Abstract
An egg parasitoid, Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae), was reared on eggs of Plautia stali scott (Hemiptera: Pentatomidae), Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), and Glaucias subpunctatus (Walker) (Hemiptera: Pentatomidae), and parasitism rates and developmental properties were compared to clarify their suitability as hosts. The parasitoids successfully emerged from more than 90% of P. stali or G. subpunctatus eggs, whereas a lower rate of T. japonicus emerged from the eggs of H. halys. The developmental period of T. japonicus in the eggs of H. halys was longer than in the other two species, and the body size of T. japonicus that emerged from the eggs of H. halys was also larger than from the other two species. From these results, factors related to parasitism and developmental properties were discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruit-piercing stink bugs are important pests that cause damage to various fruits (Tsutsumi 2001). Forty-five species of fruit-piercing stink bugs have been reported in Japan (Japanese Society of Applied Entomology and Zoology 2006), and the principal species are Plautia stali scott (Hemiptera: Pentatomidae), Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), and Glaucias subpunctatus (Walker) (Hemiptera: Pentatomidae) (Hasegawa and Umeya 1974; Kiritani 2007). They have been controlled solely by chemical spraying (Funayama 2004; Suzuki 2005); however, continuous spraying of chemicals due to frequent fruit-piercing stink bugs increases the labor and economic burden of producers. It is also responsible for the resurgence of spider mites and scale insects due to the reduction of their natural enemies in orchards (Tsutsumi 2003). Therefore, it is desirable to establish control methods that offer an alternative to spraying chemicals, and biological control by natural enemies has been attracting attention as one of the methods (Goto and Adachi 2004).
Most species of the genus Trissolcus Ashmead (Hymenoptera: Scelionidae) are egg parasitoids of bugs of the superfamily Pentatomoidea (Talamas et al. 2015; Talamas et al. 2017). Trissolcus plautiae (Watanabe) is the dominant species parasitizing egg masses of P. stali in the field, and their parasitism rate reaches more than 90% from late August to early September (Yamada and Miyahara 1979). Therefore, T. plautiae has been expected as a classical biological control agent for P. stali in Japan (Goto and Adachi 2004). Trissolcus mitsukurii (Ashmead) is the commonest species that can parasitize eggs of the southern green stink bug Nezara viridula (L.) (Hemiptera: Pentatomidae) in Japan (Kiritani and Hokyo 1962), and it is also known as a parasitoid of H. halys (Arakawa and Namura 2002). Trissolcus japonicus (Ashmead) is distributed in Japan and it has been reported to parasitize eggs of P. stali and H. halys in the field (Matsuo et al. 2014). Matsuo et al. (2016) collected T. japonicus that emerged from egg masses of G. subpunctatus in Japan and reported that T. japonicus is the dominant species among egg parasitoids of G. subpunctatus. From these reports, T. japonicus affects the population of these stink bugs. However, there is no report simultaneously comparing the suitability of fruit-piercing stink bugs as a host for T. japonicus.
In this study, eggs of three species of bugs were supplied for parasitization by T. japonicus, and parasitism rates and developmental properties were compared to clarify their suitability as hosts.
Materials and methods
Insects
A stock culture of T. japonicus was established from adults collected in Ogi city, Saga prefecture in August 2014 using egg masses of G. subpunctatus (Kuki et al. 2019). Laboratory colonies were maintained in 50 mL plastic tubes (3.0 cm diameter and 11.5 cm height) placed in incubators (MIR-153; Sanyo Electric Co., Ltd., Tokyo, Japan) set at 25 ± 1 °C and 60 ± 10% RH with a 16L:8D photoperiod. A newly laid egg mass of G. subpunctatus was supplied for oviposition and a drop of undiluted honey was applied to the inner wall every 5 days.
Laboratory stock cultures of P. stali, H. halys, and G. subpunctatus originated and were continuously refilled from Shizuoka prefecture, Akita prefecture, and Mie prefecture, respectively. Adults were continuously reared on raw peanuts (Arachis hypogaea L.), raw soybeans (Glycine max L., only for P. stail), and water containing 0.05% l-ascorbic acid sodium salt and 0.025% l-cysteine hydrochloride monohydrate in glass petri dishes (123 mm diameter × 23 mm height) placed in incubators set at 25 ± 1 °C and 60 ± 10% RH with a 16L:8D photoperiod. Food and water were replaced every 3–4 days. Newly laid egg masses were collected and transferred to nymph plastic containers (135 mm diameter × 76 mm height) with a 3 cm diameter, mesh-covered hole containing a Morella rubra Sieb. et Zucc. twig (Fagales: Myricaceae) (Shimizu 2010) under the same environmental conditions. Newly emerged adults were collected from the cages and male and female pairs were evenly distributed among adult rearing cages to obtain egg masses.
Parasitism rate and developmental period of T. japonicus
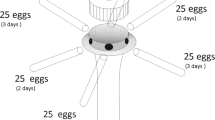
Newly laid (0–24 h) egg masses of P. stali (containing 14 eggs), H. halys (28 eggs), and G. subpunctatus (14 eggs) were used in the experiments. One egg mass laid on filter paper was stuck on the inner wall of a 50 mL plastic tube and a drop of undiluted honey was also applied to the inner wall. One female egg parasitoid (3–5 days old) was released in the tubes and kept at 25 ± 1 °C and 60 ± 10% RH with a 16L:8D photoperiod. Most hymenopterous parasitoids are haplodiploid and haploid males develop from unfertilized eggs and diploid females from fertilized ones (Colazza and Wajnberg 1998). Therefore, in this study, egg masses exposed to a mated female provided female egg parasitoids except for a few males. On the other hand, egg masses exposed to virgin females provided male egg parasitoids. Male egg parasitoids obtained from mated females were also used for experiments. After 6 h exposure, the egg parasitoids were removed, and parasitized egg masses were then kept in incubators under the same conditions. Egg masses were checked every 12 h and the time for an egg parasitoid to emerge from an egg was recorded. Observation of the eggs was continued until all egg parasitoids had emerged, and the numbers of eggs that fruit-piercing stink bug nymphs or egg parasitoid adults emerged from were counted. Eggs not showing emergence were dissected under a microscope (SZX16; Olympus Co., Tokyo, Japan) and classified into undeveloped parasitoids (egg parasitoid adult dead in egg) and dead eggs (undefined content).
Body size of T. japonicus
Emerged egg parasitoids were supplied a drop of undiluted honey and kept under the same conditions, and female and male egg parasitoids that emerged from each host egg were randomly selected. Egg parasitoids (3 days old) in the tubes were killed in a freezer (MDF-U538D; Panasonic Healthcare Co., Ltd., Tokyo, Japan) and kept until measurement. The head and forewings of the dead parasitoids were removed from the body under a microscope and photographed with a camera (HD Lite 1080P; RelyOn, Ltd., Tokyo, Japan) attached to the microscope. The head and forewings were measured using the image processing program ImageJ (Rasband 1997–2018) with reference to a micrometer similarly photographed.
Host egg size
For each host species, 25 eggs from 5 egg masses (5 eggs per egg mass) were randomly selected. After careful division by tweezers, each egg was photographed from above and the side under the microscope, and then its diameter and height were measured in the same way as the body size of T. japonicus.
Statistical analyses
The rates of parasitized eggs were analyzed by logistic regression analysis to estimate the effect of host species. Then if necessary, pairwise comparisons using the G test with Bonferroni correction were performed. The data of the developmental period, body size of T. japonicus, and host egg size were first examined using Bartlett’s test for homogeneity of variances. When significant differences were indicated, the data were analyzed using the Kruskal–Walis test followed by Dwass–Steel–Crichtlow–Fligner multiple pairwise comparisons tests. When significant differences were not indicated, the data were analyzed using one way ANOVA followed by Tukey–Kramer multiple comparisons tests. All statistical analyses were performed by SAS university edition Version 3.8 (SAS Institute 2018).
Results
Parasitism rate and developmental period of T. japonicus
The parasitoids successfully emerged from more than 90% of P. stali or G. subpunctatus eggs, whereas a lower rate of T. japonicus emerged from the eggs of H. halys (Table 1). When T. japonicus was supplied with eggs of H. halys, the numbers of dead eggs and successfully emerging nymphs of host were significantly higher. The developmental periods of both females and males in the eggs of H. halys were significantly longer than those of other fruit-piercing stink bugs (Table 2). The developmental periods of female egg parasitoids in the eggs of P. stali and G. subpunctatus were significantly different; however, the developmental periods of male egg parasitoids in the eggs of P. stali and G. subpunctatus were not significantly different.
Body size of T. japonicus and host egg size
The head width and forewing length of both females and males were significantly different depending on the host species (Table 3). The head width and forewing length of egg parasitoids emerging from H. halys eggs were significantly the largest among egg parasitoids emerging from the three fruit-piercing stink bugs, followed by those emerging from G. subpunctatus and P. stali.
The diameter and height of host eggs were significantly different among the three host species (Table 4). The diameter of H. halys was significantly the longest, followed by those of G. subpunctatus and P. stali. The heights of H. halys and G. subpunctatus were not significantly different, with P. stali being significantly the shortest.
Discussion
In this study, it was shown that parasitism and developmental properties of T. japonicus differ depending on the species of host egg. Previous studies also reported that parasitism and developmental periods in several species of Trissolcus were different depending on the species of host (Botch and Delfosse 2018; Kivan and Kilic 2002, 2004, 2005). Also, positive correlations were reported between the host egg size and parasitoid body size in other Trissolcus species (Arakawa et al. 2004; Botch and Delfosse 2018). Those previous reports strongly support the validity of the present study. Female T. japonicus reared on larger host eggs can produce more offspring as a consequence (Botch and Delfosse 2018). The relationship between the host egg size and fecundity of emerging parasitoids was also reported in other Scelionidae (Abram et al. 2016b; Arakawa et al. 2004). If this is also the case for T. japonicus, individuals emerging from eggs of H. halys should have higher reproductive capacities; therefore, H. halys might be a suitable host for T. japonicus.
Although the rates of T. japonicus parasitizing H. halys eggs under experimental conditions were previously reported to be from 50 to 90% (Haye et al. 2020; Hedstrom et al. 2017), lower success rates (25.0% for parasitism and 22.1% for emergence, respectively) were observed in this study. Over 50% of the provided H. halys eggs produced neither adult parasitoids nor host nymphs, as shown in Table 1. Egg parasitoids are known to induce host egg abortion even when the parasitism is not successful (Abram et al. 2016a). Since almost all of H. halys eggs hatch successfully in our laboratory conditions, we suggest this could also be the case in our study. Similarly, host egg abortion has been well documented in other Scelionidae (Abram et al. 2016a; Konopka et al. 2018, 2020). We consider two reasons why failure was more marked in this study. First, it may be due to a difference in the genetic background of T. japonicus. The interactions between parasitoids and hosts are influenced by various factors; such as physiological or nutritional condition of host, venom injected by adult parasitoid at oviposition, and teratocytes released by hatched larvae of parasitoid (Beckage and Gelman 2004; Konopka et al. 2020; Pennacchio and Strand 2006; Volkoff and Colazza 1992). Thus, we suggest that some differences in abovementioned factors might have induced different adaptations to H. halys eggs in the present study. Furthermore, since there is some intra-specific variation in the capacity to abort host eggs (Abram et al. 2016a), we also suggest that investigated populations that have different genetic background have caused the different adaptations. Further investigations of the intra-specific differences in parasitism and capacity to resist the host.
Another possible reason is the effect of continuous breeding. Botch and Delfosse (2018) reported that T. japonicus reared on eggs of species other than H. halys had lower reproductive success on H. halys eggs. The stock culture of T. japonicus in this study was maintained on egg masses of G. subpunctatus, not on H. halys, for a long time. Even if the stock culture has the same genetic background, it is possible that adaptation to H. halys was reduced due to continuous breeding on eggs of G. subpunctatus. Further investigation is also required to examine the effects of continuous breeding of T. japonicus.
References
Abram PK, Brodeur J, Burte V, Boivin G (2016a) Parasitoid-induced host egg abortion: an underappreciated component of biological control services provided by egg parasitoids. Biol Control 98:52–60. https://doi.org/10.1016/j.biocontrol.2016.04.002
Abram PK, Parent JP, Brodeur J, Boivin G (2016b) Size-induced phenotypic reaction norms in a parasitoid wasp: an examination of life-history and behavioural traits. Biol J Linn Soc 117:620–632. https://doi.org/10.1111/bij.12658
Arakawa R, Namura Y (2002) Effects of temperature on development of three Trissolcus spp. (Hymenoptera: Scelionidae), egg parasitoids of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Entomol Sci 5:215–218
Arakawa R, Miura M, Fujita M (2004) Effects of host species on the body size, fecundity, and longevity of Trissolcus mitsukurii (Hymenoptera: Scelionidae), a solitary egg parasitoid of stink bugs. Appl Entomol Zool 39:177–181. https://doi.org/10.1303/aez.2004.177
Beckage NE, Gelman DB (2004) Wasp parasitoid disruption of host development: implications for new biologically based strategies for insect control. Annu Rev Entomol 49:229–330. https://doi.org/10.1146/annurev.ento.49.061802.123324
Botch PS, Delfosse ES (2018) Host-acceptance behavior of Trissolcus japonicus (Hymenoptera: Scelionidae) reared on the invasive Halyomorpha halys (Heteroptera: Pentatomidae) and nontarget species. Environ Entomol 47:403–411. https://doi.org/10.1093/ee/nvy014
Colazza S, Wajnberg E (1998) Effects of host egg mass size on sex ratio and oviposition sequence of Trissolcus basalis (Hymenoptera: Scelionidae). Environ Entomol 27:329–336. https://doi.org/10.1093/ee/27.2.329
Funayama K (2004) Importance of apple fruits as food for the brown-marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). Appl Entomol Zool 39:617–623. https://doi.org/10.1303/aez.2004.617
Goto H, Adachi I (2004) Development of immature stages of Trissolcus plautiae (Hymenoptera: Scelionidae), an egg parasitoid of Plautia crossota stali (Heteroptera: Pentatomidae). Jpn J Appl Entomol Zool 48:213–218. https://doi.org/10.1303/jjaez.2004.213 (in Japanese with English summary)
Hasegawa H, Umeya K (1974) Outbreak of stink bugs in fruit trees. Plant Prot 28:279–286 (in Japanese)
Haye T, Moraglio ST, Stahl J, Visentin S, Gregorio T, Tavella L (2020) Fundamental host range of Trissolcus japonicus in Europe. J Pest Sci 93:171–182. https://doi.org/10.1007/s10340-019-01127-3
Hedstrom C, Lowenstein D, Andrews H, Bai B, Wiman N (2017) Pentatomid host suitability and the discovery of introduced populations of Trissolcus japonicus in Oregon. J Pest Sci 90:1169–1179. https://doi.org/10.1007/s10340-017-0892-6
Japanese Society of Applied Entomology and Zoology (2006) Major insect and other pests of economic plants in Japan (revised edition). Japanese Plant Protection Association, Tokyo, p 387
Kiritani K (2007) The impact of global warming and land-use change on the pest status of rice and fruit bugs (Heteroptera) in Japan. Glob Change Biol 13:1586–1595. https://doi.org/10.1111/j.1365-2486.2007.01397.x
Kiritani K, Hokyo N (1962) Studies on the life table of the southern Green Stink bug, Nezara viridula. Jpn J Appl Entomol Zool 6:124–140. https://doi.org/10.1303/jjaez.6.124
Kivan M, Kilic N (2002) Host preference: parasitism, emergence and development of Trissolcus semistriatus (Hym., Scelonidae) in various host eggs. J Appl Entomol 126:395–399. https://doi.org/10.1046/j.1439-0418.2002.00682.x
Kivan M, Kilic N (2004) Influence of host species and age on host preference of Trissolcus semistriatus. Biocontrol 49:553–562. https://doi.org/10.1023/B:BICO.0000036436.06260.19
Kivan M, Kilic N (2005) Effects of storage at low-temperature of various heteropteran host eggs on the egg parasitoid, Trissolcus semistriatus. Biocontrol 50:589–600. https://doi.org/10.1007/s10526-004-3122-0
Konopka JK, Poinapen D, Gariepy T, McNeil JN (2018) Understanding the mismatch between behaviour and development in a novel host parasitoid association. Sci Rep 8:15677. https://doi.org/10.1038/s41598-018-33756-6
Konopka JK, Poinapen D, Gariepy T, Holdsworth DW, McNeil JN (2020) Timing of failed parasitoid development in Halyomorpha halys eggs. Biol Control 141:104124. https://doi.org/10.1016/j.biocontrol.2019.104124
Kuki D, Wada Y, Tsunashima A, Itoyama K (2019) Developmental properties and parasitism capacity of the egg parasitoid Trissolcus japonicus (Hymenoptera: Scelionidae), reared on eggs of Glaucias subpunctatus (Hemiptera: Pentatomidae). Appl Entomol Zool 54:307–312. https://doi.org/10.1007/s13355-019-00627-z
Matsuo K, Hirose Y, Johnson NF (2014) A taxonomic issue of two species of Trissolcus (Hymenoptera: Platygastridae) parasitic on eggs of the brown-winged green bug, Plautia stali (Hemiptera: Pentatomidae): resurrection of T. plautiae, a cryptic species of T. japonicus revealed by morphology, reproductive isolation and molecular evidence. Appl Entomol Zool 49:385–394. https://doi.org/10.1007/s13355-014-0260-4
Matsuo K, Honda T, Itoyama K, Toyama M, Hirose Y (2016) Discovery of three egg parasitoid species attacking the shield bug Glaucias subpunctatus (Hemiptera: Pentatomidae). Jpn J Appl Entomol Zool 60:43–45. https://doi.org/10.1303/jjaez.2016.43 (in Japanese with English summary)
Pennacchio F, Strand MR (2006) Evolution of developmental strategies in parasitic Hymenoptera. Annu Rev Entomol 51:233–258. https://doi.org/10.1146/annurev.ento.51.110104.151029
Rasband WS, Image J (1997–2018) U. S. National Institutes of Health, Bethesda, Maryland, USA. https://imagej.nih.gov/ij/. Accessed 2 July 2013
SAS Institute (2018) SAS University edition, version 3.8. SAS Institute Inc., Cary
Shimizu K (2010) The trophic efect of twigs of Chinese strawberry tree (Myrica rubra) on the development and oviposition of fruit-piercing stink bugs. Ann Rept Kanto-Tosan Plant Prot Soc 57:87–90. https://doi.org/10.11337/ktpps.2010.87
Suzuki K (2005) Occurrence of the fruit-piercing stink bug Glaucias subpunctatus (Walker) (Hemiptera: Pentatomidae) in central and southern Mie prefecture. Ann Rept Kansai Plant Prot 47:161–162. https://doi.org/10.4165/kapps.47.161 (in Japanese)
Talamas EJ, Johnson NF, Bufngton M (2015) Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae). J Hymenopt Res 43:45–110. https://doi.org/10.3897/JHR.43.8560
Talamas EJ, Bufngton ML, Hoelmer K (2017) Revision of Palearctic Trissolcus Ashmead (Hymenoptera, Scelionidae). J Hymenopt Res 56:3–185. https://doi.org/10.3897/jhr.56.10158
Tsutsumi T (2001) A method for inspecting stylet sheaths of stink bugs on the cone of Japanese cypress. Plant Prot 55:560–562 (in Japanese)
Tsutsumi T (2003) Fruit-piercing stink bugs. Rural Culture Association, Tokyo, p 126
Volkoff N, Colazza S (1992) Growth patterns of teratocytes in the immature stages of Trissolcus basalis (Woll.) (Hymenoptera: Scelionidae), an egg parasitoid of Nezara viridula (L.) (Heteroptera: Pentatomidae). Int J Insect Morphol Embryol 21:323–336. https://doi.org/10.1016/0020-7322(92)90027-K
Yamada K, Miyahara M (1979) Studies on biology and control of pentatomid bugs attacking fruits. (4) Parasitoids of the brown-winged green bug. Plautia Stali Kyushu Pl Prot Res 25:147–150. https://doi.org/10.4241/kyubyochu.25.147 (in Japanese)
Acknowledgements
We sincerely thank Dr. Takayuki Mitsunaga, Central Region Agricultural Research Center, National Agriculture and Food Research Organization (NARO), for his assistance with statistical analysis. This research was supported by a Research Project Grant (B) from the Institute of Science and Technology, Meiji University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miura, A., Furuno, M., Kuki, D. et al. Comparisons of parasitism and developmental properties of Trissolcus japonicus (Hymenoptera: Scelionidae) reared on eggs of three species of fruit-piercing stink bugs. Appl Entomol Zool 56, 379–383 (2021). https://doi.org/10.1007/s13355-021-00746-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-021-00746-6