Tate DF, Conley J, Paul RH et al (2010) Quantitative Diffusion tensor imaging tractography metrics are associated with cognitive performance among HIV-infected patients. Brain Imaging Behav 4(1):68–79
Thames, A. D. (2011). Past stimulant abuse is associated with reduced basal ganglia and hippocampal integrity in older HIV+ adults: a diffusion tensor imaging study. Journal of AIDS & Clinical Research
Thurnher MM, Castillo M, Stadler A (2005) Diffusion-tensor MR imaging of the brain in human immunodeficiency virus-positive patients. AJNR Am J Neuroradiol 26(9):2275–281
Togwood K, Pitkanen M, Kulasegaram R (2012) Mapping the brain in younger and older asymptomatic HIV-1 men: frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex 48:230–241. doi:10.1016/j.cortex.2011.03.006
Tural C, Tor J, Sanvisens A et al (2009) Accuracy of simple biochemical tests in identifying liver fibrosis in patients co-infected with human immunodeficiency virus and hepatitis C virus. Clin Gastroenterol Hepatol 7(3):339–345. doi:10.1016/j.cgh.2008.11.019
Vargas HE, Laskus T, Radkowski M et al (2002) Detection of hepatitis C virus sequences in brain tissue obtained in recurrent hepatitis C after liver transplantation. Liver Transpl 8(11):1014–1019
von Giesen HJ, Heintges T, Abbasi-Boroudjeni N et al (2004) Psychomotor slowing in hepatitis C and HIV infection. J Acquir Immune Defic Syndr 35(2):131–137
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I Conceptual framework and item selection Med Care 30(6):473–483
Wu Y, Storey P, Cohen BA et al (2006) Diffusion alterations in corpus callosum of patients with HIV. AJNR Am J Neuroradiol 27(3):656–660
Wright PW, Heaps JM, Shimony JS et al (2012) The effects of HIV and combination antiretroviral therapy on white matter integrity. AIDS 26(12):1501–1508
Wright PW, Vaida FF, Fernández RJ, Rutlin J, Price RW, Lee E, Peterson J, Fuchs D, Shimony JS, Robertson KR, Walter R, Meyerhoff DJ, Spudich S, Ances BM (2015). Cerebral white matter integrity during primary HIV infection. AIDS, 29(4), 433–442
Zhang XD, Zhang LJ, Wu SY, Lu GM (2014) Multimodality magnetic resonance imaging in hepatic encephalopathy: an update. World journal of gastroenterology: WJG 20(32):11262
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The WUSTL Human Research Protection Office approved this study. All participants provided informed consent for this study.
Conflict of interests
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 59 kb)
Rights and permissions
About this article
Cite this article
Heaps-Woodruff, J.M., Wright, P.W., Ances, B.M. et al. The impact of human immune deficiency virus and hepatitis C coinfection on white matter microstructural integrity. J. Neurovirol. 22, 389–399 (2016). https://doi.org/10.1007/s13365-015-0409-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-015-0409-0