Abstract
The potato-rot nematode Ditylenchus destructor (Tylenchida: Anguinidae) causes severe damage to garlic production in Japan. Nematode-contaminated garlic seed cloves are often used for planting, because nematode-infested cloves are difficult to discriminate from healthy ones. To be able to find nematode-infested garlic in a nondestructive way, here this study introduced a novel analytical system of GC–O and GC–MS with heart-cut enrichment technique. The objective of this research was to find an analytical approach to identify the existence of subjectively perceptible odors which derive from the garlic infested with the nematode and to evaluate the effectiveness of those novel analytical systems. Ten odor activities associated with nematode-infested garlic were detected by the system, and “allyl methyl sulfide” and “2-methyl-1-butanol” were identified as major odor compounds (1-propanethiol was candidate). This result suggests that the novel analytical technique introduced in this study delivered sufficient data to identify the odor compounds from infested garlics. Two distinctive odor compounds might be used as indicators to detect nematode-infested garlic cloves with nondestructive way.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The potato-rot nematode Ditylenchus destructor (Tylenchida: Anguinidae) causes serious damage to garlic (Allium sativum) production in the Aomori Prefecture, a major garlic production area in Japan. Since the first discovery of damage by this nematode in 1984 (Fujimura et al. 1986), fields infested with D. destructor have been spreading in Aomori and other areas in Japan. It was reported that the seed garlic planted in fields infested with D. destructor is facing an infestation of 100% in the future (Fujimura et al. 1986; Kitano and Yamashita 2011). Garlic plants heavily infested by the nematode wither and die. Lightly infested plants can grow but sometimes show yellowish leaves and nematodes reproduced in those cloves. After harvest, nematodes propagate in the cloves, and some nematode-infested cloves go rotten during storage. Because the appearance of a recently infested garlic bulb or clove is the same as a healthy one, nematode-contaminated garlic cloves are inadvertently used as seed garlic, which causes spreading of nematode-infested fields and increases yield loss. There is no established method that completely kills the nematode in the garlic cloves: although chemical fumigation using chloropicrin effectively lowers the nematode density in an infested field (Kitano and Yamashita 2011); it is not efficient enough and harmful to the environment. These problems make control of D. destructor on garlic difficult, and a procedure to discriminate infested from non-infested garlic is necessary.
It is empirically known among garlic farmers that nematode-infested garlic has distinctive odors. If these odors can be used to distinguish nematode-infested cloves from healthy ones, only healthy garlic could be used as a seed garlic. In addition, the quality of garlic not only for propagation but also for food could be guaranteed. However, there is no information available on relationship between odors compounds and nematode-infested garlic.
A gas chromatography–olfactometry (GC–O) technique, which couples traditional gas chromatographic analysis with sensory detection, has been used previously to study and identify odor compounds in complex mixtures (Brattoli et al. 2013). In this technique, panelists (sensory evaluators) sit at the olfactometry outlet (sniffing port) and record what they smell in the humid air stream (Friedrich and Acree 1998). GC–O in combination with GC mass spectrometry (GC–MS) not only enables the olfactory evaluation of odor compounds, but also their identification with mass spectral information (Brattoli et al. 2013). Thus, we considered that the use of GC–O and GC–MS with heart-cut enrichment technique in combination could be useful for detecting and identifying odors specific for nematode-infested garlic.
To be able to detect nematode-infested garlic in a nondestructive way, here this study introduced a novel analytical system of GC–O and GC–MS with heart-cut enrichment technique. The objective of this research was to find an analytical approach to identify the existence of subjectively perceptible odors which derive from nematode-infested garlic and to evaluate the effectiveness of the novel analytical system.
Materials and methods
Samples
To obtain D. destructor-infested and non-infested garlic bulbs, the garlic cultivar “Fukuhci White” was grown conventionally in D. destructor-infested and non-infested areas, respectively, in a field of Aomori Prefectural Industrial Technology Research Center under conventional conditions in 2016–2017. The cultivar Fukuchi White is the standard garlic cultivar and has the largest areal distribution in Japan. Just after harvesting in August 2017, raw garlic bulbs were sent to Saga University at 4 °C. After dissecting two garlic bulbs (infested and non-infested) into cloves and removal of protective leaves within a week after harvesting, the base of each garlic clove was carefully observed under a dissecting microscope to confirm the presence or absence of nematodes [Fig. 1(A) c, d, e, f]. Three garlic cloves in the early nematode-infested stage confirmed by visual observation [Fig. 1(B) a, b] were used as “infested” for analyses. As a control, three garlic cloves in nematode free healthy condition were used as “non-infested.”
A Appearance of garlic. Nematode-infested (a, c, e) and non-infested (b, d, f) fresh garlic bulbs and cloves used for experiments. Nematodes (black arrow) and cavities (blue arrow) were observed. B Nematode-infested garlic cloves. Two months after harvest (partial rot: g, h) and four months later (total rot: i, j). Bar = 1 cm
Nematodes were extracted from the garlic cloves by the Baermann method using nematode-extraction equipment (Fujiwara Scientific Co., Ltd., Tokyo, Japan) soon after odor collection and mincing of garlic cloves. The extracted nematodes were confirmed to be D. destructor under the microscope, and the number of nematodes was counted.
Visual and sensory evaluation
Panelists for olfactory analysis were three healthy, non-smoker volunteers (1 male aged 43 years and 2 females aged 22 and 24 years) from Saga University, Japan. Prior to participation in the experiments, panelists were examined their olfactory functions (e.g., anosmia and hyposmia) with authentic standard odor compounds of “T&T Olfactometer” (Daiichi Yakuhin Sangyo Co., Ltd, Tokyo, Japan), following the method of “Olfactory Measurement Operator” in “Offensive Odor Control Law” under the jurisdiction of the Ministry of the Environment, Japan (MOE 1971). In addition, panelists were continually trained to distinguish 80 reference smells which were selected to cover a wide range of aroma with “Olfactory training kit” (Daiichi Yakuhin Sangyo Co., Ltd, Tokyo, Japan).
For sensory analysis, each garlic clove without protective leaves was individually placed in a 100-mL glass culture bottle that had previously been heated to 200 °C for 12 h and confirmed to have no odor by panelists. Each bottle was covered with a glass Petri dish and kept at room temperature (25 °C). Three nematode-infested garlic cloves and three nematode non-infested garlic cloves were used for sensory evaluation. The appearance, odor character, and odor intensity of the samples were sensory evaluated. Odor character and intensity were evaluated with direct sniffing by the three selected panelists on 0, 1, and 2 weeks after the start of incubation. Odor intensity was described as rating scale below: 0 = no odor; 1 = weak; 2 = moderate; 3 = strong; and 4 = very strong, and average values of three panelists were used. Odor characters that three panelists showed similar description were used as “odor character”.
Olfactory detection by GC–O analysis
Glass culture bottles (150 mL) were heated to 200 °C for 12 h and confirmed to have no odor by panelists. Protective leaves were removed, and D. destructor-infested or non-infested cloves were individually placed in the glass bottles. The bottles containing garlic cloves were settled for 1 h at room temperature (25 °C) to allow the odor compounds to equilibrate. Volatile organic compounds (VOCs) including various odor compounds from garlic samples were collected using MonoTrap (RGC18 TD; GL Sciences Co., Ltd, Tokyo, Japan), which is a graphite carbon containing solid phase extraction device designed for a thermal desorption system. Three units of MonoTrap were placed into each bottle and incubated for 1 h, and then removed from the bottle and immediately placed in an adsorption tube with a tight cap (GL Sciences Co., Ltd, Tokyo, Japan). As an internal standard, 10 ng of toluene-d8 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was spiked onto the MonoTrap prior to GC analysis.
The odor compounds collected from garlic were sensory analyzed using a GC–O system consisting of a sniffing port (Olfactory Detector Port OP275; GL Sciences Co., Ltd, Tokyo, Japan) with a voice recording system (Olfactory Voicegram; GL Sciences Co., Ltd, Tokyo, Japan). The GC–O system used a 6890 series GC (Agilent Technology, CA, USA) equipped with a flame ionization detector (FID). The whole system for the sensory analysis with olfaction is termed GC–O/FID. The odor compounds collected by MonoTrap were introduced into the GC–O/FID using a thermal desorption system (TurboMatrix 650; PerkinElmer, USA). The introduced odor compounds were separated on an Agilent J&W DB-5MS column (60 m length, 0.32 mm internal diameter, 0.5 μm film thickness; Agilent Technology, CA, USA) with the following temperature program: 40 °C for 1 min, temperature rising to 250 °C at a rate of 10 °C/min, and then 250 °C for 20 min. Helium was used as carrier gas, and flow ratio was 2 ml/min. The effluent from the column was split into the FID and the sniffing port at a ratio of 1:9. Sniffing sensory of GC–O analysis was conducted by one trained panelist (female, 22 years old) with multiple trials.
Identification of odor compounds
A GC-fractionation system (VPS-2800; GL Sciences Co., Ltd, Tokyo, Japan) attached to a GC–FID (6890; Agilent Technology, CA, USA) was employed for heart-cut enrichment technique. The GC-fractionation system is “fraction collector” for GC instrument which is used to obtain enough amount of the pure chemicals for their identification. At the end of the GC–FID capillary column, effluent was split into the FID and fractionation system at a ratio of 1:9; the transfer line was kept at 240 °C. The heart-cut was conducted for ± 15 s of retention time on odor activity. The effluent from GC column kept into outlet flow of fraction system was switched into fractionation flow connected into the absorption tube (Tenax-TA packed liner) with the configured heart-cut timing. Three units of MonoTrap were injected into GC-fractionation system with 10 times.
Enriched heart-cut fractions were injected into GC–MS (6890 N/5973 N, GL Sciences Co., Ltd, Tokyo, Japan) to obtain mass spectra of individual odor compounds. Injection was conducted using a portable thermal desorption system (HandyTD TD265; GL Sciences Co., Ltd, Tokyo, Japan). The separation column and GC temperature program were the same as used with the GC–O/FID. Mass spectra were collected in full-scan mode (m/z range 33–350) with an electron impact (EI) mode energy of 70 eV. Chromatograms were processed by chemistation (Agilent Technology, CA, USA) with a peak processing threshold (signal/noise ratio, > 3). The retention time of n-alkanes mixture (C6–C20: GL Sciences Co., Ltd, Tokyo, Japan) injected prior to the sample analysis was used to calculate retention index (RI). The comprehensive aroma chemical databases, AromaOffice (Nishikawa Keisoku, Tokyo, Japan) and AroChemBase (Alpha M.O.S., France), which include names, formulas, molar masses, RIs, and odor characters were used for searching candidates. Then, mass spectra of detected peaks on the GC–MS chromatograms were compared to the NIST Mass Spectral Search Program 2014 (NIST, Washington, DC, USA), and the compounds with high similarity (> 70) were classed as identified. To confirm the identification of those compounds, authentic standards: 1-propanethiol (95%); (±)-2-methyl-1-butanol (98%) (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) and allyl methyl sulfide (Tokyo Chemical Industry Co., LTD, Tokyo, Japan) were purchased from chemical industry. Those were analyzed by GC–O and GC–MS under the same conditions. If RI, odor characters, and mass spectra on the candidate compound were identical to those on the authentic standard, the odor compound was classed as identified compound. If the mass spectra of candidate compound could not be obtained, the odor compound was still classed as “candidate compound.”
Since the two odor chemicals were identified (one was candidate) in this study, the odor of a mixture of three authentic standards (allyl methyl sulfide, 2-methyl-1-butanol, and 1-propanethiol) was olfactory confirmed by comparing with those on infested garlics. A portion of each authentic standard (10 mg) was mixed in a glass culture bottle (150 mL) with glass cap, and it was settled for 1 h at room temperature (27 °C) to allow the odorants to equilibrate. Odor character was evaluated with direct sniffing by the three selected panelists.
Results
Visual and sensory evaluation
Fresh nematode-infested and non-infested garlic bulbs and cloves were used for measurements. Appearance of garlics is shown in Fig. 1. There was no difference in appearance of infested and non-infested fresh bulbs [Fig. 1(A): a, b]. However, many nematodes and cavities caused by the nematodes at the base of the clove were observed in the freshly infested cloves after removal of the protective leaves [Fig. 1(A): c, e], whereas no nematodes or cavities were observed in the non-infested ones [Fig. 1(A): d, f]. By comparing to fresh garlic, nematode-infested garlic cloves 2 months after harvest showed partial rot [Fig. 1(B): g, h] and those of 4 months later showed total rot [Fig. 1(B): i, j].
The scheme of analytical system to identify specific odor compounds from nematode-infested garlic by sensory evaluation, GC–O, and GC–MS with heart-cut enrichment technique is shown in Fig. 2. The results for sensory evaluation by direct sniffing of infested and non-infested garlic samples (n = 3, each group) are summarized in Table 1. The odor from non-infested garlic was described as “vegetable like” or “green”; the odor intensity was 1 (weak) at day 0 and only increased to 2 (moderate) by day 14. On the other hand, the odor from nematode-infested garlic was characterized as “rotten garlic” or “old garlic” throughout the period of the experiment, and the odor intensity increased from 2 to 3 (moderate to strong) at day 0, to 3 to 4 (strong to very strong) at day 14. Equivalent results were obtained among three panelists. The odor characters of nematode-infested garlic were defined as “rotten garlic” or “old garlic,” and those were used on GC–O analysis to find specific odor activities.
The numbers of nematodes in the nematode-infested garlic cloves ranged from 168 to 443 at day 14, whereas no nematodes were detected in non-infested garlic samples (Table 1).
Olfactory detection by GC–O analysis
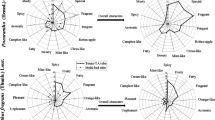
The odor compounds from infested and non-infested garlic were collected with MonoTrap, and GC–O/FID analysis was conducted to specify the distinctive odor compounds following the scheme shown in Fig. 2. Although the GC–O analysis found 16 and 18 odor activities from non-infested and infested garlic samples, respectively, only a few peaks were detected on the GC–FID chromatogram (Fig. 3).
The odor characters detected in non-infested garlic by the GC–O analysis were “garlic,” “vegetable like,” and “grass like,” which were similar to the results of direct sniffing of non-infested garlic samples (i.e., “vegetable like” or “green”). On the other hand, the odor characters detected in infested garlic were not only “rotten garlic” (as detected by direct sniffing) but also “roasted onion,” “mold like,” and “rubber like.” By comparing the odor activities of GC–O analysis between infested and non-infested garlic samples, 10 specific odor activities in infested garlic were found (Fig. 3). Among these, odor numbers 1, 2, and 3, which showed strong intensity and specific odor characters similar to direct sniffing of infested garlic (Fig. 3), were chosen for further experiments.
Identification of odor compounds
MonoTrap-collected garlic odor compounds were directly injected into GC–FID and GC–MS; no peak was detected on the chromatogram at the retention time corresponding to odor numbers 2 or 3. Although a small peak was found for the odor number 1 on the GC–FID chromatogram (Fig. 3), a peak at the retention time was also found on the chromatogram of the control (blank) sample, and it was identified as hexane as laboratory background. Actually, strong odor intensities were detected by GC–O analysis, and the amount of odor compounds appeared to be much lower than the detection limit of GC–FID and GC–MS. We therefore enriched the odor compounds to obtain clear mass spectra in GC–MS analysis. By using the GC-fractionation system, three heart-cut fractions corresponding to odor numbers 1, 2, and 3 were obtained from chromatogram of infested garlic following the scheme in Fig. 2. The three heart-cut fractions were enriched into three separate Tenax-TA packed liners by a 10 times injection of MonoTrap collecting the odor compounds from infested garlic into GC-fractionation system. The three heart-cut fractions enriched into Tenax-TA were injected into GC–MS to obtain mass spectra.
The GC–MS total ion chromatograms of enriched heart-cut fractions are summarized in Fig. 4. As expected, in the chromatograms of the heart-cut fractions 2 and 3, peaks were detected at RIs corresponding to odor numbers 2 and 3, respectively. When the obtained mass spectra from the peaks of odor numbers 2 and 3 were compared with those in the NIST library, several compounds showed high similarity (> 80). Among them, odor compounds were further specified by using the information of odor characters (rotten garlic, roasted onion, and sweet) and RIs in the aroma chemicals databases. As the result of these database searches, odor numbers 2 and 3 were matched with allyl methyl sulfide and 2-methyl-1-butanol as candidates, respectively (Fig. 4). The chromatogram of the enriched heart-cut fraction 1 was overlapped with a hexane background peak, and mass spectra were not obtained. Therefore, only the aroma chemical databases could be used to find this odor compound by using obtained RI and odor characters (rotten garlic). As one candidate in heart-cut fraction 1, 1-propanethiol was matched by this database search.
To confirm those candidates, authentic standards of the above three chemicals were purchased and analyzed by GC–O and GC–MS following the scheme in Fig. 2. The RIs and odor characters analyzed by GC–O, and RIs and mass spectra analyzed by GC–MS in authentic standards were matched with those in odor numbers 2 and 3 in the infested garlic. Thus, the odor numbers 2 and 3 were identified compounds as allyl methyl sulfide and 2-methyl-1-butanol, respectively (Table 2). Although the odor character and RI of odor numbers 1 obtained in GC–O analysis were matched with those in authentic standard of 1-propanethiol, mass spectra of this chemical were not obtained. 1-Propanethiol was still “a candidate compound” of this odor active compound in heart-cut fraction 1 (Table 2). To make them identify accurately, those should be analyzed by using two or more chromatography columns with different stationary phases, such as a wax column.
Since the three odor chemicals were identified (one of them was a candidate), the odor on mixture of three authentic standards was olfactory compared with those on infested garlics by three panelists. As a result of olfactory comparison, the odor of authentic standards mixture was similar to infested garlics, and it was obviously different from that on non-infested garlics.
Discussion
In the present study, we hypothesized that nematode-infested garlic has distinctive odors compared with non-infested garlic. Although strong odor intensity was obtained by olfactory detected using GC–O analysis, it was not possible to detect mass spectra of those substances because their concentration was lower than the sensitivity of GC–MS. Therefore, we used the technique of heart-cutting on GC chromatograms and enrichment of individual odor compounds using the GC-fractionation system. In addition, we employed comprehensive aroma chemical databases, which include chemical names, formulas, RIs, and odor characters to facilitate identification. These series of novel techniques made it possible to identify the distinctive “odor-active substances (odor compounds)” from infested garlic. As the results of those trials, two distinct odor compounds with strong odors were identified as allyl methyl sulfide and 2-methyl-1-butanol (1-propanethiol was candidate: mass spectra could not be obtained). The odor of a mixture of three authentic standards was prepared and olfactory compared with those on infested. It was similar to those with infested, and it was apparently different with non-infested garlics.
Those distinctive odor chemicals identified from infested garlics in this study seem plausible enough because those chemicals have been detected from garlic and related crop in previous reports. Allyl methyl sulfide (odor 2) is the odorant that is strongly detected from black garlic (thermal- and fermentation-processed garlic) (Molina-Calle et al. 2017). The 1-propanethiol (odor 1) is one of the VOCs emitted from onion bulbs infected by the pathogenic fungus Fusarium oxysporum (Wang et al. 2019), suggesting that 1-propanethiol could be a common chemical related to the host response against infection by pathogens or metabolites in Allium plants rather than a product of the nematode itself. In addition to nematodes, various pathogens such as fungi and mites can infect and damage garlic, and they may also produce the same substances. The 2-methyl-1-butanol (odor 3) is often detected as a yeast-derived compound in various wine products (Crandles et al. 2015; Synos et al. 2015). The 2-methyl-1-butanol has a chiral center so that two enantiomers exist. It has been known that odors could be different because of its chirality. To make them identify accurately, those compounds should be analyzed by using two or more chromatography columns with different stationary phases of chiral columns. Further research on the chirality of odor chemicals is required.
Our results are the first step in the establishment of a nondestructive method for finding nematode-infested garlic by their distinctive odors as indicators. As future possibility, nematode infestation can be detected on site automatically using the odor sensor (called “Electric nose”) which has sensitivity on those specific odor compounds from the infested garlic. Further steps are needed to attribute the identified compounds from nematode-infested garlic as a diagnostic property. This would require the conduction of controlled and repeated experiments following the Koch’s postulates and a validation of odor detection verses true infestation rates using the analytical approach introduced in this study.
References
Brattoli M, Cisternino E, Rosario Dambruoso P, de Gennaro G, Giungato P, Mazzone A, Palmisani J, Tutino M (2013) Gas chromatography analysis with olfactometric detection (GC–O) as a useful methodology for chemical characterization of odorous compounds. Sensors 13:16759–16800. https://doi.org/10.3390/s131216759
Crandles M, Reynolds AG, Khairallah R, Bowen A (2015) The effect of yeast strain on odor active compounds in Riesling and Vidal blanc icewines. LWT Food Sci Technol 64:243–258. https://doi.org/10.1016/j.lwt.2015.05.049
Friedrich JE, Acree TE (1998) Gas chromatography olfactometry (GC/O) of dairy products. Int Dairy J 8:235–241. https://doi.org/10.1016/S0958-6946(98)80002-2
Fujimura T, Washio S, Nishizawa T (1986) Garlic as a new host of the potato-rot nematode, Ditylenchus destructor THORNE. Jpn J Nematol 16:38–47. https://doi.org/10.14855/jjn1972.16.38
Kitano N, Yamashita K (2011) Effect of soil fumigant on damage of garlic caused by Ditylenchus destructor. Ann Rep Plant Prot North Japan 62:144–147 (in Japanese)
MOE (1971) (Ministry of the Environment), Offensive odor control law. http://www.env.go.jp/en/laws/air/odor/index.html. Accessed in April 2020 (in Japanese)
Molina-Calle M, Priego-Capote F, Luque de Castro MD (2017) Headspace− GC–MS volatile profile of black garlic vs fresh garlic: evolution along fermentation and behavior under heating. LWT Food Sci Technol 80:98–105. https://doi.org/10.1016/j.lwt.2017.02.010
Synos K, Reynolds AG, Bowen AJ (2015) Effect of yeast strain on aroma compounds in Cabernet franc icewines. LWT Food Sci Technol 64:227–235. https://doi.org/10.1016/j.lwt.2015.05.044
Wang A, Islam MN, Johansen A, Haapalainen M, Latvala S, Edelenbos M (2019) Pathogenic Fusarium oxysporum f. sp. cepae growing inside onion bulbs emits volatile organic compounds that correlate with the extent of infection. Postharvest Biol Technol 152:19–28. https://doi.org/10.1016/j.postharvbio.2019.02.010
Acknowledgements
This work was supported by Fund of garlic quality improvement project, Aomori Prefecture Government. The authors especially thank Ms. Misaki Matsuo for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Human and animal rights
The authors obtained informed consent from human panelists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsumoto, M., Ueno, D., Aoyama, R. et al. Novel analytical approach to find distinctive odor compounds from garlic cloves infested by the potato-rot nematode Ditylenchus destructor using gas chromatography–olfactometry (GC–O) with heart-cut enrichment system. J Plant Dis Prot 127, 537–544 (2020). https://doi.org/10.1007/s41348-020-00349-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-020-00349-3