Abstract
Seventy-seven EPA priority environmental pollutants were analyzed using gas chromatography-mass spectrometry (GC-MS) equipped with an optimized atmospheric pressure photoionization (APPI) and an atmospheric pressure laser ionization (APLI) interface with and without dopants. The analyzed compounds included e.g., polycyclic aromatic hydrocarbons (PAHs), nitro compounds, halogenated compounds, aromatic compounds with phenolic, acidic, alcohol, and amino groups, phthalate and adipatic esters, and aliphatic ethers. Toluene, anisole, chlorobenzene, and acetone were tested as dopants. The widest range of analytes was ionized using direct APPI (66/77 compounds). The introduction of dopants decreased the amount of compounds ionized in APPI (e.g., 54/77 with toluene), but in many cases the ionization efficiency increased. While in direct APPI the formation of molecular ions via photoionization was the main ionization reaction, dopant-assisted (DA) APPI promoted ionization reactions, such as charge exchange and proton transfer. Direct APLI ionized a much smaller amount of compounds than APPI (41/77 compounds), showing selectivity towards compounds with low ionization energies (IEs) and long-lived resonantly excited intermediate states. DA-APLI, however, was able to ionize a higher amount of compounds (e.g. 51/77 with toluene), as the ionization took place entirely through dopant-assisted ion/molecule reactions similar to those in DA-APPI. Best ionization efficiency in APPI and APLI (both direct and DA) was obtained for PAHs and aromatics with O- and N-functionalities, whereas nitro compounds and aliphatic ethers were the most difficult to ionize. Halogenated aromatics and esters were (mainly) ionized in APPI, but not in APLI.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric pressure photoionization (APPI) is one of the three most important ionization techniques for liquid chromatography-mass spectrometry (LC-MS), together with electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). After its introduction in 2000 [1, 2], APPI has been shown to widen the group of compounds that can be analyzed by LC-MS towards neutral and nonpolar compounds. As an LC-MS interface, APPI has been applied widely in e.g., pharmaceutical, biological, and environmental analysis [3, 4]. Although APPI was originally developed for LC-MS, there is also an increasing number of reports using it as an interface for GC-MS [5–12]. A major advantage of GC compared with LC is its much higher peak capacity, which allows the separation of a much higher number of compounds in one chromatographic run. Traditional methods of ionization for GC-MS are electron ionization (EI) and chemical ionization (CI), but APPI has been shown to provide softer ionization, producing mainly molecular ions (M+.) or protonated molecules ([M + H]+), which can be utilized for e.g., single ion or multiple reaction monitoring. Atmospheric pressure laser ionization (APLI) is another ionization technique for LC-MS, which has been shown to be highly selective towards completely nonpolar, highly conjugated molecules, such as polycyclic aromatic hydrocarbons (PAHs) [13]. APLI is much less explored, but its suitability as an interface for both LC-MS and GC-MS has been demonstrated [13, 14].
Recently, a novel API interface for GC-MS tailored towards APPI operation was introduced [15]. In this design, the crucial parameters for efficient direct ionization and chromatographic performance have been carefully considered. The key part of the interface is a small, airtight, and conically shaped ionization unit. Inside, a vortex is maintained by a highly purified (99.999999%) nitrogen flow, to the eye of which the minor GC gas stream is placed. Optical access is provided by a MgF2 window, on which the photoionization lamp is directly set. The interface has been shown to give very high sensitivity for trace analysis, with LODs in the femtogram range in direct photoionization [16]. This is similar to what has been reported with GC-EI/CI-Orbitrap in a recent study [17]. In addition, APLI is compatible with the interface operation, however, only for the purpose of mechanistic comparison studies. A dedicated GC-APLI interface is currently under construction.
In a previous study, the ionization mechanisms in direct and dopant-assisted APPI and direct and dopant-assisted APLI with the above-mentioned API interface was investigated using eight compounds [15]. It was shown that (1) in direct APPI and direct APLI, mainly molecular ions were formed; (2) direct APPI was shown to be more universal than direct APLI, whereas APLI was highly selective towards compounds that have favorable two-photon ionization cross sections; (3) introduction of dopants hindered direct photoionization in APPI and two-photon ionization in APLI and, instead, dopant-mediated reactions took place that led to more variety in the type of ions formed; (4) the dopant-mediated reactions were mainly the same in APPI and APLI; and (5) introduction of dopants can enhance the ionization efficiency; (6) although in APPI, the presence of dopants in many cases decreased the number of compounds that were ionized; (7) whereas in APLI many compounds that were not ionized by resonance enhanced (1 + 1) multiphoton ionization (REMPI) were ionized with one or several of the dopants. The reactions shown in Table 1 were identified as fundamental processes operative in the direct and dopant mediated ionization schemes when using ultra-pure buffer gases.
The aim of the present study was to analyze a wider group of analytes with environmental interest using the novel API interface, and again APPI and APLI for mechanistic studies in direct and dopant-assisted modes. An EPA standard mixture with 77 environmental pollutants was chosen for the study, with a wide variety of functionalities that were thought to give a comprehensive view about the feasibility of the novel interface in environmental GC-MS analysis as well as a possibility to discuss the effect of molecule structure in the ionization route and ionization efficiency in different conditions. A similar sample mixture has been previously analyzed in GC-APPI-MS without a dopant [6].
Experimental
Chemicals
Toluene (99.9%), acetone (99.9%), and toluene-d8 (99%) were purchased from Sigma-Aldrich (Steinheim, Germany), chlorobenzene (>99%) and anisole (>99%) from Merck (Hohenbrunn, Germany), and dichloromethane (HPLC grade) from VWR (Leuven, Belgium). The sample was a 8270 LCS Mix 1 (Supelco, Bellefonte, PA, USA) containing 100 μg/mL of 77 EPA priority pollutants in acetone/methylene chloride (9/1). A complete list of the EPA mix compounds is given in Table 2. For the GC-MS analyses, the sample was diluted in dichloromethane to a final concentration of 100 pg/μL.
Instrumentation
Gas Chromatography
The samples were separated using a Thermo Scientific 450 Series gas chromatograph and a TR-Dioxin 5MS column (30 m × 0.25 mm i.d. × 0.1 μ; Milan, Italy). The GC temperature program was as follows: initial temperature was 50°C for 1 min, 30°C/min up to 150°C, 20°C/min up to 200°C, 30°C/min up to 300°C, and 20°C/min up to 320°C, hold time 5 min. Helium at constant 1.50 mL/min flow rate was used as the carrier gas. The GC transfer line and injector temperatures were both set to 325°C. Splitless injection of 0.5 μL was used (corresponding to 50 pg on column/compound).
Mass Spectrometry
The mass spectrometer was a Thermo Scientific (Bremen, Germany) Exactive Orbitrap, equipped with a custom-made API interface, which has been described in detail before [15]. Shortly, a commercial transfer line (Thermo Scientific) was used to guide the sample flow from the GC column to an air-tight, conically-shaped ion source, which was heated to 325°C. The ion source used high purity N2 make-up gas at 850 mL/min. The capillary temperature was 300°C, and capillary, tube lens, and skimmer voltages were 25, 45, and 16 V, respectively. The mass range was set to m/z 50–1000. All measurements were performed in positive ion mode. For the dopant experiments, an additional line was connected via a t-piece directly to the make-up gas entrance of the source enclosure. Headspace of the dopant (toluene, acetone, anisole, or chlorobenzene) was added to the make-up gas with a gas syringe and a syringe pump at a flow rate of 100 μL/min.
For the APPI measurements, a low-pressure Kr discharge lamp with a radio frequency (rf) driver from Syagen (Santa Ana, CA, USA) provided radiative output at 10.0 and 10.6 eV. The entire ionization volume was irradiated. For the APLI measurements, an OEM laser device from CryLaS (Berlin, Germany) was used. The laser was a frequency-quadrupled diode-pumped solid-state (DPPS) Nd:YAG laser radiating at 266 nm (4.66 eV) wavelength with a pulse duration of 0.9 ns, a repetition rate of 60 Hz, a spot size of 0.5 mm, a pulse energy of 200 μJ, resulting in a power density of roughly 1 × 108 W/cm2. The laser beam pointed straight down the cone, sweeping past the exit of the GC column.
Results and Discussion
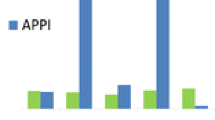
Table 3 shows the total number of compounds that were ionized with and without dopant in APPI and APLI. In direct APPI, altogether 66 (out of 77) compounds were ionized, and in direct APLI 41. The introduction of dopants decreased the number of compounds ionized in APPI but increased the number of compounds ionized in APLI. Due to the very high number of compounds in the sample, they were categorized into seven groups depending on their chemical functionalities, to simplify the discussion. For compounds with several functionalities, the group was chosen depending on the functionality that had the most effect on the compound’s ionization energy (IE) or proton affinity (PA). The groups were (1) polycyclic aromatic hydrocarbons (PAHs), (2) halogenated compounds, (3) nitro compounds, (4) other N-containing compounds, (5) esters, (6) aliphatic ethers, and (7) other O-containing compounds. Some common ionization pathways for different groups could be identified, and these are discussed rather than all the compounds individually. Some individual compounds are shown as examples for the ionization behavior of the entire group, or as particularly interesting cases. Retention indices and Ref. [20] were used to aid in the determination of the elution order and identification of the observed ions.
PAH Compounds
The 18 PAH compounds contained only C and H, with different numbers of aromatic rings, some in addition aliphatic rings or methyl groups. PAHs are highly conjugated compounds with large π-systems, and their IEs are well below the energy of the VUV lamp photons. As expected, the PAH compounds were efficiently ionized in direct APPI (Table 2). Similarly to a previous study [15], all the PAHs showed molecular ions (M+.) in direct APPI, as well as with toluene, anisole, and chlorobenzene dopants in DA-APPI. Addition of dopants enhanced the ionization of the PAH compounds due to charge exchange between the dopant M+. and the analyte (Table 1, Reaction 8), which replaces direct photoionization (Table 1, Reaction 1). Acetone photo-ions form protonated molecules ([M + H]+) by self-protonation [22] (Table 1, Reaction 6), and therefore most compounds with PAs above the PA of acetone (812 kJ/mol) formed protonated molecules ([M + H]+) in reaction with acetone [M + H]+ (Table 2). The only exception was phenanthrene (1-methylnaphthalenes formed both M+. and [M + H]+), for which only the M+. ion was observed. The proton transfer from [M + H]+ of acetone to phenanthrene is less exothermic than it is for the other PAHs, which may explain why the reaction was not observed.
In direct APLI all PAHs were ionized, forming M+. ions, similarly to APPI. APLI is based on resonance-enhanced multi-photon ionization (REMPI), where the ionization is caused by absorption of two or more photons. In APLI, typical laser densities are in the range of 106–108 W/cm2, which basically restricts the REMPI process to resonant two photon excitation (Table 1, Reaction 2). The first photon excites the molecule to an intermediate electronic state, when favorable linear absorption coefficients are present. Absorption of a second photon becomes feasible if (1) the absorption cross-section from the intermediate level into the ionization continuum is favorable, (2) the lifetime of the intermediate level is well above 1 ns, and (3) the IE of the compound is below the two-photon energy. Note, though, that ultra-fast relaxation processes from the initially populated intermediate state in the Sn ← S0 manifold to S1 and/or efficient singlet-triplet coupling may lead to unexpected results. The laser wavelength in the current setup is 266 nm, which corresponds to 4.66 eV, and the summed energy of two photons available for ionization is 9.32 eV. All the PAHs of the present study have IEs well below the two-photon energy. PAHs have been reported to be efficiently ionized by REMPI in several publications, including APLI [13, 14, 23–25]. However, significantly lower signals were achieved for acenaphthylene, anthracene, and fluorantherene than the seemingly similar acenaphthene, phenanthrene, and pyrene, respectively. For example, acenaphthylene gave a 500 times lower signal than acenaphtene because the lifetime of the first excited singlet (S1) state for acenaphtylene has been reported to be in picosecond time domain (in condensed phase), due to deactivation of S1 state by internal conversion [26]. This makes ionization in the current conditions highly unlikely. Similarly, the lifetimes of the S1 states for anthracene and phenanthrene have been reported as 8 and 76 ns, respectively, and those for fluoranthene and pyrene as 38 and 1400 ns, respectively [23], readily explaining the higher ionization efficiencies for phenanthrene and pyrene.
In dopant-assisted APLI, the PAH compounds formed M+. ions in all cases. Unlike in APPI, the introduction of dopants did not increase the analyte signal markedly (see Figure 1 for acenaphthene). Acenaphtylene, however, was an exception to the rule: while it showed a very weak signal in direct APLI, the signal increased significantly with toluene, chlorobenzene, and anisole dopants: e.g., toluene caused a 500-fold increase in the acenaphthylene signal (Figure 1). This is because in the presence of these dopants the ionization takes place through collisionally induced charge exchange with the dopant M+. (Table 1, Reaction 8), where the IE and the reaction cross section of the compounds determine the efficiency of the process. Acetone has IE above the two-photon energy of the laser and is thus not ionized in APLI. As a consequence, proton transfer reactions with acetone [M + H]+ ions are not observed in DA-APLI as they are in DA-APPI, but the ionization proceeds by direct APLI also in the presence of acetone (Table 1, Reaction 2). Similarly, (linear) absorption of the laser light by matrix constituents such as LC solvents or water is also not observed in APLI and, thus, the entire laser energy is available for driving two-photon ionization processes in the target analyte group.
Halogenated Compounds
The 19 halogenated compounds had 1-6 chlorine or bromine substituents attached either to aromatic ring or to aliphatic core; some of the halogenated compounds were also phenols or phenyl ethers. In direct APPI, 17 out of 19 of the halogenated compounds were ionized, all forming M+. ions. The known IEs of these compounds are in the range of 8.11–9.28 eV (Table 2), all below the energy of the VUV lamp photons, which explains their efficient ionization in APPI. For the two compounds not ionized, hexachloroethane and pentachlorophenol, the IEs are unknown.
In DA-APPI, the number of halogenated compounds that were ionized decreased. The lowest number of compounds was ionized with anisole (four compounds), because of the low IE of anisole (8.20 eV), which is below the IEs of many of the analytes. Chlorobenzene and acetone, on the other hand, ionized 11 compounds because of their substantially higher IEs. For the compounds that were ionized in the presence of dopants, the ionization efficiency increased, especially with toluene and chlorobenzene dopants. Also in the presence of dopants the halogenated compounds formed M+. ions, even with acetone. The signals in the presence of acetone were at least an order of magnitude lower than with direct APPI, indicating suppression of analyte ionization by the acetone dopant, probably because of the consumption of photons by acetone, or neutralizing reactions between the analyte ions and acetone. The same was observed in a previous study for naphthalene [15].
With direct APLI, only six out of 19 of the halogenated compounds were ionized (Tables 2 and 3). Although the (known) IEs of the halogenated compounds are below the two-photon energy of the laser radiation used, halogenated compounds with several halogen substituents have very short excited-state lifetimes in REMPI, because of strong intersystem crossing (crossover between electronic states of different multiplicity; most commonly observed for compounds with heavy atoms) [22, 27]. For chlorophenols, it has been reported that the ionization cross-section decreases as the number of chlorine substituents increases, and this was also observed here (Figure 2, Table 2).
The addition of toluene and chlorobenzene dopants in APLI increased the number of compounds that could be ionized to 7 and 13, respectively, whereas with anisole and acetone, only four compounds were ionized (Tables 2 and 3). The compounds ionized are mainly the same as those ionized in DA-APPI with the same dopants, indicating ionization through the same dopant-mediated reactions (Table 1, Reaction 8, acetone being an exception, for reasons explained above).
Nitro Compounds
The nine compounds in the nitro group were all aromatic compounds with one or two nitro substituents. In addition to the nitro groups, some of the compounds also contained phenolic functionalities. The nitro compounds gave poor ionization efficiencies in both direct APPI and APLI: in direct APPI only four out of nine of the nitro compounds were ionized, in direct APLI none. Aromatic nitro substituents are strongly electron-withdrawing, which increases the IE of the compound compared with a non-substituted compound [28]. The known IEs for the compounds in this group are in the range of 9.10–10.71 eV, being much higher than the IEs for corresponding compounds in other groups. As a consequence, most of the nitro compounds cannot be ionized by the VUV lamp photons. With DA-APPI an even smaller number of nitro compounds was ionized (Tables 2 and 3).
With direct APLI, none of the nitro compounds was ionized. Of the compounds with known IEs, all except 2-nitrophenol have IEs above the two-photon energy of the here used 266 nm laser, and therefore ionization by (1 + 1) REMPI is not possible. 2-Nitrophenol showed also no signal, although its IE is 9.10 eV and, thus, below the two-photon energy of the current setup. However, it is very likely that ultra-fast relaxation and/or ultra-fast dissociation processes driven at the energy level of the first photon, which have been reported for nitrotoluene [29], hold also true for 2-nitrophenol and render the required absorption of a second photon highly unfavorable. Chlorobenzene with highest IE was the only dopant that enabled the ionization of 2-nitrophenol by DA-APLI.
Other Nitrogen-Containing Compounds
The 12 other nitrogen-containing compounds had all nitrogen moieties that can be protonated, and the compounds are thus expected to have rather high PAs (see Table 2; although not all were found in the literature). Some of these compounds exhibit also nitro groups or halogens. In direct APPI, the nitrogen-containing compounds were ionized mainly as M+. ions (Table 1, Reaction 1), except N-nitrosodimethylamine, pyridine, and N-nitroso-di-n-propylamine, which formed [M + H]+. Interestingly, in a previous study using the same setup, pyridine showed both M+. and [M + H]+ in direct APPI [15]. In direct APPI the proton was suggested to originate from self-protonation of pyridine (Table 1, Reaction 3), whereas in the presence of dopants the proton was shown to originate from the dopants (Table 1, Reactions 9 and 10). The main difference between the earlier study and this study is that in Ref. [15] neat pyridine headspace was injected without a dilution solvent, to insure clean and well-defined ionization conditions. Here, pyridine was injected as a component of the diluted EPA sample mixture, with other analytes and solvent constituents eluting at the same time. There are, therefore, plenty of possible proton donors present, and the conditions are no longer well-defined, as they were in [15]. Similarly to [15], the experiment was repeated using toluene-d8 as the dopant, but also in its presence, pyridine showed [M + H]+, instead of [M + D]+ as in [15]. Isophorone, however, showed a [M + D]+, N-nitrosodimethylamine a [M + H]+, and N-nitroso-di-n-propylamine showed both [M + H]+ and [M + D]+ ions. The source of the proton is, therefore, likely to depend on the conditions (i.e., the presence of possible proton-donors) that exist in the ion source at the time of elution. It should be noted that the conditions will change during a chromatographic run, and it seems that at least here, more possible proton-donors are present in the beginning of the run, where the non-retaining solvent components elute.
With toluene, anisole, and chlorobenzene dopants the N-containing compounds formed mainly identical ions as in direct APPI. With acetone dopant, all the compounds of this group were protonated, mainly because of their PAs that are above the PA of acetone (Table 1, Reaction 10). In addition to M+. and [M + H]+ ions, some of the compounds in this group were fragile enough to fragment, and with chlorobenzene dopant, pyridine showed a [M + 77]+ ion, also observed in a previous study [15].
Although direct APPI was able to ionize all the N-containing compounds, in direct APLI pyridine, nitroanilines and N-nitrosodimethylamine were not ionized. Pyridine has only short-lived accessible electronically excited states, as already noted in the previous study [15] and, therefore, it cannot be ionized at the current conditions (i.e., wavelength and power density). This is most probably also true for N-nitrosodimethylamine and the nitroanilines, as has been reported for nitrotoluene [29]. In the presence of toluene, anisole, and/or chlorobenzene dopants, also the compounds that were not ionized in direct APLI could be ionized via dopant-mediated reactions.
Esters
The sample contained six phthalate esters and one adipate ester. The phthalate esters contain both aromatic and aliphatic moieties, whereas the adipate ester is completely aliphatic. In direct APPI, the esters were efficiently ionized and observed as typical phthalate ester fragments, such as m/z 149 and 163 [30]. In direct APLI, no signals for the esters were recorded. Only the IE of dimethyl phthalate was found from the literature, and it is reported to be 9.64 eV, which is below the energy of the VUV lamp photons in APPI, but above the two-photon energy in 266 nm APLI. This could explain why the esters were not ionized in APLI, besides possible unfavorable two-photon cross sections. In the presence of toluene and chlorobenzene dopants, all the esters were ionized also in DA-APLI. In DA-APPI the ionization efficiency was especially high in the presence of toluene and acetone, and in DA-APLI in the presence of toluene. [M + H]+ was observed as the base peak in the spectra of some of the esters in acetone-assisted APPI.
Ethers
The three ethers in the ether group are all aliphatic compounds. There were also some aromatic ethers in the sample, but they were divided into different groups because of their different ionization behavior. The three aliphatic ethers of the sample were not ionized under any circumstance, either in APPI or APLI, with or without dopants (Tables 2 and 3). The IEs of the ethers are not known, but they are expected to be quite high since the compounds are fairly small and do not contain any delocalized electron density. The PAs of the ethers, on the other hand, are probably rather low since the compounds do not possess pronounce basic sites. This probably explains the poor sensitivity for these compounds in both APPI and APLI.
Other O-Containing Compounds
The nine other O-containing compounds are a rather diverse group containing C, H, and O, consisting of benzyl alcohol, several phenols, benzoic acid, dibenzofuran, and isophorone (a ketone). All the compounds of this group were ionized in direct APPI and APLI, except for benzoic acid, which is likely to form deprotonated molecules in negative ion mode. All compounds formed M+. as the main ion in both direct APPI and APLI, except for isophorone, which formed a fragment at m/z 82 (and [M + H]+) with APPI, and [M + H]+ (with very low intensity) with APLI. The PA of isophorone is considerably high (893.5 kJ/mol), and since also the IE is high (9.07 eV), proton transfer is likely to be a preferable ionization route for isophorone. In direct APPI and APLI, the proton may originate either from isophorone itself (Table 1, Reaction 3), or other compounds eluting at the same time, similarly to the case of pyridine (see above).
In DA-APPI, the addition of toluene and chlorobenzene dopants had a significant effect on the ionization of most of the O-containing compounds, showing an up to 20-fold increase in the abundance of the M+. ion. In DA-APLI, the difference was not as pronounced, although for most compounds the signal was generally doubled by the addition of dopant. The only exception to the rule was isophorone, for which approximately 250 times higher signal was observed with toluene-assisted APLI than with direct APLI. This is probably due to the very low efficiency of the 2-photon REMPI process for the aliphatic isophorone. Also in the presence of dopants, the O-containing compounds formed mostly M+. ions (Table 1, Reaction 8), except with acetone dopant in APPI, where [M + H]+ was observed for isophorone and two of the methylphenols (Table 1, Reaction 10).
Conclusions
The number of EPA compounds ionized in direct APPI was significantly higher than in their number direct APLI. This is explained by lower sum energy available for ionization in APLI as well as unfavorable excited state properties of many of the analytes. This is in full accord with the remarkable selectivity of direct APLI for large aromatic systems, such as PAHs. The introduction of dopants decreased the number of ionized compounds in DA-APPI and increased it in DA-APLI. The ionization in both cases was observed to proceed through dopant-mediated reactions, whereas direct photoionization and REMPI were quenched. The compounds that were ionized in DA-APPI and DA-APLI were nearly the same, indicating that the ionization in dopant-assisted case takes place through the same route, independent of the initial ionization reaction.
Nitro compounds and aliphatic ethers were found to be challenging compounds with both APPI and APLI, with and without dopants. In the future, GC-API-MS in negative ion mode will be studied for e.g., phenols, carboxylic acids, nitro-, and halogenated compounds that have either positive EAs or high gas-phase acidities. In APLI, ionization by high intensity or faster (femtosecond or picosecond) pulse laser will be investigated, and is expected to increase the ionization efficiency as well as the number of compounds that can be ionized in direct APLI.
References
Robb, D.B., Covey, T.R., Bruins, A.P.: Atmospheric pressure photoionization: an ionization method for liquid chromatography-mass spectrometry. Anal. Chem. 72, 3653–3659 (2000)
Syage, J.A., Evans, M.D., Hanold, K.A.: Photoionization mass spectrometry. Am. Lab. 32, 24–29 (2000)
Raffaelli, A., Saba, A.: Atmospheric pressure photoionization mass spectrometry. Mass Spectrom. Rev. 22, 318–331 (2003)
Marchi, I., Rudaz, S., Veuthey, J.-L.: Atmospheric pressure photoionization for coupling liquid-chromatography to mass spectrometry: a review. Talanta 78, 1–18 (2009)
Revelsky, I.A., Yashin, Y.S., Sobolevsky, T.G., Revelsky, A.I., Miller, B., Oriedo, V.: Electron ionization and atmospheric pressure photochemical ionization in gas chromatography-mass spectrometry analysis of amino acids. J. Mass Spectrom. 9, 497–507 (2003)
McEwen, C.N.: GC/MS on an LC/MS instrument using atmospheric pressure photoionization. Int. J. Mass Spectrom. 259, 57–64 (2007)
Haapala, M., Luosujärvi, L., Saarela, V., Kotiaho, T., Ketola, R.A., Franssila, S., Kostiainen, R.: Microchip for combining gas chromatography or capillary liquid chromatography with atmospheric pressure photoionization-mass spectrometry. Anal. Chem. 79, 4994–4999 (2007)
Luosujärvi, L., Karikko, M.-M., Haapala, M., Saarela, V., Huhtala, S., Franssila, S., Kostiainen, R., Kotiaho, T., Kauppila, T.J.: Gas chromatography-mass spectrometry of polychlorinated biphenyls using atmospheric pressure chemical ionization and atmospheric pressure photoionization microchips. Rapid Commun. Mass Spectrom. 22, 425–431 (2008)
Hintikka, L., Haapala, M., Franssila, S., Kuuranne, T., Leinonen, A., Kostiainen, R.: Feasibility of gas chromatography-microchip atmospheric pressure photoionization-mass spectrometry in analysis of anabolic steroids. J. Chromatogr. A 217, 8290–8297 (2010)
Kersten, H., Derpmann, V., Barnes, I., Brockmann, K.J., O’Brien, R., Benter, T.: A novel APPI-MS setup for in situ degradation product studies of atmospherically relevant compounds: capillary atmospheric pressure photo ionization (cAPPI). J. Am. Soc. Mass Spectrom. 22, 2070–2081 (2011)
Revelsky, I.A., Yashin, Y.S.: New approach to complex organic compounds mixtures analysis based on gas chromatography-atmospheric pressure photoionization-mass spectrometry. Talanta 102, 110–113 (2012)
Haapala, M., Suominen, T., Kostiainen, R.: Capillary photoionization: a high sensitivity ionization method for mass spectrometry. Anal. Chem. 85, 5715–5719 (2013)
Constapel, M., Schellentraeger, M., Schmitz, O.J., Gaeb, S., Brockmann, K.J., Giese, R., Benter, T.: Atmospheric-pressure laser ionization: a novel ionization method for liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 19, 326–336 (2005)
Stader, C., Beer, F.T., Achten, C.: Environmental PAH analysis by gas chromatography-atmospheric pressure laser ionization-time-of-flight-mass spectrometry (GC-APLI-MS). Anal. Bioanal. Chem. 405, 7041–7052 (2013)
Kauppila, T.J., Kersten, H., Benter, T.: Comparison of the ionization mechanisms in direct and dopant-assisted atmospheric pressure photoionization and atmospheric pressure laser ionization. J. Am. Soc. Mass Spectrom. 25, 1870–1881 (2014)
Peterson, A.C., Kersten, H., Krumwiede, D., Quarmby, S., D’Silva, K., Kroll, K., Haberer, K., Bromirski, M., Makarov, A., Benter, T.: Analytical performance of a novel, dopant-free GC-APPI source with femtogram-level sensitivity for quadrupole-Orbitrap GC/MS. Proceedings of the 62nd ASMS Conference on Mass Spectrometry and Allied Topics, Baltimore, MD (June 15–19, 2014)
Peterson, A.C., Hauschild, J.-P., Quarmby, S.T., Krumwiede, D., Lange, O., Lemke, R.A.S., Grosse-Coosmann, F., Horning, S., Donohue, T.J., Westphall, M.S., Coon, J.J., Griep-Raming, J.: Development of a GC/quadrupole-orbitrap mass spectrometer, Part I: design and characterization. Anal. Chem. 86, 10036–10043 (2014)
Klee, S., Albrecht, S., Derpmann, V., Kersten, H., Benter, T.: Generation of ion-bound solvent clusters as reactant ions in dopant-assisted APPI and APLI. Anal. Bioanal. Chem. 405, 6933–6951 (2013)
P.J. Linstrom, W.G. Mallard, Eds., NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg MD, 20899, http://webbook.nist.gov, (retrieved September 9, 2014).
Method 8270C semivolatile organic compounds by gas chromatography/mass spectrometry (GC/MS), www.caslab.com/EPA-MEthods/PDF/8270c.pdf, Dec-1996 (retrieved September 9, 2014)
Kolakowski, B.M., Grossert, J.S., Ramaley, L.: The importance of both charge exchange and proton transfer in the analysis of polycyclic aromatic compounds using atmospheric pressure chemical ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 15, 301–310 (2004)
Uchimura, T., Hafner, K., Zimmermann, R., Imasaka, T.: Multiphoton ionization mass spectrometry of chlorophenols as indicators for dioxins. Appl. Spectrosc. 57, 461–465 (2003)
Haefliger, O.P., Zenobi, R.: Laser mass spectrometric analysis of polycyclic aromatic hydrocarbons with wide wavelength range laser multiphoton ionization spectroscopy. Anal. Chem. 70, 2660–2665 (1998)
Mühlberger, F., Hafner, K., Kaesdorf, S., Ferge, T., Zimmermann, R.: Comprehensive on-line characterization of complex gas mixtures by quasi-simultaneous resonance-enhanced multiphoton ionization, vacuum-UV single-photon ionization, and electron impact ionization in a time-of-flight mass spectrometer: setup and instrument characterization. Anal. Chem. 76, 6753–6764 (2004)
Schiewek, R., Schellenträger, M., Mönnikes, R., Lorenz, M., Giese, R., Brockmann, K.J., Gäb, S., Benter, T., Schmitz, O.J.: Ultrasensitive determination of polycyclic aromatic compounds with atmospheric-pressure laser ionization as an interface for GC/MS. Anal. Chem. 79, 4135–4140 (2007)
Samanta, A., Devadoss, C., Fessenden, R.: Picosecond time-resolved absorption and emission studies of the singlet excited-states of acenaphthylene. J. Phys. Chem. 94, 7106–7110 (1990)
Zimmermann, R., Lenoir, D., Kettrup, A., Nagel, H., Boesl, U.: On-line emission control of combustion processes by laser-induced resonance-enhanced multi-photon ionization/mass spectrometry. Pittsburgh, Combustion Institute, 2859–2868 (1996)
Crable, G.F., Kearns, G.L.: Effect of substituent groups on the ionization potentials of benzenes. J. Phys. Chem. 66, 436–439 (1962)
Boesl, U.: Laser mass spectrometry for environmental and industrial chemical trace analysis. J. Mass Spectrom. 35, 289–304 (2000)
Guo, X., Bruins, A.P., Covey, T.R.: Characterization of typical chemical background interferences in atmospheric pressure ionization liquid chromatography-mass spectrometry. Rapid Commun. Mass Spectrom. 20, 3145–3150 (2006)
Acknowledgments
Financial support by the Academy of Finland (projects 218150, 255559, and 268757), Magnus Ehrnrooth Foundation and iGenTrax UG (Haan, Germany), is gratefully acknowledged. Thermo Scientific (Bremen, Germany), is acknowledged for supplying the Orbitrap, the GC and the consumables, and Morpho Detection (Santa Ana, CA, USA) for supplying the APPI source. Dr. Amelia Peterson from Thermo Scientific is acknowledged for advice concerning the elution order and identification of the analytes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kauppila, T.J., Kersten, H. & Benter, T. Ionization of EPA Contaminants in Direct and Dopant-Assisted Atmospheric Pressure Photoionization and Atmospheric Pressure Laser Ionization. J. Am. Soc. Mass Spectrom. 26, 1036–1045 (2015). https://doi.org/10.1007/s13361-015-1092-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-015-1092-3