Abstract
The Neotropical region hosts one of the highest levels of small non-volant mammal species diversity worldwide, but sampling therein is often intractable due to high logistic and labour costs. While most common sampling methods include live trapping (LT) and pitfall trapping (PT), camera trapping (CT) is potentially a useful technique. Studies assessing data acquisition efficiency for neotropical small mammals are mostly limited to LT and PT, and no small mammal study to date included CT. We provide a comparative assessment of the efficiency of LT (Sherman and wire-mesh traps), PT and CT in surveying small mammal species across 25 sites in an Amazonian archipelagic landscape. Based on 26,184 trap nights, we obtained 782 small mammal records representing at least 18 species. Most species were detected by both LT (72.2%) and PT (83.3%), but each of these methods exclusively recorded additional species, whereas CT detected only nearly one-fourth (N = 4) of all species recorded. Nevertheless, for nearly all species detected by CT, the probability of detecting individual species was similar or higher than that of LT. Species detected by CT represented the largest-bodied rodents and marsupials (> 200 g). Pitfall traps are an important complement to LT, and CT comprises an efficient technique to sample large-bodied small mammals. Improvements in the efficiency of camera traps in recording and identifying small-bodied species are both needed and possible, but we recommend the combination of LT and PT methods to enhance the completeness of community-wide small mammal sampling in neotropical forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Identifying cost-effective methods for biological surveys, including innovative and automated approaches whenever applicable, is of great importance to assess threats and monitor changes in forest landscapes (Larsen 2016). In the Neotropics, small non-volant mammals represent over half of the mammalian species diversity, at least at a local scale, yet comprise one of the taxa associated with the highest survey costs (Gardner et al. 2008). This broad taxonomic group comprises a variety of species and lineages of marsupials and rodents that occupy the entire vertical forest strata (Vieira and Monteiro-Filho 2003; Hannibal and Caceres 2010), feed on a wide spectrum of resources, act as key seed predators and seed dispersers (Mangan and Adler 2000; Terborgh et al. 2001), and comprise vital prey for a wide range of carnivores (Emmons and Feer 1997). Therefore, small mammals play important roles in ecosystem functioning, and information on their species diversity can elucidate broad ecological processes, such as forest regeneration (Terborgh et al. 2001).
In tropical forests, small mammals are typically sampled using metal live traps, hereafter LTs (i.e. most frequently Sherman and Tomahawk/wire-mesh traps), placed at different heights of the forest vertical strata (Vieira and Monteiro-Filho 2003; Hannibal and Caceres 2010). LTs are baited and all traps need to be checked at least once each day. More recently, studies started to include unbaited pitfall traps (PTs), preferentially of 100 L (Ribeiro-Júnior et al. 2011), for sampling small mammals. In comparison to LTs, pitfalls are less affected by factors such as food availability (Adler and Lambert 1997), species preferences for different baits (Laurance 1992), bait consumption by other animals (McClearn et al. 1994), and the tendency to capture only adult individuals (Boonstra and Krebs 1978). Indeed, PTs in many studies record an overall higher small mammal species diversity compared to LTs (Bovendorp et al. 2017). Yet different species may be differentially captured by either one of these methods (Santos-Filho et al. 2015; Ardente et al. 2017).
Additionally, the use of large numbers of LTs or PTs requires carrying heavy loads into the field, and sufficient manpower to dig many holes for 100 L buckets. The logistics involved limit sampling of remote field sites, the size of the sampling area at each site, and restrict the duration of sampling (i.e. number of days in each trapping session; Castleberry et al. 2014; McCleery et al. 2014). LTs and PTs also require long periods of time to complete the field work, and many experienced people involved in data collection. Moreover, exceptionally low trapping success is widespread in the Neotropics, even when placing LTs at different heights of the vertical forest strata (e.g. 5%: Woodman et al. 1996; 1%: Palmeirim et al. 2018). This renders small mammal sampling a gruelling and costly mission that often discourages field investigators. For those reasons, small mammal studies are severely limited, and often excluded from rapid biodiversity assessments. Therefore, in addition to the need for a better understanding of the self-sufficiency of PTs in relation to LTs, particularly in terms of detectability of individual species, there is a major critical need to develop simpler alternative techniques to sample small mammal assemblages, such as camera trapping.
Camera trapping comprises an excellent tool for assessing terrestrial vertebrate communities, having the great advantages of reduced field work time, 24 h per day of operation, placement in even inaccessible areas, and enabling species identification with certainty (Silveira et al. 2003). Although this technique has been vastly used to survey large terrestrial mammals worldwide (O’Connell et al. 2010; Ahumada et al. 2011), some studies have shown the potential of camera trapping for surveying small mammals in temperate forest landscapes (Larrucea and Brussard 2008; De Bondi et al. 2010). So far, camera traps (CTs) have been used in tropical forests to assess activity patterns of small mammals (Oliveira-Santos et al. 2008; Norris et al. 2010). Although not all small mammal species in tropical forests can be readily identified using external characters, complementary data provided by CTs may be potentially useful to increase the sample completeness of small mammal assemblages, depending on study objectives (e.g., those focusing on functional diversity or on a particular set of species). However, the efficiency of CTs has yet to be compared to the most commonly used LTs to sample small mammals.
In this context, to establish simpler and more efficient methods to survey small mammal assemblages, we compare the efficiency of live, pitfall and camera traps placed near the ground within islands and continuous mainland forest sites of a neotropical fragmented landscape. We also evaluate the relative efficiency of these methods in terms of species body mass and locomotion mode (terrestrial, scansorial, arboreal species). To do so, we compared the number of small mammal species recorded, as well as the detection rate of each species, their body mass and locomotion mode, using each of these three trap types—LTs, PTs and CTs.
Methods
Study area
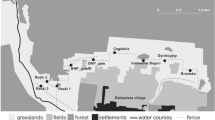
We carried out this study at 23 tropical land-bridge islands (ranging from 0.83 to 1466 ha) in addition to two mainland continuous forest sites within the vast archipelagic landscape of the Balbina Hydroelectric Reservoir, located in the Central Brazilian Amazon (1° 48′ S, 59° 29′ W; Fig. 1). This 28-year-old reservoir was flooded following the damming of the Uatumã River, a left bank tributary to Amazon River. The Balbina Dam created 3546 islands, corresponding to the former elevated ridges of the once continuous forest, within a 443772-ha reservoir (FUNCATE/INPE/ANEEL 2000). The neighbouring mainland continuous forest and most islands consist of dense closed-canopy terra firme forest. The local mean annual temperature is 28 °C and the mean annual rainfall is ~ 2376 mm (IBAMA 1997). The area within and around the former left bank of the Uatumã River has been legally protected since 1990 by the 942786-ha Uatumã Biological Reserve, the largest reserve in its category in Brazil. This contributes to low levels of post-damming human disturbance across the reservoir.
Location of the study area and survey sites within and around the Balbina Hydroelectric Reservoir in Central Brazilian Amazonia: 23 land-bridge islands (red coloured and highlighted by a 1 km-buffer) and two continuous forest sites (indicated by red rectangles). The inset figure illustrates the sampling design of each trapping plot to survey small mammals: (a) camera trapping, (b) pitfall trapping and (c) live trapping. Distances between the same types of traps are indicated in black, and distances between different trap types are grey coloured
Small mammal sampling
We collected data during two different time periods within 43 sampling plots, distributed across 23 islands and two mainland continuous forest sites differing in terms of spatial configuration such as island size (Table S1), which were spaced by at least 1 km from one another (Fig. 1). In 2011–2012, we deployed two CT stations at each plot using two Reconyx Hyperfire digital cameras (model HC500 HyperFire Semi-Covert IR; Holmen, WI, USA). All CTs were deployed at 30 cm above ground along transects, without the use of baits, aiming to survey all medium and large-sized terrestrial vertebrates (see further details in Benchimol and Peres 2015). We configured each CT to obtain a sequence of five photographs for each animal or animal cluster recorded, using 15-s intervals between records. When the same species was recorded consecutively, we defined independent detections as those records spread by at least 60-min intervals.
In 2014–2015, we established live and pitfall trapping plots placed in the same sampling plots as CTs. Live and pitfall trapping plots consisted of a set of nine single-catch LTs placed on the forest ground, followed by a set of three PTs. At each trapping plot, two types of LTs were alternatively placed 20 m apart—Sherman (23 × 9 × 8 cm, H. B. Sherman Traps, Inc., Tallahassee, Florida) and wire-mesh traps (30 × 17.5 × 15 cm, Metal Miranda, Curitiba, Paraná). Therefore, each plot comprised five Sherman and four wire-mesh traps (Fig. 1). A mix of bananas, peanut powder, sardines and oak flocks was used to bait all LTs. Unbaited pitfall traps (100 L, 68 × 57 cm) were also spaced at 20-m intervals and connected by a plastic fence of 50-cm height and 10-cm underground, with 10 m of overhanging fence farther extended after the two external pitfalls. Floating platforms were left within each pitfall trap in case of sudden flooding due to heavy rain. All traps were inspected daily in the early morning, to minimise the period of time spent by small mammals within traps. Although a variety of species can enter pitfall traps, potential diurnal predators of small mammals such as many raptor species are not able to enter the buckets; therefore, this does not represent a major issue for small mammals. Non-target animals captured on pitfalls, including lizards, leaf-litter amphibians, small snakes and species from many different groups of arthropods (e.g. ants and beetles), were also removed each morning. Whenever live captures could not be identified in the field, voucher specimens were collected, but a maximum of five voucher specimens per species per site was collected during the first season. Voucher specimens were euthanized in the field using anaesthetics (Comissão de Ética, BioÉtica e Bem-Estar Animal/CFMV 2012; American Veterinary Medical Association 2013) and preserved in formyl, and subsequently deposited at the Mammal Collection of the Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Brazil. All other individuals recorded were weighted and tagged (fish and small animal tag, size 1; National Band and Tag Co., Newport, Kentucky), so that any subsequent recaptures could be distinguished. Each individual was carefully handled at the same site where it had been captured, released within a few minutes after being transferred from the trap into an appropriate bag and inspected, without the need of sedation. We classified individuals according to two age classes—juvenile and adult. This classification was based on the individual reproductive status, for rodents, and on the tooth-eruption sequence, for marsupials (Macedo et al. 2006). We were not always able to identify at the species-level records of Proechimys spp. (P. cuvieri and P. guyanensis) and Oecomys spp. 1 (O. roberti and O. bicolor) at all sites. As these congeners are ecologically very similar (Jones et al. 2009), we further refer to those as ecospecies. To streamline, we refer to both species and ecospecies as species. This research followed ASM guidelines (Sikes 2016) and was approved by the appropriate institutional animal care and use committee in Brazil (SISBIO License No. 39187-4).
The number of transects placed at different sites varied according to their area. This allowed us to obtain a higher number of individuals at larger forest sites, where overall trap density, and consequently the probability of an individual passing near a trap, was lower (Table S1). Due to spatial restrictions in small islands, alternative smaller transects were established therein. Thus, all islands smaller than 2 ha and those between 2 and 10 ha were sampled by transects containing only three LTs followed by an array of one pitfall, and by six LTs followed by an array of two pitfalls, respectively. Larger islands were sampled by as many as four transects, according to their size classes: 10–50, 50–200, 200–500, and > 500 ha, respectively; CF sites were sampled by six transects. Despite the positive effect of island area on small mammal species richness at Balbina (Palmeirim et al. 2018), in this study, we compare the efficiency of different trapping methods within the same site, so we do not consider that such area effect on species richness affects our results. CTs were deployed over two periods of 30 consecutive days each, totalling 6600 trap nights. LT and PT trapping plots were operated over periods of 16 consecutive days during each trapping session, totalling 14688 and 4896 trap nights, respectively.
Data analysis
Both LTs types—Sherman and wire-mesh traps—were similar in terms of number of records and species richness, hence we did not distinguish between different types of LTs in subsequent analyses (Table S2). Rarefaction curves were used to evaluate sampling efficiency by each trapping method at each sampling site, using the R package vegan (Oksanen et al. 2007). Asymptote curves were reached for most sites using LTs and CTs, yet that was not the case of PTs (Fig. S1). Despite the lower number of PT-nights in comparison to that of LTs, PTs still performed as efficiently as LTs in detecting as many small mammal species per site. Therefore, we decided to use PT data, albeit interpreted with caution.
We compared the total number of species recorded by each method, considering species body size (g) and locomotion mode (i.e. terrestrial, scansorial and arboreal species) (Table S2). We considered the body mass of each species as measured for specimens collected during field work. Also, because PTs are considered to capture a higher proportion of juvenile individuals (Umetsu et al. 2006), we compared the proportion of juvenile individuals recorded using LT and PT for each species. The overall efficiency of each trapping method was evaluated by the number of species detected at each sampling plot by CTs and PTs compared to traditional live trapping, using paired t tests (Zar 1999). We further analysed the performance of each method to detect individual species by bootstrapping the estimates of species detection, considering only species detected by at least two trapping methods within the same sampling site. For each species and method, the same number of sites where the species had been detected was randomly drawn from the 25 sampled sites, obtaining an estimate of detection rate. This procedure was repeated 1000 times with replacement, resulting in a mean (± SE) detection rate for each species and method, using the boot R package (Ripley and Canty 2017). All data analyses were performed in R (R Development Core Team 2015).
Results
Considering all three sampling methods, a total of 18 small mammal species (10 rodents and 8 marsupials) was recorded across 43 sampling plots nested within our 25 forest sites. Trapping success ranged between 2.8% and 3.6% (4.8% including unidentified records) for LTs and CTs, respectively (Table 1). The total number of species (S) recorded by each method was higher for PTs (S = 15), followed by LTs (S = 13) and CTs (S = 4; Table 1). In addition to the 238 CT records identified to species or genus level, it was not possible to identify 80 additional records due to poor photo quality. Some of these unidentified records matched a cricetid rodent (N = 2 records) and an arboreal marsupial (N = 1). However, unidentified records were not included in further comparative analyses between methods.
LTs and PTs recorded both smaller- (< 200 g) and larger-bodied species (> 200 g), while CTs recorded only larger-bodied small mammals (Table 1). In fact, the rodent Echimys chrysurus, weighing ~ 430 g (Table S2), recorded only once throughout the sampling using LTs, was the only small mammal larger than 200 g that was not recorded by CTs. Although LTs and PTs were placed on the forest floor, they captured not only terrestrial and scansorial species, but also arboreal ones. CTs instead recorded terrestrial and scansorial species, but not arboreal species (Table 1). Moreover, LTs exclusively recorded two species, the terrestrial cricetidae Euryoryzomys macconnelli and the arboreal echimyidae E. chrysurus. PTs exclusively allowed the capture of four species, including two marsupials, the terrestrial Monodelphis arlindoi and the scansorial Marmosops pinheiroi, and two arboreal rodents (i.e., Isothrix pagurus, and Rhipidomys mastacalis). The proportion of juvenile individuals recorded using PTs (mean ± SD: 48.2 ± 36.4%) was on average similar to that recorded using LTs (51.8 ± 36.4%). Yet for three species—Marmosops parvidens, Metachirus nudicaudatus, Oecomys spp. 1—pitfall trapping was the most efficient technique to record juvenile individuals (Table 2).
When comparing the number of species recorded per sampled site by each of the sampling methods, LTs (3.1 ± 2.3 species) performed similarly to PTs (2.6 ± 2.3; t = 1.553, d.f. = 24, P = 0.134), but better than CTs (1.8 ± 1.3; t = 4.151, d.f. = 24, P < 0.001). For some species, detections were recorded by more than one method, and in a large proportion of sites (Fig. 2). This was the case of Philander opossum, Hylaeamys megacephalus, and M. nudicaudatus, which were simultaneously detected by both LTs and PTs in ≥ 50% of the sites where those species were recorded. Also, at the two sites where Neacomys guinae was recorded, its detection was revealed by both LTs and PTs (Fig. 2a). Two species were detected by LTs and CTs at most sites, but not by PTs: M. nudicaudatus and Proechimys spp. (Fig. 2b).
Number of sites occupied by each species across all 25 sampling sites in the Balbina Hydroelectric Reservoir landscape, as detected from (a) live and pitfall trapping, and (b) live and camera trapping. Bars are colour-coded according to the method(s) through which a species has been detected at any individual site (i.e. live traps (LT), pitfall traps (PT), camera traps (CT) or a combination of either LT and PT or LT and CT). Species names are coded as: Philander opossum (Phil), Hylaeamys megacephalus (Hyla), Proechimys spp. (Proe), Oecomys spp. 1 (Oeco1), Marmosa demerarae (Mdem), Marmosops parvidens (Mparvi), Didelphis marsupialis (Didel), Monodelphis arlindoi (Mono), Metachirus nudicaudatus (Meta), Euryoryzomys macconnelli (Eury), Mesomys hispidus (Meso), Isothrix pagurus (Iso), Marmosa murina (Mmuri), Marmosops pinheiroi (Mpin), Neacomys guianensis (Ngui), Oecomys cf. rex (Oeco2), Echimys chrisurus (Echi) and Rhipidomys mastacalis (Rhipi)
Sampling methods further differed in their performance in detecting some species at any given site. In addition to the species exclusively recorded by any of the methods, LTs performed better than PTs in recording Didelphis marsupialis, P. opossum, and M. demerarae. In turn, PTs ensured a higher detection rate of the marsupial M. parvidens. CTs detected D. marsupialis and Proechimys spp. more efficiently than LTs, while LTs detected P. opossum more efficiently than CTs (Table 3).
Discussion
Sampling highly diverse small non-volant mammal assemblages across the tropics is often intractable due to high logistic and labour costs of the methods commonly used (Gardner et al. 2008). Yet, attempts to use alternatively simpler methods to survey small mammals have been recently carried out in temperate regions (e.g. De Bondi et al. 2010; Castleberry et al. 2014; Welbourne et al. 2015; Villette et al. 2017), including the development of camera traps appropriate to detect small-bodied species (McCleery et al. 2014; Hobbs and Brehme 2017). In the neotropical region, studies examining the efficiency of small mammal sampling methods are mostly limited to either live or pitfall trapping (Santos-Filho et al. 2015; Ardente et al. 2017; Bovendorp et al. 2017) and, to our knowledge, no study to date has examined the combined sampling efficiency of live, pitfall and camera trapping across the same set of sites. Although camera traps were not primarily set to survey small-bodied species, this is the first study to address the potential benefits of this simple and cost-effective method to survey neotropical small mammals. Most species were recorded by LTs (72.2%) and PTs (83.3%), with each of these methods exclusively recording additional species, while CTs detected only nearly one-fourth of all species, corresponding to the largest species (> 200 g). Nevertheless, for nearly all species detected by CTs, the probability of detecting individual species was similar or higher than that of LTs.
We do not expect that the 2-year gap between CT (e.g. set in 2011/12) and PT and LT (set in 2014/15) significantly affected our results, as those are based on species incidence data. Local species densities considerably change over relatively short-time periods (Krebs 1966), but species incidence take longer to change, involving extinction-recolonization processes. Additionally, in our study system, larger forest sites harbour complete or nearly complete small mammal assemblages, while smaller forest sites contained only a set of matrix-tolerant species (Palmeirim et al. 2018). Major changes in species composition due to extinction-recolonization are unlikely over the 2-year gap, particularly at larger forest sites. In small islands, where species extinction is more likely, the remaining species or colonisers would belong to the same set of matrix-tolerant species (Palmeirim et al. 2018), further leading to only minor changes in species composition therein. The use of species incidence data also allowed us to deal with the lack of independence between LTs and PTs, set simultaneously at the same sites. An individual caught in a LT or PT cannot then be detected by the other method on that same night, affecting abundance estimates, but unlikely to affect species incidence at the site.
Each of the trapping methods tested in this study operates differently, and their comparison requires acknowledging such differences. Single-catch live traps attract individuals on an olfactorial basis, and individuals from certain species are known to take advantage of the baits used, which may change according to local food availability (Adler and Lambert 1997). Indeed, along the 16 nights of each trapping session, we noted that the same individuals of D. marsupialis, P. opossum and M. demerarae were recurrently recaptured within LTs (Palmeirim et al. under review). In contrast, other species are known to avoid live traps, perhaps because they are rare or not attracted by any bait used (Umetsu et al. 2006). For example, the terrestrial marsupials M. arlindoi and M. nudicaudatus were recorded more often or exclusively by pitfall traps than live traps. In contrast to LTs, pitfall and camera traps are multi-catch methods that randomly record individuals that are moving through the area. However, pitfall and camera traps also have drawbacks: while some species are able to jump or climb out of the pitfall buckets (e.g., Proechimys spp. and adult M. nudicaudatus; Palmeirim pers. obs.), individuals recorded by camera traps are not handled by the researcher, rendering the identification process to species level difficult to obtain for some species. In this way, the probability of an individual being recorded by LTs, PTs or CTs is affected by different factors that are intrinsic to each of these methods. Likewise, each multi-catch trap (pitfall and camera traps) allows more than one individual to be recorded on the same night, while the same is not possible for single-catch traps (live traps). Such characteristics of each trap-type were incorporated in our sampling design in which three pitfall traps, two camera traps and nine live traps were deployed at each trapping plot. Our sampling design further reflects the number of traps of each type that is commonly used to survey small mammals across the Neotropics, so that the overall efficiency of each trap type, as usually deployed, can be compared with other studies.
Live vs pitfall trapping
Using the combination of live, pitfall and camera trapping placed on the forest floor within the same sites, we were able to detect all six strictly terrestrial small mammal species likely to occur throughout the study landscape, in addition to 12 scansorial and arboreal species (for a full species list, see Malcolm 1991). Arboreal species were detected by both LTs and PTs, hence those species, regardless of their arboreal habits, also use the forest ground to move around. Although arboreal species were previously recorded on the forest floor, those were mainly captured by PTs, which proved to be a more efficient method in recording a larger number of species (Umetsu et al. 2006; Santos-Filho et al. 2015; Ardente et al. 2017; but see Santos-Filho et al. 2006). In this study, PTs also recorded an overall slightly higher number of species compared to LTs, further exclusively recording four species (M. arlindoi, M. pinheiroi, I. pagurus, and R. mastacalis), which are probably not that attracted by the bait used. Yet, LTs exclusively recorded two additional species, including spiny rats (Proechimys spp.), which are able to leap out of even 100 L-pitfall buckets (Palmeirim pers. obs.). Moreover, for comparative purposes, both LTs and CTs traps were intentionally set on the forest floor. Nevertheless, the deployment of these trap types across the vertical forest strata would likely increase the total number of species detected (Vieira and Monteiro-Filho 2003). Indeed, three of the four species exclusively detected by PT were also detected by LTs placed in the understorey and the (sub)canopy (Palmeirim et al. 2018), but not considered in this study. Also, LTs in this study were three times more numerous than PTs, suggesting that, for the same number of trap-nights, PTs are likely more efficient in recording species richness than were LTs. Indeed, a recent comprehensive review of the sampling efficiency between LTs and PTs across the Brazilian Atlantic forest shows that PTs set alone, or in combination with LTs, provide higher estimates of species richness requiring less sampling effort than sites that were exclusively sampled by LTs (Bovendorp et al. 2017).
At individual sampling sites, LTs and PTs recorded a similar small mammal species richness, with some species proving to be more efficiently detected by either LTs (N = 3) or PTs (N = 1). Similarly, both methods tended to be selective towards the distinct set of species they attracted at other Amazonian forests (Santos-Filho et al. 2015; Ardente et al. 2017). In general, PTs are considered more efficient to capture species that are rarely or never captured using traditional live traps (Voss et al. 2001; Hice and Schmidly 2002; Umetsu et al. 2006). In this study, that was the case of the terrestrial marsupial M. arlindoi, only recorded on PTs. Nonetheless, our findings do not support a small mammal sampling strategy based entirely on pitfall trapping, as some species were not well sampled by this technique as those were by LTs (i.e., D. marsupialis, P. opossum and M. demerarae). Furthermore, contrary to previous findings (Umetsu et al. 2006), LTs and PTs detected an overall similar proportion of juvenile individuals, that differed according to the species considered. In sum, given that trapping success was very similar between LTs and PTs, and that the effort in placing one PT is much higher than that of one LT, we suggest the consistent use of PTs in exhaustive small mammal inventories, further complementing LT records (Santos-Filho et al. 2006, 2015; Ardente et al. 2017). If the study is focused on a single population, the choice can be defined by using one or two techniques that are more powerful in sampling that target species (see Table 2 and Fig. 2).
Advantages and limitations of camera trapping
In this study, CTs recorded up to one-fourth of the total number of species detected by LTs, largely failing to provide sufficient resolution in surveys of small-bodied species < 200 g. In 25.2% of all CT records, images were not of sufficiently high quality to ensure reliable identification to the species or genus level. Indeed, given that our camera trapping protocol had not been primarily designed to record small mammals, their setup near the ground did not favour small-bodied species that move below or above the forest leaf litter, and appropriate baits were not used to attract small mammals. These factors likely contribute to the low number of species detected by CTs in this study. In contrast to the present findings, studies designed to detect small mammals but carried out outside the Neotropics have obtained similar results comparing CTs and LTs (Castleberry et al. 2014; McCleery et al. 2014), further considering CTs to be more cost-effective than LTs (De Bondi et al. 2010; Welbourne et al. 2015). Therefore, future improvements to increase image definition will likely increase the efficiency of CTs to survey small mammals (e.g. Glen et al. 2013).
Notwithstanding, the CT detectability of the largest four species of the small non-volant mammal fauna we sampled—D. marsupialis, P. opossum, M. nudicaudatus, and Proechimys spp.—was just as efficient as that revealed by LTs. CTs may therefore be a better option for neotropical studies focused on that size class of rodents and marsupials. Moreover, we show that the larger-bodied small mammal species are also accurately recorded even when CTs are positioned to record midsize to large mammal species. In this regard, camera trapping studies focused on medium and large-sized vertebrates could provide valuable information on the upper size subset of small mammal species (> 200 g) information that should not be disregarded. When medium and large-sized vertebrates and small mammals are surveyed within the same study area, as frequently occurs in Rapid Biological Field Assessment surveys (RAP; Larsen 2016), supplementary small mammal data obtained by CTs would increase the detection of those largest small mammal species. Another possibility would be deploying those CTs already used in RAP (for medium- and large-sized mammal sampling) within the same sites, but reducing the height above ground and using attractive baits for small mammal species. Moreover, the efficient detection of those four largest small mammal taxa observed here also suggests that CTs can be potentially useful if placed at other heights of the forest strata. For example, in the Brazilian Pantanal, Oliveira-Santos et al. (2008) successfully used platforms to place CTs in the forest understorey, aiming to investigate the activity patterns of small mammals, including small-bodied arboreal rodents.
Identifying neotropical small mammal species in the field remains an intractable problem that goes beyond the trapping method used. For instance, we were unable to identify four taxa to the species level, even after careful handling live specimens captured by LTs and PTs. Even considering well-resolved species for which the alpha taxonomy is widely accepted, many species cannot be teased apart based on external morphological characters, requiring examination of skull morphology, karyotypes, or molecular markers (e.g. Patton et al. 2000). Each of those taxa identified to the genus level in the field may likely include more than one sympatric species, a problem that will persist. Nevertheless, alternative studies considering functional diversity may overcome this issue. Functional diversity is closely linked to ecosystem processes (Tilman 2000), and has been assumed to be a better predictor of ecosystem functioning than species richness (Hillebrand and Matthiessen 2009). Such studies may not require detailed identification to the level of species, and congeners, particularly those of smaller-bodied species, also tend to be ecologically similar (see Wilman et al. 2014).
Our results highlight the importance of live traps as the primary method to survey small mammals across the Neotropics. However, pitfalls proved to be an important complement to LTs, particularly when the aim of the study is to obtain a robust assessment of the small mammal assemblage. Furthermore, camera trapping may also be helpful as a complementary technique or when the research objectives of behavioural and population studies are focused on the largest species (Buckner 1964). Nevertheless, the deployment of CTs at lower positions near the ground (10–15 cm) and at the forest understory, with appropriate changes to improve photoquality (e.g. trigger speed, passive infrared vs. microwave sensor, white vs. infrared flash, and still photographs vs. video), and the use of appropriate baits can improve the detection of smaller-bodied small mammal species (Glen et al. 2013). This would then contribute to camera trapping serving as a viable supplementary method to sample small mammal communities in Neotropical regions.
References
Adler GH, Lambert TD (1997) Ecological correlates of trap response of a neotropical forest rodent Proechimvs semispinosus. J Trop Ecol 13:59–68
Ahumada JA, Silva CEF, Gajapersad K, Hallam C, Hurtado J, Martin E, McWilliam A, Mugerwa B, O’Brien T, Rovero F, Sheil D, Spironello WR, Winarni N, Andelman SJ (2011) Community structure and diversity of tropical forest mammals: data from a global camera trap network. Philos Trans R Soc Lond Ser B Biol Sci 366:2703–2711
American Veterinary Medical Association (2013) AVMA guidelines for the euthanasia of animals. https://www.avma.org/KB/Policies/Documents/euthanasia.pdf. Accessed Jan 2014
Ardente NC, Ferreguetti AC, Gettinger D, Leal P, Martins-Hatano F, Bergallo HG (2017) Differential efficiency of two sampling methods in capturing non-volant small mammals in an area in eastern Amazonia. Acta Amazon 47:123–132
Benchimol M, Peres CA (2015) Widespread forest vertebrate extinctions induced by a mega hydroelectric dam in lowland Amazonia. PLoS One 10:e0129818
Boonstra R, Krebs CJ (1978) Pitfall trapping of Microtus townsendii. J Mammal 59:136–148
Bovendorp RS, McCleery RA, Galetti M (2017) Optimising sampling methods for small mammal communities in Neotropical rainforests. Mammal Rev 47:148–158
Buckner CH (1964) Preliminary trials of a camera recording device for the study of small mammals. Can Field Nat 78:77–79
Castleberry SB, Mengak MT, Menken TE (2014) Comparison of trapping and camera survey methods for determining presence of allegheny woodrats. Wildl Soc Bull 38:414–418
Comissão de Ética, BioÉtica e Bem-Estar Animal/CFMV (2012) Guia Brasileiro de Boas Práticas em Eutanásia em Animais - Conceitos e Procedimentos Recomendados. Brasília, Brazil. http://portal.cfmv.gov.br/uploads/files/Guia%20de%20Boas%20Práticas%20para%20EEutanasi.pdf. Accessed Jan 2014
De Bondi N, White JG, Stevens M, Cooke R (2010) Comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities. Wildl Res 37:456–465
Emmons L, Feer F (1997) Neotropical rainforest mammals: a field guide. University of Chicago Press, Chicago
FUNCATE/INPE/ANEEL (2000) Mapeamento por satélite das áreas inundadas por reservatórios de hidrelétricas brasileiras. Unpublished Report. Convênio FUNCATE/INPE/ANEEL, São Paulo
Gardner TA, Barlow J, Araujo IS, Ávila-Pires TCS, Bonaldo AB, Costa JE, Espósito MC, Ferreira LV, Hawes J, Hermandez MI, Hoogmoed M, Leite RN, Lo-Man-Hung NF, Malcolm JR, Martins MB, Mestre LAM, Miranda-Santos R, Nunes-Gutjahr A, Oveal WL, Parry LTW, Peters SL, Ribeiro-Júnior MA, Silva MNF, Silva-Motta C, Peres CA (2008) The cost-effectiveness of biodiversity surveys in tropical forests. Ecol Lett 11:139–150
Glen AS, Cockburn S, Nichols M, Ekanayake J, Warburton B (2013) Optimising camera traps for monitoring small mammals. PLoS One 8:e67940
Hannibal W, Caceres NC (2010) Use of vertical space by small mammals in gallery forest and woodland savannah in South-Western Brazil. Mammalia 74:247–255
Hice CL, Schmidly DJ (2002) The effectiveness of pitfall traps for sampling small mammals in the Amazon basin. Mastozool Neotrop 9:85–89
Hillebrand H, Matthiessen B (2009) Biodiversity in a complex world: consolidation and progress in functional biodiversity research. Ecol Lett 12:1405–1419
Hobbs MT, Brehme CS (2017) An improved camera trap for amphibians, reptiles, small mammals, and large invertebrates. PLoS One 12:e0185026
IBAMA (1997) Plano de manejo fase 1: Reserva Biológica do Uatumã. Eletronorte/Ibama. Brasília/DF. http://www.icmbio.gov.br/portal/images/stories/docs-planos-de-manejo/rebio_uatuma_pm.pdf. Accessed 10 Sept 2017
Jones KE, Bielby J, Cardillo M, Fritz SA, O'Dell J, Orme CDL, Safi K, Sechrest W, Boakes EH, Carbone C, Connolly C, Cutts MJ, Foster JK, Grenyer R, Habib M, Plaster CA, Price SA, Rigby EA, Rist J, Teacher A, Bininda-Emonds ORP, Gittleman JL, Mace GM, Purvis A (2009) PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90:2648–2648
Krebs CJ (1966) Demographic changes in fluctuating populations of Microtus californicus. Ecol Monogr 36:239–273
Larrucea ES, Brussard PF (2008) Efficiency of various methods used to detect presence of pygmy rabbits in summer. West N Am Nat 68:303–310
Larsen TH (2016) Core standardized methods for rapid biological field assessment. Conservation International, Arlington
Laurance WF (1992) Abundance estimates of small mammals in Australian tropical rainforest: a comparison of four trapping methods. Wildl Res 19:651–655
Macedo J, Loretto D, Vieira MV, Cerqueira R (2006) Classes de desenvolvimento em marsupiais: um método para animais vivos. Mastozool Neotrop 13:133–136
Malcolm JR (1991) The small mammals of Amazonian forest fragments: pattern and process. PhD thesis, University of Florida
Mangan SA, Adler GH (2000) Consumption of arbuscular mycorrhizal fungi by terrestrial and arboreal small mammals in a panamanian could forest. J Mammal 81:563–570
McClearn D, Kohler J, McGowan KJ, Cedeno E, Carbone LG, Miller D (1994) Arboreal and terrestrial mammal trapping on Gigante peninsula, Barro Colorado nature monument, Panama. Biotropica 26:208–213
McCleery RA, Zweig CL, Desa MA, Hunt R, Kitchens WM, Percival HF (2014) A novel method for camera-trapping small mammals. Wildl Soc Bull 38:887–891
Norris D, Michalski F, Peres CA (2010) Habitat patch size modulates terrestrial mammal activity patterns in Amazonian forest fragments. J Mammal 91:551–560
O’Connell AF, Nichols JD, Karanth KU (2010) Camera traps in animal ecology: methods and analyses. Springer, London
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH (2007) Vegan: the community ecology package. R package version 2, pp 4–2
Oliveira-Santos LG, Tortato MA, Graipel ME (2008) Activity pattern of Atlantic Forest small arboreal mammals as revealed by camera traps. J Trop Ecol 24:563–567
Palmeirim AF, Benchimol M, Vieira MV, Peres CA (2018) Small mammal responses to Amazonian forest islands are modulated by their forest dependence. Oecologia 187:1–14. https://doi.org/10.1007/s00442-018-4114-6
Patton JL, Da Silva MNF, Malcolm JR (2000) Mammals of the Rio Juruá and the evolutionary and ecological diversification of Amazonia. Bull Am Mus Nat Hist 244:1–306
R Core Team (2015) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ribeiro-Júnior MA, Rossi RV, Miranda CL, Ávila-Pires TC (2011) Influence of pitfall trap size and design on herpetofauna and small mammal studies in a Neotropical Forest. Zoologia (Curitiba) 28:80–91
Ripley B, Canty A (2017) Boot: bootstrap functions. R package version 1, pp 3–19
Santos-Filho M, Silva DJ, Sanaiotti TM (2006) Efficiency of four trap types in sampling small mammals in forest fragments. Mato Grosso, Brazil Mastozool Neotrop 13:217–225
Santos-Filho MD, Lázari PRD, Sousa CPFD, Canale GR (2015) Trap efficiency evaluation for small mammals in the southern Amazon. Acta Amaz 45:187–194
Sikes RS (2016) 2016 guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97:663–688
Silveira L, Jácomo ATA, Diniz-Filho JAF (2003) Camera trap, line transect census and track surveys: a comparative evaluation. Biol Conserv 114:351–355
Terborgh J, Lopez L, Nuñes PV, Rao M, Shahabuddin G, Orihuela G, Riveros M, Ascanio R, Adler GH, Lambert TD, Balbas L (2001) Ecological meltdown in predator-free forest fragments. Science 294:1923–1926
Tilman D (2000) Causes, consequences and ethics of biodiversity. Nature 405:208–211
Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. J Mammal 87:757–765
Vieira EM, Monteiro-Filho ELA (2003) Vertical stratification of small mammals in the Atlantic rain forest of South-Eastern Brazil. J Trop Ecol 19:501–507
Villette P, Krebs CJ, Jung TS (2017) Evaluating camera traps as an alternative to live trapping for estimating the density of snowshoe hares (Lepus americanus) and red squirrels (Tamiasciurus hudsonicus). Eur J Wildl Res 63(1):7
Voss RS, Lunde DP, Simmons NB (2001) The mammals of Paracou, French Guiana: a neotropical lowland rainforest fauna part 2. Nonvolant species. Bull Am Mus Nat Hist 263:3–236
Welbourne DJ, MacGregor C, Paull D, Lindenmayer DB (2015) The effectiveness and cost of camera traps for surveying small reptiles and critical weight range mammals: a comparison with labour-intensive complementary methods. Wildl Res 42:414–425
Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W (2014) EltonTraits 1.0: species-level foraging attributes of the world's birds and mammals. Ecology 95:2027–2027
Woodman N, Timm RM, Slade NA, Doonan TJ (1996) Comparison of traps and baits for censusing small mammals in Neotropical lowlands. J Mammal 77:274–281
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Acknowledgements
We are grateful to all 19 volunteers and field assistants who helped during field campaigns and to M. N. da Silva for helping with species identification.
Funding
Funding was provided by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro–FAPERJ (grant Cientistas do Nosso Estado to MVV), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Grant to MVV), Natural Environment Research Council (NERC; grant NE/J01401X/1 to CAP) and the Post-Graduate Program in Ecology of the Federal University of Rio de Janeiro. The Uatumã Biological Reserve provided logistic support during fieldwork, and additional resources or equipment were provided by The Rufford Small Grants Foundation, RFP-Wildlife Conservation Society, The Conservation, Food and Health Foundation, and Idea Wild. AFP and MB were funded by the Coordenação de Aperfeiçoamento Pessoal de Nível Superior scholarship from the Brazilian Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Magdalena Niedziałkowska
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 167 kb)
Rights and permissions
About this article
Cite this article
Palmeirim, A.F., Benchimol, M., Peres, C.A. et al. Moving forward on the sampling efficiency of neotropical small mammals: insights from pitfall and camera trapping over traditional live trapping. Mamm Res 64, 445–454 (2019). https://doi.org/10.1007/s13364-019-00429-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-019-00429-2