Abstract
The nematode Ditylenchus destructor Thorne (Tylenchida: Anguinidae) is a serious garlic pathogen in Japan; cloves are infested by the nematode rot during storage, and there is no method that will completely eliminate the nematode from the clove. In the present study, we examined the survival of D. destructor under hypoxic and anoxic conditions to assess the use of controlled atmospheres as control strategies. Under hypoxic condition at 25 °C, the survival rate of D. destructor at 5 weeks was 60%, whereas the majority of Caenorhabditis elegans Maupas (Rhabditida: Rhabditidae) died in a week. Under anoxic condition at 25 °C, the survival rate of D. destructor at 2 weeks was approximately 80%, whereas that of Meloidogyne incognita (Kofoid and White) Chitwood (Tylenchida: Meloidogynidae) was 30% at the same time point. Under hypoxic and anoxic conditions at 35 °C, the survival rate of D. destructor dramatically decreased: most died within 14 and 7 days, respectively. These results suggest that D. destructor has a high tolerance to hypoxia/anoxia, but a combination of low oxygen with heat stress can cause high mortality in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ditylenchus destructor Thorne (Tylenchida: Anguinidae) causes serious damage to a wide variety of crops, such as potato, sweet potato, carrot, iris, and garlic, worldwide (EPPO 2017). In Japan, it is one of the main garlic pathogens that cause substantial damage in major garlic production areas such as Aomori and Hokkaido. The D. destructor rot infested the garlic clove during storage, causing garlic yield loss (Fujimura et al. 1986). Although several control methods can be applied, eradicating the nematode from fields and cloves remains difficult.
At the early stage of infestation, a distinction with the appearance of garlic cloves infested by D. destructor from healthy cloves, is difficult. Thus, nematode-infested garlic cloves are mixed with healthy seed garlic cloves and are used for planting, resulting in the spread of D. destructor infestation in fields. Recently, a method that can detect nematode-infested cloves using specific odors has been studied (Matsumoto et al. 2020). However, other methods for detecting nematode-infested cloves or eliminating nematodes from cloves have yet to be established.
Oxygen is critical for the survival of most of the multicellular organisms; thus, hypoxia and/or anoxia can be lethal stressors. Controlled atmospheres, e.g., controlled atmospheres with depleted O2 or elevated CO2, are environmentally friendly alternative fumigation methods for the control of pests such as stored grain insect pests (Banks and Annis 1990; Cao et al. 2019; Navarro 2012). However, the data on the survival of nematodes under hypoxic/anoxic conditions (Föll et al. 1999; Kitazume et al. 2018; Qiu and Bedding 2000) and controlled atmospheres (Van Kruistum et al. 2012, 2015) are limited. Therefore, the present study aimed at examining hypoxia/anoxia tolerance in D. destructor and obtaining data on the potential control of this nematode via controlled atmospheres.
Materials and methods
Nematodes
Ditylenchus destructor (strain: HK1) originally isolated from garlic was cultured using a fungus (Haraguchi and Yoshiga 2020). Briefly, nematodes were inoculated onto a fungal mat of Botrytis cinerea growing on autoclaved barley grain medium (10 g of barley grains and 10 g of distilled water) in a 100-ml medium bottle. After propagation, nematodes were recovered from the bottle using the Baermann funnel method (Hooper 1990).
The bacterial-feeding nematode Caenorhabditis elegans Maupas (Rhabditida: Rhabditidae) was used for comparison with D. destructor because it is a popular model organism and has been used as a model to study survival under a range of oxygen levels (Padilla et al. 2012; Van Voorhies et al. 2000). This nematode was obtained from Caenorhabditis Genetics Center (University of Minnesota, St. Paul, MN, USA) and maintained on nematode growth medium plates (1.7% agar) seeded with Escherichia coli strain OP50 (Stiernagle 2006) at 20 °C. Mixed stages of the nematode were used for experiments.
The sedentary plant parasitic nematode Meloidogyne incognita (Kofoid and White) Chitwood (Tylenchida: Meloidogynidae), which is an endoparasite to a wide variety of crops and one of the most serious plant-parasitic nematodes worldwide, was also used for comparison. It was maintained on tomato (Solanum lycopersicum; variety: Chibikko; Marutane Co., Ltd., Kyoto, Japan), and second-stage juveniles (J2s) were obtained using a hydroponic culture system (Yoshiga and Umezaki 2016). Freshly hatched J2s were used for experiments.
Survival under hypoxic and anoxic conditions
Hypoxia experiments were conducted using mixed stages of D. destructor and C. elegans. An aliquot of 500 µl of nematode suspension containing approximately 70 individuals was added to a 12-ml glass test tube with a screw cap (NR-10; Maruemu Corporation, Osaka, Japan), and CO2 gas was introduced into the nematode suspension for 3 min through a Pasteur pipette. The O2 concentration under this condition was 0.3%, as measured by a fiber-optic oxygen transmitter system with an oxygen dipping probe (Fibox 3: PreSens; Precision Sensing GmbH, Regensburg, Germany). It was the lowest O2 concentration achieved by this method. Immediately after the introduction of CO2 for 3 min, the tube was closed with a cap and maintained at 25 °C in the dark. As a control, normoxic condition (21% O2 concentration) was prepared as described above without the introduction of CO2 gas. After incubation, the test tube was opened to air, the nematode suspension was mixed, and it was left overnight. The next day, 500 µl of distilled water was added to the tube, and the nematode suspension was transferred to a Syracuse watch glass; nematode survival was examined via the addition of 50 µl of 1-N NaOH (Harada and Yoshiga 2015). Moving and stationary nematodes were counted as live and dead, respectively. Death was confirmed by touching the nematode with a pin. In the case of C. elegans, only adults were counted because offspring was produced during the treatment. Survival data were calculated as follows: survival (%) = (number of live nematodes / total number of nematodes on a plate) × 100.
To examine nematode survival under the anoxic condition, mixed stages of D. destructor and J2s of M. incognita were used. An aliquot of 20-µl nematode suspension containing approximately 60 individual nematodes and antibiotics (penicillin–streptomycin–neomycin antibiotic mixture; Gibco, Thermo Fisher Scientific, Massachusetts, USA; used to suppress microbial propagation) was placed onto the center of a 6-cm Petri dish containing 1% water agar. The anoxic condition was set by placing plates with nematodes in an AnaeroPack W pouch (A-65; Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan) together with AnaeroPack-Anaero (A-13), which generates CO2 while simultaneously producing an anaerobic atmosphere. The O2 concentration was < 0.1% within 2 h, which was confirmed using an O2 indicator (Anaero-Indicator, A-66). Control (normoxic condition) was prepared without AnaeroPack-Anaero. After placing the plates in the pouch, the latter was maintained in an incubator at 25 °C in the dark. At 3, 7, and 14 days after setting the anoxic condition, the pouch was opened to air. The next day, the survival of the nematodes was examined under a dissecting microscope (Olympus SZX16, Tokyo, Japan). To evaluate heat stress under lowered oxygen concentrations, survival at 35 °C was also examined for D. destructor at 1, 3, 5, 7, and 14 days after setting the anoxic condition. A temperature of 35 °C was chosen because garlic is usually heat-dried at 35 °C for 2 weeks after harvest to dry the skin and prevent microbial infestation; moreover, this temperature is the maximum for garlic heat tolerance. Droplet-like structures observed in nematodes subject to anoxic conditions were stained by Oil Red O (Katamaya Chemical Industries, Osaka, Japan) as described by William and Marc (1995) to confirm the presence of lipids in the structure.
Statistical analysis
All analyses were performed using R version 4.1.1 (R Development Core Team 2021). We employed a generalized linear model (GLM) with binomial distribution and logit link function and evaluated oxygen condition, time, and their interactions on nematode survival. To compare survival between D. destructor and M. incognita, species was included as a factor in the model. After GLM was created, statistical significance of models was assessed using type II ANOVA from car package (Fox et al. 2009).
Results
Nematode survival under hypoxic condition
Soon after hypoxic condition exposure, D. destructor and C. elegans stopped moving. There were significant interaction effects between the treatment and time in both nematodes (GLM; D. destructor: χ2 = 24.4, p = 0.001; C. elegans: χ2 = 24.4, p < 0.05, Table 1). The survival of D. destructor under hypoxic condition remained high (> 80%) for 3 weeks and then gradually began to decrease thereafter (Fig. 1a). On the other hand, the survival of C. elegans dramatically decreased one day after hypoxic condition exposure, and all nematodes died by day 4 (Fig. 1b). Internal hatching in C. elegans adults was often observed under normoxic condition, which resulted in high mortality.
Nematode survival under anoxic condition
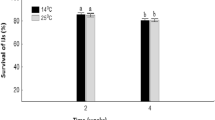
Soon after anoxic condition exposure, nematodes stopped moving. There was no significant three-dimensional interaction effect among the species, the treatment and time (GLM; χ2 = 1.24, p = 0.26, Table 2). However, there were significant interaction effects between species and treatment (χ2 = 5.86, p < 0.05), and species and days (χ2 = 24.4, p < 0.01), except treatment and time (χ2 = 1.07, p = 0.30, Table 2). The survival of D. destructor remained high even 14 days after anoxic conditions at 25 °C were begun (Fig. 2). The survival of M. incognita, however, gradually decreased under the same conditions; the survival rate at 14 days was approximately 30%.
Effect of heat stress on survival under hypoxic and anoxic conditions
There were significant interaction effects between the treatment and time at 35 °C under hypoxic and anoxic conditions (GLM; hypoxia: χ2 = 111.31, p < 0.001; anoxia: χ2 = 579.0, p < 0.001, Table 3). When nematodes were exposed to the hypoxic condition at 35 °C, the survival of D. destructor gradually decreased, and only 23% and 2% of nematodes survived at 7 and 14 days after treatment initiation, respectively (Fig. 3a). Under anoxic condition at 35 °C, nematode survival decreased more rapidly, reaching 0% survival at 7 days after treatment initiation (Fig. 3b). Droplet-like structures were observed inside most nematodes exposed to anoxic conditions at 35 °C for > 7 days. Oil Red O staining indicates the presence of lipids in the structure (Fig. 4).
Discussion
In the present study, we examined hypoxia/anoxia tolerance in D. destructor to understand the survival of nematodes under low-oxygen conditions, and to establish new D. destructor control methods for the protection of garlic cloves under controlled atmospheres. CO2 treatment is often used to control insect pests, and exposure to > 60% CO2 typically kills insects in a couple of days (Cao et al. 2019). In the present study, however, the survival of D. destructor was kept high even in a tube filled with CO2 gas (0.3% O2 concentration) at least for 3 weeks. In addition, the majority of D. destructor individuals and approximately 30% of M. incognita individuals survived even when subjected to the anoxic condition for > 14 days at 25 °C. In contrast, most C. elegans individuals died in 2 days under hypoxic conditions. These results suggest that the plant-parasitic nematodes D. destructor and M. incognita have a higher tolerance to hypoxia/anoxia than do C. elegans and insects; D. destructor, in particular, has strong hypoxia/anoxia tolerance. Soil nematodes are often subjected to flooding and, therefore, hypoxic conditions after heavy rain; these nematodes cannot escape because of their limited mobility. Thus, it is possible that the nematodes have developed the ability to survive under hypoxic/anoxic conditions. The differences in hypoxia/anoxia tolerance among nematodes are not well studied but could be related to their life histories and survival strategies. Further studies of different types of nematodes are needed to understand the difference.
Although the survival of D. destructor was high at 25 °C under anoxic condition, anoxia at 35 °C caused high mortality in this species. Because 35 °C is not a lethal temperature for this nematode at least in 14-days exposure under normoxic condition, mortality seems to have been elevated when this temperature was combined with anoxia. Some animal parasitic nematodes, such as adults of the pig roundworm Ascaris suum Goeze (Ascaridida: Ascarididae), which live under low-oxygen conditions in the small intestine of pigs, obtain their energy by anaerobic metabolism (Behm 2002). An entomopathogenic nematode Steinernema carpocapsae (Weiser) (Rhabditida: Steinernematidae) utilizes glycogen to survive hypoxic conditions (Qiu and Bedding 2000). Similarly, D. destructor might survive under hypoxic/anoxic conditions using glycogen as a survival resource. In the present study, the higher temperature might have stimulated the metabolism of D. destructor and exhausted the energy resources necessary for anaerobic metabolism, such as glycogen. A combination of anaerobic and heat stresses might also have interrupted tissue homeostasis, resulting in the damage of tissue and the formation of lipid droplet-like structures in the nematodes. Similar structures are often observed in nematodes after recovering from desiccation stress (Otsubo et al. 2006). Further study will be necessary to understand anoxybiosis in D. destructor and the effect of temperature on anoxia.
The effectiveness of controlled atmospheres combined with higher temperatures has been studied for the control of various insect pests (reviewed by Cao et al. 2019). In nematodes, however, controlled atmosphere/temperature treatments have only been studied for Meloidogyne hapla Chitwood (Tylenchida: Meloidogynidae) and Pratylenchus penetrans (Cobb) Filipjev and Schuurmans Stekhoven (Tylenchida: Pratylenchidae) in strawberry planting stock (Van Kruistum et al. 2012, 2015). In the present study, for the first time, we demonstrated that hypoxia and anoxia conditions at 35 °C increase mortality in D. destructor. After harvest, garlic bulbs are usually heat-dried at 35 °C for 2 weeks, which is an effective method to kill fungi and nematodes (Fujimura et al. 1989). Higher temperatures are more effective for killing D. destructor, but temperatures > 35 °C cause heat damage to garlic; thus, 35 °C is the maximum treatment temperature for garlic. Together with our results, this information suggests that nematodes, such as D. destructor, that infest cloves and bulbs of garlics can potentially be controlled by the combination of anoxic/hypoxic conditions and higher temperatures, i.e., 35 °C. Additional studies, including the development of equipment that can achieve appropriate conditions for garlic, and effects of stresses such as low-oxygen, heat and desiccation on survival of the nematode in garlic bulb are needed before our novel control method of D. destructor can be applied in garlic production.
References
Banks HJ, Annis PC (1990) Comparative advantages of high CO2 and low O2 types of controlled atmospheres for grain storage. In: Calderon M, Barkai-Golan R (eds) Food preservation by modified atmospheres. CRC Press, Florida, pp 93–122
Behm CA (2002) Metabolism. In: Lee DL (ed) The biology of nematodes. Taylor & Francis, London, pp 261–290
Cao Y, Xu K, Zhu X, Bai Y, Yang W, Li C (2019) Role of modified atmosphere in pest control and mechanism of its effect on insects. Front Physiol 10:206. https://doi.org/10.3389/fphys.2019.00206
EPPO (2017) Ditylenchus destructor and Ditylenchus dipsaci. Bull OEPP/EPPO Bull 47:401–419. https://doi.org/10.1111/epp.12433
Fujimura T, Washio S, Nishizawa T (1986) Garlic as a new host of the potato-rot nematode, Ditylenchus destructor Thorne. Jpn J Nematol 16:38–47
Fujimura T, Ichita T, Kimura T (1989) Occurrence of potato-rot nematode, Ditylenchus destructor Thorne, in garlic and control. 1. Evaluation of treatments applied before planting and after harvest for control. Jpn J Nematol 18:22–29
Föll RL, Pleyers A, Lewandovski GJ, Wermter C, Hegemann V, Paul RJ (1999) Anaerobiosis in the nematode Caenorhabditis elegans. Comp Biochem Physiol B 124:269–280. https://doi.org/10.1016/S0305-0491(99)00130-3
Fox J, Bates D, Firth D, Friendly M, Gorjanc G, Graves S, Heiberger R, Monette G, Nilsson H, Ogle D, et al. (2009) CAR: companion to applied regression, R Package version 1.2-16
Harada Y, Yoshiga T (2015) Distinguishing between inactivated and dead second stage juveniles of Meloidogyne incognita using the NaOH method. Nematol Res 45:51–55. https://doi.org/10.3725/jjn.45.51
Haraguchi S, Yoshiga T (2020) Potential of the fungal feeding nematode Aphelenchus avenae to control fungi T and the plant parasitic nematode Ditylenchus destructor associated with garlic. Biol Control 143:104203. https://doi.org/10.1016/j.biocontrol.2020.104203
Hooper DJ (1990) Extraction and processing of plant and soil nematodes. In: Luc M, Sikora RA, Bridge J (eds) Plant parasitic nematodes in subtropical and tropical agriculture. CAB International, Wallingford, Oxon, UK, pp 45–68
Kitazume H, Dayi M, Tanaka R, Kikuchi T (2018) Assessment of the behaviour and survival of nematodes under low oxygen concentrations. PLoS ONE 13:e0197122. https://doi.org/10.1371/journal.pone.0197122
Matsumoto M, Ueno D, Aoyama R, Sato K, Koga Y, Higuchi T, Matsumoto H, Nishimuta K, Haraguchi S, Miyamoto H, Haraguchi T, Yoshiga T (2020) Novel analytical approach to find distinctive odor compounds from garlic cloves infested by the potato-rot nematode Ditylenchus destructor using gas chromatography–olfactometry (GC–O) with heart-cut enrichment system. J Plant Dis Protect 127:537–544. https://doi.org/10.1007/s41348-020-00349-3
Navarro S (2012) The use of modified and controlled atmospheres for the disinfestation of stored products. J Pest Sci 85:301–322. https://doi.org/10.1007/s10340-012-0424-3
Qiu L, Bedding R (2000) Energy metabolism and survival of the infective juveniles of Steinernema carpocapsae under oxygen-deficient conditions. J Nematol 32:271–280
Otsubo R, Yoshiga T, Kondo E, Ishibashi N (2006) Coiling is not essential to anhydrobiosis by Aphelenchus avenae on agar amended with sucrose. J Nematol 38:41–45
Padilla PA, Goy JM, Hajeri VA (2012) Anoxia-induced suspended animation in Caenorhabditis elegans. In: Padilla P (ed) Anoxia. Europe, InTech, pp 25–58
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Stiernagle T (2006) Maintenance of C. elegans. In: The C. elegans Research Community (ed) WormBook. https://doi.org/10.1895/wormbook.1.101.1
Van Kruistum G, Hoek H, Verschoor J, Molendijk L (2012) Controlled atmosphere temperature treatment as sustainable alternative to control strawberry tarsonemid mites and plant parasitic nematodes in strawberry plants. Acta Hortic 926:601–608
Van Kruistum G, Evenhuis A, Hoek J, Kastelein P, van der Wolf JM, Verschoor JA (2015) CATT: a new and non-chemical pest and nematode control method in strawberry planting stock. Acta Hortic 1105:189–196
Van Voorhies WA, Ward S (2000) Broad oxygen tolerance in the nematode Caenorhabditis elegans. J Exp Biol 203:2467–2478. https://doi.org/10.1242/jeb.203.16.2467
William TS, Marc JL (1995) A rapid and simple method for staining lipid in fixed nematodes. J Nematol 27:244–248
Yoshiga T, Umezaki U (2016) A simple and small-scale hydroponic culture system to prepare second-stage juveniles of the root-knot nematode. Appl Entomol Zool 51:151–154. https://doi.org/10.1007/s13355-015-0365-4
Acknowledgements
The authors wish to thank the staff of the Vegetable Research Institute, Aomori Prefectural Industrial Technology Research Center, Aomori, Japan, for providing the opportunity to begin this study and for their support. We also thank the Caenorhabditis Genetics Center for providing C. elegans.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sugita, Y., Sobagaki, T. & Yoshiga, T. Low-oxygen tolerance of Ditylenchus destructor (Tylenchida: Anguinidae). Appl Entomol Zool 57, 131–136 (2022). https://doi.org/10.1007/s13355-021-00769-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-021-00769-z