Abstract
Gathering knowledge on the breeding ecology of species in wild-living conditions is critical to set baselines from which to analyse population trends and design appropriate conservation actions. This is particularly challenging when studying elusive animals like carnivores, as breeding events are difficult to detect and monitor. Based on direct sightings of wildcats, we provide the first scientific information on the breeding ecology in wild conditions of European wildcats as well as hunting success and provisioning rates of female wildcats. Mean litter size at weaning was two with most observations occurring between July and September. Auxiliary dens were mostly located inside thick vegetation in the proximities of pastoral fields, although anthropogenic constructions were occasionally used. Two cases of different female wildcats rearing their respective litters closer than 500 m were recorded. Hunting success of breeding females (66%) was higher than that of non-breeding females (33%) and males (40%). Breeding females provided around 80% of the captured prey to their kittens. In conclusion, direct observations of wild-living wildcats in the Cantabrian Mountains (NW Spain) allowed us to find that anthropogenic mosaic-structured landscapes combining open pastoral fields providing prey, and areas with thick vegetation such as shrub and forest patches providing shelter, encompass conditions required by the wildcats to successfully breed in human-dominated environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The breeding ecology of species can be defined by a set of behavioural parameters, such as den selection (Fernández and Palomares 2000; Sazatornil et al. 2016), prey provisioning rates (Nour et al. 1998) or spatial behaviour (Palomares et al. 2017), and fitness parameters, such as age at first and last reproduction (Krüger 2005; Wikenros et al. 2021), probability of reproduction, litter size, or juvenile survival (Sikes et al. 1998; López-Bao et al. 2010, 2019). Variation in such parameters can determine fitness, ultimately influencing species demography (Anthony and Blumstein 2000). Several environmental and human-related factors can constrain the outcomes of breeding events (Steidl and Anthony 2000; Sazatornil et al. 2016) by altering the availability of prey (Sherley et al. 2013) and suitable breeding spots (Shamoon and Shapira 2019). For instance, human activities can influence breeding processes negatively throughout enhanced mortality risk of offspring due to disturbances while rearing (Zuberogoitia et al. 2008) or positively throughout increased food availability (Šálek et al. 2015). In this context of increased human presence (Milner and Boldsen 2023), primary knowledge on the natural history of the species, including breeding ecology, is critical to set baselines from which to analyse population trends and design appropriate conservation actions (Fernández and Palomares 2000; Morales-González et al. 2022).
Despite the necessity of obtaining data on breeding parameters, research on breeding ecology in wild conditions is generally scarce, as breeding events are difficult to detect and monitor, particularly when studying elusive and nocturnal animals like carnivores (Swenson 1999; Theuerkauf 2009). Although research on captive carnivores can provide preliminary results on the breeding ecology of the species (Ruiz-Olmo et al. 2018), gathering information in wild-living individuals is crucial to obtain accurate results determined by individual and environmental factors, particularly in human-dominated scenarios.
The European wildcat (Felis silvestris) is a medium-sized carnivore that inhabits landscapes with different degrees of human presence across Europe (Gerngross et al. 2022). Although some of their populations recovered during the last decades, others show clear signs of decline (Senn et al. 2019; Gil-Sánchez et al. 2020). Due to its elusive behaviour, very little is known about wildcat breeding ecology, with the existing information obtained across Europe mainly based on captive or dead individuals (Daniels et al. 2002; Ruiz-Olmo et al. 2018) or published in grey literature (Stahl et al. 1992). In particular, information regarding behaviourally related reproductive parameters in wildcats, such as den selection and reutilization or food provisioning rates is mostly inexistent. Although several works have highlighted the importance of suitable breeding conditions for female wildcats, these conclusions are generally inferred from habitat selection approximations, instead of actual detection and monitoring of breeding dens (Monterroso et al. 2009; Oliveira et al. 2018).
Wildcats use open areas in the pastoral fields of the Cantabrian Mountains with hunting purposes, creating a good opportunity to observe their behaviour (Ruiz-Villar et al. 2021; Ruiz-Villar et al. 2022), which facilitates the detection and observation of breeding females and their offspring allowing for an approximation on describing reproductive parameters in a wild living population of this felid.
In this article, we aimed at providing the first scientific information on the breeding ecology of European wildcats in natural conditions. Specifically, we described the seasonality, number and size of kittens during weaning, den location and description of surrounding landscape, duration of den use, and frequency of their reuse. Furthermore, we also studied the hunting success and provisioning rates of female wildcats and compared it with non-breeding females and males.
Methods
Study area
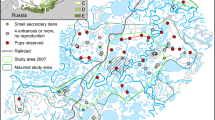
We observed European wildcats in the Western Cantabrian Mountains (NW Spain) inside an area of ca. 1800 km2 between the provinces of Asturias and León (Fig. 1), although additional information on wildcat reproduction from collaborators was gathered for the whole Cantabrian range (Fig. 1). The Cantabrian Mountains, which experience rough winters with considerable snow cover (Arenillas et al. 2008), belong to the temperate oceanic bioclimatic region with a few Mediterranean locations (Martínez and Arregui 1999) with altitudes ranging from 0 to 2650 m.a.s.l. The landscape is characterized by a mosaic of broadleaf forests (oak (Quercus sp.), beech (Fagus sylvatica), birch (Betula sp., etc.), broom and heather, and pasturelands. The valley bottoms are occupied by human settlements and fields derived from traditional farming activities (Fig. 1; García et al. 2005). In these mountains, wildcats predate upon ten species of rodents, with Arvicola monticola encompassing most rodent consumption by wildcats in pastoral fields (Ruiz-Villar et al. 2022).
Location of the wildcat reproductive dens recorded in the Cantabrian Mountains (NW Spain; top right). Orange circles show location of own sightings and pale yellow circles show locations of dens recorded by collaborators. The bottom map shows the main land use categories found in the Western Cantabrian Mountains (WCM), where we actively searched and monitored wildcat breeding events. To facilitate map interpretation land use categories were reclassified from the Third Spanish Forest Inventory (1997–2007) of the Spanish Ministry of Agriculture, Fisheries and Food (https://www.miteco.gob.es/es/biodiversidad/servicios/banco-datos-naturaleza/informacion-disponible/ifn3_bbdd_descargas.htm.aspx). ‘Anthropic’ refers to human settlements, highways and mines. ‘Fields’ refers to anthropogenic meadows and pasturelands (i.e. pastoral fields). ‘Shrub’ and ‘Forest’ encompass all the shrub and forest types, respectively. ‘Other’ includes water bodies and bare ground
Wildcat observations
Based on the regular use of open fields, meadows and pasturelands by European wildcats with predation purposes in the Cantabrian Mountains (Rodríguez et al. 2020; Ruiz-Villar et al. 2021; Ruiz-Villar et al. 2022) we aimed at observing wildcats, at sunrise and sunset when they are more active and visible in pastoral fields (Jiménez-Albarral et al. 2021), during the mid and late breeding periods (July to November; Ruiz-Olmo et al. 2018) from 2014 to 2022. To optimize our efforts, we did not look for wildcats during the early breeding season (April to June) as very young kittens remain in the birthing den and are consequently very difficult to detect when following wildcat females without using any specific tracking system (e.g. telemetry). Moreover visits to birthing dens can be a source of disturbance to the studied individuals. We did not run night observations as the use of spotlights can alter wildlife behaviour (Wilson 1999) and thermal viewers do not allow for reliable differentiation between wildcats and domestic cats. We detected wildcats through opportunistic surveys either along transects in cars on paved roads or from stationary viewing points. Once we detected the wildcat, we ran all observations from stationary viewing points using binoculars and a telescope (Swarovski Habitch 7×42 and Swarovski ATS 65HD + Zoom 20×60, Swarovski Optik KG, Austria) to observe wildcats from the distance (>200 m) without altering their behaviour, and recorded the individuals using a bridge camera Canon PowerShot SX60 HS (Canon Inc., Japan). Specifically, we were interested in observing wildcat breeding females but recorded all individuals seen across the mentioned period.
We identified wildcats based on diagnostic pelage characteristics presented by Ragni and Possenti (1996). The chances of misidentifying a wildcat with a hybrid in the Cantabrian Mountains are very low based on the lack of wildcat-domestic cat hybrids detected there by previous researchers (Tiesmeyer et al. 2020). Nevertheless, as morphology solely does not fully allow wildcat differentiation from hybrids (Devillard et al. 2014), and we did not analyse our focal animals genetically, we use the term wildcat or European wildcat referring to phenotypic European wildcats. We assigned each observation to a specific individual based on its unique pelage characteristics as presented by Jiménez-Albarral et al. (2021). Sex was determined when possible through the observation of the genital area. Observations of non-sexed individuals were discarded from the analysis.
Detection of breeding females and collection of breeding parameters
We determined the breeding status of females by observing wildcat females in the company of kittens. Accordingly, we considered as breeding females only those observed in the company of kittens. These observations stemmed from previous behaviours potentially indicating the presence of kittens in the surroundings. For instance, we confirmed the presence of kittens for all the cases, when undisturbed wildcat females repeatedly carried captured prey outside the field by leaving the meadow through the same area. Nonetheless, disturbed individuals could behave differently and leave the field with the captured prey for different reasons (e.g. to feed upon the captured prey hidden from potential threats (Ruiz-Villar et al. n.d.)). Our method allows gathering breeding-related information during the weaning period, when kittens are transitioning their diets from milk to solid prey (Gittleman 1986).
To count kittens and estimate their size, we waited until late dawn when they could roam outside the auxiliary den into the open pastoral field more likely. Most litters were observed in multiple occasions and different environments, which reduced the chances of underestimating the number of kittens. We assigned three age categories to the kittens based on the wildcat size and other external traits that allowed for approximate age estimation based on the body growth rate established in the domestic cats (DiGangi et al. 2020): (1) kittens 1/4 the size of the mother, with poor movement capabilities and very striped body sides; (2) kittens half the size of the mother with good movement capabilities and maintaining the striped pattern characteristic of young kittens (Fig. 2b); and (3) kittens 3/4 the size of the mother that have already developed the adult pelage lacking stripes on the sides (Fig. 2a and c).
Figures a to c show wildcat females with a varying number of kittens of different sizes: a Female with one kitten of category 3 (3/4’s of the mother); b female with 2 kittens of category 2 (1/2 of the mother); and c female with 3 kittens of category 3. As seen, kittens from category 2 present marked stripes on sides that are lost as they grow. Figure d shows the typical vegetation cover under riparian vegetation (Salix sp.) commonly used by European wildcats as maternal dens. Figure e shows a wildcat kitten in an abandoned hut used with breeding purposes by a female wildcat. Wildcat pictures were obtained by Héctor Ruiz-Villar from large distances without disturbing the individuals
We monitored den use by recording the minimum number of days spent using each den. To minimize disturbance, den description during breeding season was based on distant observations of the place used by the kittens, although dens were visited outside breeding season to determine if they were using enclosed structures inside vegetation and undetectable from the distance. We also recorded the type of den, i.e. birthing den: where the female gave birth (Fernández and Palomares 2000); or auxiliary den: where the female moved the kittens after leaving the birthing site (Fernández and Palomares 2000). As we did not use GPS tagging or radio-tracking of females to locate dens, most information obtained regarded auxiliary dens involved situations when the kittens were older, prey-fed and visible, although information on a few birthing dens was compiled as well. We included opportunistic observations of female wildcats moving the kittens (i.e. female kittens followed by already mobile kittens) despite they were not using any den to increase information of number of kittens with females. We only included observations, for which we were sure that we did not miss previous or later movement of kittens to minimize the risk of underestimating the number of kittens.
Finally, we collected information from collaborators regarding nine breeding events in relation to size and number of kittens, observation date, and litter location and type across the whole breeding period (May to November from 2009 to 2022).
Collection of hunting behaviour and provisioning rates
We recorded hunting behaviour of all observed wildcats and determined the hunting success per individual and observation (i.e. number of successful hunting attempts per observation divided by number of total hunting attempts per observation). We recognized hunting behaviours based upon the definition provided by Stanton et al. (2015), i.e. the cat actively pursues live prey including movements such as crouching, stalking, or any other species-specific behaviour. The main species captured by wildcats during our observations was Arvicola monticola (Ruiz-Villar et al. 2022), a large rodent species easy to identify from the distance due to its much larger size in comparison with the rest of rodent species. To determine the prey provisioning rates, we recorded the number of captured prey provided by the female to the kittens divided by the total amount of captured prey by the female and represented it as a percentage. Similarly we calculated the percentage of captured prey consumed by the mother as the number of consumed prey divided per the total number of captured prey multiplied by 100.
Statistical analysis
To compare the hunting success of breeding females, non-breeding females and males during the mid and late breeding period (July to November) we fitted a generalized additive mixed model (GAMM; ‘mgcv’ package in R statistical software (Wood 2015), with a logarithmic link and binomial distribution with hunting success as a response variable and wildcat status (i.e. breeding female, non-breeding female, and male) as an explanatory variable. To consider the effects associated to varying number and different durations of observation per individual we included such variables as smoothing term and offset respectively. To account for potential differences on hunting success associated to individuals and years (the latter potentially determined by different prey abundances), we included wildcat ID and year as random effects. We set statistical significance levels at p < 0.05.
Results
We obtained 40 observations of 10 breeding female wildcats (and their litters) using 19 different dens during the study period (Table 1). Additionally, we compiled information on the number of kittens and use of 10 dens by 8 female wildcats from collaborators (Table 1). Number of kittens varied from 1 (16%) to 3 (16%), with 2 being the most frequent number (68%) (Table 1, Fig. 3a). Most kittens observed were half (58%) or 3/4 (37%) the size of the mother (Table 1). Most observations of kittens occurred between July and September (Table 1). Observations in May correspond with very young kittens whereas observations in October and November correspond with late litters. Most auxiliary dens were located inside shrub (mainly broom (Cytisus sp., 28%)) and riparian vegetation (Salix sp., 38%; Figs. 2d and 3b), although wildcats occasionally used anthropogenic constructions like huts or stone walls with breeding purposes (10%, Table 1, Figs. 2e and 3b). We compiled information on two birthing dens, which were inside enclosed structures (hollow trunk and rock crevice, Table 1). The majority of monitored dens were located in the proximities of pastoral fields (97%, Table 1). Nonetheless, shrub patches and hedges were present in close proximity in all the cases females were using pastoral fields (Table 1). Duration of den use varied between 1 and 18 days, with the majority of dens being used during periods between 1 and 3 days (45 %, Table 1). Distance between observed dens used by the same female during the same breeding period varied between 35 and 507 m. Two cases of different female wildcats rearing their respective litters closer than 500 m from each other were recorded. Seventeen percent of the dens were reused either by the same female during the same breeding period or by the same or other female during consecutive breeding periods (Table 1). Length of monitoring periods varied between 1 and 27 days.
Frequency of (a) litters composed by 1, 2, and 3 kittens (n = 19 litters); and (b) different den locations (n = 29 dens) documented for breeding wildcat females using pastoral fields of the Cantabrian Mountains. Figure c represents the hunting success (number of successful hunting attempts divided by total number of attempts per observation) of breeding (n = 40 observations) and non-breeding (n = 12 observations) wildcat females, and males (n = 89 observations) during the mid and late breeding period (July to November). Only observations longer than 10 min were considered
We obtained 40, 12, and 89 observations of 10 breeding females, 6 non-breeding females, and 25 male individuals and recorded 189, 40, and 458 their hunting attempts respectively. Hunting success of breeding females (66%) was significantly higher than hunting success of non-breeding females (33%; p<0.001) and males (40%; p<0.05; Fig 3c). Regarding prey provisioning to kittens, females provided around 80% of the captured prey to the kittens and consumed the remaining 20%.
Discussion
We provided the first scientific data on the breeding ecology of wild-living European wildcats as well as the first information on hunting success and prey provisioning rates of breeding females (i.e. percentage of the captured prey that was brought to the kittens by their mother).
The litter size of wildcats at weaning found in our study in the Cantabrian Mountains (mean = 2) fell within the range obtained in captive animals, which spanned from one to six kittens at birth (usually 3–4) and halved after weaning due to kitten mortality (Ruiz-Olmo et al. 2018). However, we do not know whether litter size at birth in the wild differs from that recorded in captivity due to the difficulties of finding recently born kittens in untagged wildcat individuals. Furthermore, it is possible that mortality in the wild differs from that in captivity due to different sources of mortality recorded for both scenarios. For instance, wild kittens may be more vulnerable to interspecific predation or extreme weather conditions and captive individuals may be more susceptible to disease and infanticide, generally linked to the maintenance of dense and enclosed populations (Ruiz-Olmo et al. 2018).
Breeding seasonality was also similar to that recorded previously for the species based on other methods, which showed females to breed most litters between spring and summer (Stahl et al. 1992; Daniels et al. 2002; García 2006; Ruiz-Olmo et al. 2018). Nonetheless, most litters in captivity were born in April (Ruiz-Olmo et al. 2018), and considering the size and dates of most kittens observed in the Cantabrian Mountains, it is possible that most parturition in the wild occurs between May and June. This could be related with the climatic conditions of the Cantabrian Mountains as well as synchronization between parturition and peaks in prey availability. For instance, the snow cover can persist until late spring in medium to high elevations of the Cantabrian Mountains (Arenillas et al. 2008). Snow cover is a limiting factor for wildcat occurrence (Mermod and Liberek 2002), and one would expect that females synchronize their parturition with more adequate snowless conditions. In addition, wildcats using pastoral fields mostly feed upon montane water voles (Arvicola monticola), which are more abundant and accessible after the grass harvest in July (Ruiz-Villar et al. 2022). It is thus likely that kitten rearing and weaning overlaps with prey abundance and favourable weather conditions in order to increase kitten survival as seen in other felid species (Jansen and Jenks 2012). We recorded one case of a late litter in October. Late litters were recorded as replacement litters in captive wildcat females that lost their kittens or were in their first reproductive year (Ruiz-Olmo et al. 2018). Although literature says that kitten survival is higher in replacement litters in captivity (Ruiz-Olmo et al. 2018), this kind of litters in an environment with long and snowy winters like the Cantabrian Mountains are probably less likely to survive.
Auxiliary dens were mostly recorded inside thick vegetation like shrub (mainly broom (Cytisus sp.)) and willow (Salix sp.). This agrees with the literature on wildcats and other felids showing that females rely on availability of areas with dense vegetation for breeding purposes (Fernández and Palomares 2000; White et al. 2015; Oliveira et al. 2018). Although adult wildcats may not suffer many predation in our latitudes, vegetation cover may protect kittens which are more vulnerable to attacks from potential predators or encounters and disturbance from humans, as seen in the Eurasian lynx (Lynx lynx; Andrén et al. 2006). We detected the use of anthropogenic constructions by wildcats with breeding purposes, including an abandoned hut and two stone walls. Such constructions may resemble cave or rock deposits naturally used by medium felids for breeding (Boutros et al. 2007). This contrasts with literature recorded for other felid species, generally selecting inaccessible areas to reduce encounters with humans (White et al. 2015). High prey availability in pastoral fields (Ruiz-Villar et al. 2022) may ensure prey provisioning to kittens which could promote the use by females of the available breeding spots in the surrounding environment, including human constructions. The two recorded birthing dens were inside a cavity (hollow tree and rock crevice) with one entrance. This is common in other felid species breeding in European landscapes, and they may provide safety to the kittens during the first stages of life (Fernández and Palomares 2000; White et al. 2015). However, when kittens are large enough, females move them to auxiliary dens following the increasing mobility of kittens and their requirements of space (Fernández et al. 2002).
Pastoral fields were present around most dens, although there is a bias in this regard as we monitored open areas were females could be visible when hunting. Shrub and wooded linear structures such as riparian vegetation or hedges were present around most dens. As previously stated for the species, such structures increase landscape heterogeneity and are crucial for wildcat survival in open and fragmented landscapes like agricultural central European areas (Jerosch et al. 2018; Ruiz-Villar et al. 2023). As a predator selecting for mosaic-structured environments, wildcats need simultaneous availability of open areas with prey abundance to hunt (such as pastoral fields) and vegetation cover (such as broom, willow, and linear wooded structures; Lozano 2010; Ruiz-Villar et al. 2023) to rest, breed, or hide from potential threats. In this regard, the Cantabrian scenario of pastoral fields embedded in a matrix of large surfaces of shrub and forest interconnected by linear vegetation structures can encompass the requirements demanded by wildcats.
The use of auxiliary dens was generally short with most dens used between 1 and 3 days. As recorded for other felid species, females can move auxiliary dens regularly as a direct consequence of disturbance or to reduce probabilities of both detection by predators and parasite infestation of kittens (Fernández et al. 2002). It is also possible that breeding females move dens to avoid overexploitation of prey in a given point as they may use different hunting grounds around each den. The duration of den use seems generally shorter than that detected in the Iberian lynx (Fernández et al. 2002), although in some cases it was considerably long (up to 18 days). It is possible that the use of anthropogenic landscapes were sources of disturbance are widespread may push female wildcats to move their kittens more regularly, as seen in other carnivores (Thiel et al. 1998). Besides this, several dens were reused either during the same period or in successive breeding periods, which may point to a limited availability of suitable breeding spots in human-modified landscapes.
Wildcat females are highly territorial, very rarely presenting overlapping territories that are defended from neighbouring females (Biró et al. 2004; Beugin et al. 2016). In addition, female wildcats were shown to use home ranges of 7.74 km2 in the study area (Ruiz-Villar et al. 2023), which makes it surprising that we detected females breeding their respective litters closer than 500 m from each other in two separate areas and occasions. Pastoral fields provide abundance of prey during pup rearing periods (Ruiz-Villar et al. 2022), and as a consequence, it is possible that wildcat females relax their territoriality under such circumstances, as it was described in other territorial vertebrates (Maher and Lott 2000).
We found that the hunting success of breeding females was significantly higher than that of non-breeding females and males. Breeding females become highly effective at capturing prey during the weaning period. Hunting success in vertebrates has been previously shown to increase during the breeding period, as for instance, hunting success was higher for red-backed shrikes (Lanius colliuro) during the nestling feeding period (Morelli et al. 2016). In this case, such increase was explained by a simultaneous increase in prey availability. In our study, however, the prey availability was presumed to be the same for males, breeding and non-breeding-females during the same monitoring period. It is possible that wildcat females selected those micro-scale areas with higher prey availability during breeding periods thus increasing their hunting success. From the prey captured, females fed 79% to the kittens and ate the rest. This draws attention to the energetic costs for female individuals during the pup rearing process, which double the basic energetic requirements (Natural Research Council 2006), making the selection of suitable sites a determining factor for a successful reproductive outcome.
In conclusion, our study showed that pastoral fields embedded in a matrix of vegetation cover can provide both prey abundance to support the energetic requirements as well as the refuges for successful breeding by wildcats (Ruiz-Villar et al. 2022). Nevertheless, human-modified landscapes present multiple sources of risk and mortality that may compromise kitten survival. For instance, kittens may be vulnerable to road mortality while moving between dens or after independence (Bastianelli et al. 2021). In addition, they can suffer the consequences of frequent disturbance by humans (Barja et al. 2012). Direct non-intrusive observations of wild-living wildcats proofed efficient to acquire representative and unique data on the reproductive parameters of wildcats using human-modified landscapes in the Cantabrian Mountains (NW Spain). Future research should aim at studying differences in breeding ecology and success in wildcats using landscapes with different degrees of human presence to investigate to which extent and in which direction (positive or negative) humans are influencing the breeding ecology of wildcats.
References
Andrén H, Linnell JDC, Liberg O et al (2006) Survival rates and causes of mortality in Eurasian lynx (Lynx lynx) in multi-use landscapes. Biol Conserv 131:23–32. https://doi.org/10.1016/j.biocon.2006.01.025
Anthony LL, Blumstein DT (2000) Integrating behaviour into wildlife conservation: the multiple ways that behaviour can reduce N(e). Biol Conserv 95:303–315. https://doi.org/10.1016/S0006-3207(00)00037-9
Arenillas M, Cobos G, Navarro J (2008) Datos sobre la nieve y los glaciares en las cordilleras españolas. El programa ERHIN (1984–2008), Madrid
Barja I, Silvan G, Illera JC (2012) Effects of tourist pressure and reproduction on physiological stress response in wildcats: management implications for species conservation. Wildl Res 39:532–539. https://doi.org/10.1071/WR10218
Bastianelli ML, Premier J, Herrmann M et al (2021) Survival and cause-specific mortality of European wildcat (Felis silvestris) across Europe. Biol Conserv 261:109239. https://doi.org/10.1016/j.biocon.2021.109239
Beugin MP, Leblanc G, Queney G et al (2016) Female in the inside, male in the outside: insights into the spatial organization of a European wildcat population. Conserv Genet 17:1405–1415. https://doi.org/10.1007/s10592-016-0871-0
Biró Z, Szemethy L, Heltai M (2004) Home range sizes of wildcats (Felis silvestris) and feral domestic cats (Felis silvestris f. catus) in a hilly region of Hungary. Mamm Biol 69:302–310. https://doi.org/10.1078/1616-5047-00149
Boutros D, Breitenmoser-Würsten C, Zimmermann F, et al. (2007) Characterisation of Eurasian lynx Lynx lynx den sites and kitten survival. Wildlife Biol 13:417–429. https://doi.org/10.2981/0909-6396(2007)13[417:COELLL]2.0.CO;2
Daniels MJ, Wright TCM, Bland KP, Kitchener AC (2002) Seasonality and reproduction in wild-living cats in Scotland. Acta Theriol (Warsz) 47:73–84. https://doi.org/10.1007/BF03193568
Devillard S, Jombart T, Léger F et al (2014) How reliable are morphological and anatomical characters to distinguish European wildcats, domestic cats and their hybrids in France? J Zool Syst Evol Res 52:154–162. https://doi.org/10.1111/jzs.12049
DiGangi BA, Graves J, Budke CM et al (2020) Assessment of body weight for age determination in kittens. J Feline Med Surg 22:322–328. https://doi.org/10.1177/1098612X19844846
Fernández N, Palomares F (2000) The selection of breeding dens by the endangered Iberian lynx (Lynx pardinus): implications for its conservation. Biol Conserv 94:51–61. https://doi.org/10.1016/S0006-3207(99)00164-0
Fernández N, Palomares F, Delibes M (2002) The use of breeding dens and kitten development in the Iberian lynx (Lynx pardinus). J Zool 258:1–5. https://doi.org/10.1017/S0952836902001140
García D, Quevedo M, Obeso JR, Abajo A (2005) Fragmentation patterns and protection of montane forest in the Cantabrian range (NW Spain). For Ecol Manage 208:29–43. https://doi.org/10.1016/j.foreco.2004.10.071
García FJ (2006) El gato montés Felis silvestris Schreber, 1775. Galemys 16:1–14
Gerngross P, Ambarli H, Angelici FM, et al. (2022) Felis silvestris
Gil-Sánchez JM, Barea-Azcón JM, Jaramillo J et al (2020) Fragmentation and low density as major conservation challenges for the southernmost populations of the European wildcat. PLoS One 15:e0227708. https://doi.org/10.1371/journal.pone.0227708
Gittleman JL (1986) Carnivore life history patterns: allometric, phylogenetic, and ecological associations. Am Nat 127:744–771. https://doi.org/10.1086/284523
Jansen BD, Jenks JA (2012) Birth timing for mountain lions (Puma concolor); testing the prey availability hypothesis. PLoS One 7:e44625. https://doi.org/10.1371/journal.pone.0044625
Jerosch S, Kramer-Schadt S, Götz M, Roth M (2018) The importance of small-scale structures in an agriculturally dominated landscape for the European wildcat (Felis silvestris silvestris) in central Europe and implications for its conservation. J Nat Conserv 41:88–96. https://doi.org/10.1016/j.jnc.2017.11.008
Jiménez-Albarral JJ, Urra F, Jubete F et al (2021) Abundance and use pattern of wildcats of ancient human-modified cattle pastures in northern Iberian Peninsula. Eur J Wildl Res 67:1–11. https://doi.org/10.1007/s10344-021-01533-y
Krüger O (2005) Age at first breeding and fitness in goshawk Accipiter gentilis. J Anim Ecol 74:266–273. https://doi.org/10.1111/j.1365-2656.2005.00920.x
López-Bao JV, Aronsson M, Linnell JDC et al (2019) Eurasian lynx fitness shows little variation across Scandinavian human-dominated landscapes. Sci Rep 9:8903. https://doi.org/10.1038/s41598-019-45569-2
López-Bao JV, Palomares F, Rodríguez A, Delibes M (2010) Effects of food supplementation on home-range size, reproductive success, productivity and recruitment in a small population of Iberian lynx. Anim Conserv 13:35–42. https://doi.org/10.1111/j.1469-1795.2009.00300.x
Lozano J (2010) Habitat use by European wildcats (Felis silvestris) in central Spain: what is the relative importance of forest variables? Anim Biodivers Conserv 33:2–5
Maher CR, Lott DF (2000) A review of ecological determinants of territoriality within vertebrate species. Am Midl Nat 143:1–29. https://doi.org/10.1674/0003-0031(2000)143[0001:AROEDO]2.0.CO;2
Martínez S, Arregui J (1999) Bioclimatology of the Iberian Peninsula. Itinera Geobot 13:41–47
Mermod CP, Liberek M (2002) The role of snowcover for European wildcat in Switzerland. Z Jagdwiss 48:17–24
Milner GR, Boldsen JL (2023) Population trends and the transition to agriculture: Global processes as seen from North America. Proc Natl Acad Sci U S A 120:e2209478119. https://doi.org/10.1073/pnas.2209478119
Monterroso P, Brito JC, Ferreras P, Alves PC (2009) Spatial ecology of the European wildcat in a Mediterranean ecosystem: dealing with small radio-tracking datasets in species conservation. J Zool 279:27–35. https://doi.org/10.1111/j.1469-7998.2009.00585.x
Morales-González A, Fernández-Gil A, Quevedo M, Revilla E (2022) Patterns and determinants of dispersal in grey wolves (Canis lupus). Biol Rev 97:466–480. https://doi.org/10.1111/brv.12807
Morelli F, Mróz E, Pruscini F et al (2016) Habitat structure, breeding stage and sex affect hunting success of breeding Red-backed Shrike (Lanius collurio). Ethol Ecol Evol 28:136–147. https://doi.org/10.1080/03949370.2015.1022907
Natural Research Council (2006) Nutrient requirements of dogs and cats. National Academies Press, Washington DC, USA
Nour N, Currie D, Matthysen E et al (1998) Effects of habitat fragmentation on provisioning rates, diet and breeding success in two species of tit (great tit and blue tit). Oecologia 114:522–530. https://doi.org/10.1007/s004420050476
Oliveira T, Urra F, López-Martín JM et al (2018) Females know better: sex-biased habitat selection by the European wildcat. Ecol Evol 8:9464–9477. https://doi.org/10.1002/ece3.4442
Palomares F, Lucena-Pérez M, López-Bao JV, Godoy JA (2017) Territoriality ensures paternity in a solitary carnivore mammal. Sci Rep 7:1–6. https://doi.org/10.1038/s41598-017-04820-4
Ragni B, Possenti M (1996) Variability of coat-colour and markings system in felis silvestris. Ital J Zool 63:285–292. https://doi.org/10.1080/11250009609356146
Rodríguez A, Urra F, Jubete F et al (2020) Spatial segregation between red foxes ( Vulpes vulpes ), European Wildcats ( Felis silvestris ) and domestic cats ( Felis catus ) in pastures in a livestock area of Northern Spain. Diversity 12. https://doi.org/10.3390/d12070268
Ruiz-Olmo J, Pinyol C, Sánchez D, Such-Sánz Á (2018) Breeding pattern of wildcat Felis silvestris (Schreber, 1777) studied in captivity in the iberian peninsula. Hystrix Ital J Mammal 29:202–210. https://doi.org/10.4404/hystrix
Ruiz-Villar H, Bastianelli ML, Heurich M et al (2023) Agriculture intensity and landscape configuration influence the spatial use of wildcats across Europe. Biol Conserv 277:109854. https://doi.org/10.1016/j.biocon.2022.109854
Ruiz-Villar H, Jubete F, Revilla E et al (2021) Like cat and fox: diurnal interactions between two sympatric carnivores in pastoral landscapes of NW Spain. Eur J Wildl Res 67:1–6. https://doi.org/10.1007/s10344-021-01469-3
Ruiz-Villar H, Morales-González A, López-Bao JV, Palomares F Humans and traffic influence European wildcat behaviour in pastoral landscapes. Under Rev
Ruiz-Villar H, Urra F, Jubete F et al (2022) Presence of pastoral fields in mountain landscapes influences prey consumption by European wildcats. J Zool 319:63–75. https://doi.org/10.1111/jzo.13027
Šálek M, Drahníková L, Tkadlec E (2015) Changes in home range sizes and population densities of carnivore species along the natural to urban habitat gradient. Mamm Rev 45:1–14. https://doi.org/10.1111/mam.12027
Sazatornil V, Rodríguez A, Klaczek M et al (2016) The role of human-related risk in breeding site selection by wolves. Biol Conserv 201:103–110
Senn HV, Ghazali M, Kaden J et al (2019) Distinguishing the victim from the threat: SNP-based methods reveal the extent of introgressive hybridization between wildcats and domestic cats in Scotland and inform future in situ and ex situ management options for species restoration. Evol Appl 12:399–414. https://doi.org/10.1111/eva.12720
Shamoon H, Shapira I (2019) Limiting factors of striped hyaena, Hyaena hyaena, distribution and densities across climatic and geographical gradients (Mammalia: Carnivora). Zool Middle East 65:189–200. https://doi.org/10.1080/09397140.2019.1596589
Sherley R, Underhill L, Barham B et al (2013) Influence of local and regional prey availability on breeding performance of African penguins Spheniscus demersus. Mar Ecol Prog Ser 473:291–301. https://doi.org/10.3354/meps10070
Sikes RS, Ylönen H, Ylonen H (1998) Considerations of optimal litter size in mammals. Oikos 83:452. https://doi.org/10.2307/3546673
Stahl P, Leger F, Société française pour l’étude et la protection des mammifères (1992) Le Chat sauvage (Felis silvestris Schreber, 1777). Société franc̜aise pour l’étude et la protection des mammifères
Stanton LA, Sullivan MS, Fazio JM (2015) A standardized ethogram for the felidae: a tool for behavioral researchers. Appl Anim Behav Sci 173:3–16. https://doi.org/10.1016/j.applanim.2015.04.001
Steidl RJ, Anthony RG (2000) Experimental effects of human activity on breeding bald eagles. Ecol Appl 10:258–268. https://doi.org/10.1890/1051-0761(2000)010[0258:eeohab]2.0.co;2
Swenson JE (1999) Does hunting affect the behavior of brown bears in Eurasia? Ursus 11:157–162
Theuerkauf J (2009) What drives wolves: fear or hunger? Humans, diet, climate and wolf activity patterns. Ethology 115:649–657. https://doi.org/10.1111/j.1439-0310.2009.01653.x
Thiel RP, Merrill S, Mech DL (1998) Tolerance by denning wolves, Canis lupus, to human disturbance. Can Field-Naturalist 122:340–342
Tiesmeyer A, Ramos L, Manuel Lucas J et al (2020) Range-wide patterns of human-mediated hybridisation in European wildcats. Conserv Genet 21:247–260. https://doi.org/10.1007/s10592-019-01247-4
White S, Briers RA, Bouyer Y et al (2015) Eurasian lynx natal den site and maternal home-range selection in multi-use landscapes of Norway. J Zool 297:87–98. https://doi.org/10.1111/jzo.12260
Wikenros C, Gicquel M, Zimmermann B et al (2021) Age at first reproduction in wolves: different patterns of density dependence for females and males. Proc R Soc B Biol Sci 288. https://doi.org/10.1098/rspb.2021.0207
Wilson R (1999) Possums in the spotlight. Nat Aust 26:34–41
Wood S (2015) Package “mgcv.”. R Packag Version 29:729
Zuberogoitia I, Zabala J, Martínez JA et al (2008) Effect of human activities on Egyptian vulture breeding success. Anim Conserv 11:313–320. https://doi.org/10.1111/j.1469-1795.2008.00184.x
Acknowledgements
We thank Bernardino López, Alberto Pola, Jonathan Rodriguez, and Roberto Rodríguez for their collaboration by providing breeding data on European wildcats across the Cantabrian Mountains. HRV is beneficiary of a PhD scholarship “Severo Ochoa” from the Regional Government of Principality of Asturias.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by: Krzysztof Schmidt
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruiz-Villar, H., López-Bao, J.V. & Palomares, F. Insights into the breeding ecology of wild-living European wildcats in the Cantabrian Mountains, Spain. Mamm Res 68, 495–505 (2023). https://doi.org/10.1007/s13364-023-00708-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-023-00708-z