Abstract
The relationship between the temperature and developmental period was investigated in an egg parasitoid, Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae), reared on eggs of the fruit-piercing stink bug Glaucias subpunctatus (Walker) (Hemiptera: Pentatomidae). Adults of Tr. japonicus steadily emerged under 22, 25, 28, and 31°C conditions, and their normal development was affected under 18 and 34°C conditions. From these results, the developmental zero temperatures for females and males were estimated to be 12.43 and 11.65 °C, respectively. The calculated effective cumulative temperatures in females and males were 153.85 and 140.85 degree-days, respectively. Females fully matured in 3 days after emergence, and could lay 80.4 eggs throughout their lifetime. Based on these results, the relationship between the life histories of this parasitoid and G. subpunctatus was discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruit-piercing stink bugs are important pests that cause damage to various fruits (Tsutsumi 2001). Three species of Pentatomidae, Plautia stali Scott, Glaucias subpunctatus (Walker), and Halyomorpha halys (Stål), are the principal pests in Japan (Kiritani 2007). They grow mainly on cones of Japanese cedar Cryptomeria japonica D. Don (Pinales: Cupressaceae) and cypress Chamaecyparis obtusa Sieb. et Zucc. (Pinales: Cupressaceae) (Oda et al. 1981; Tanaka 1979). After the cones become unsuitable for food due to the disappearance of endosperm or the embryo by their sucking, the fruit-piercing stink bugs fly to orchards (Takimoto and Ogasawara 2003; Tsutsumi 2001). Since the extent of damage caused by fruit-piercing stink bugs depends on the shortage of food in montane forests, it is an effective control measure to spray chemicals after confirming the arrival of the bugs in an orchard (Suzuki 2005). However, continuous spraying of chemicals due to frequent fruit-piercing stink bugs increases the labor and economic burden of producers. It is also responsible for the resurgence of spider mites and scale insects due to the reduction of natural enemies in orchards (Tsutsumi 2003).
We need to examine environmentally friendly and self-sustaining control measures, such as using natural enemies, and explore and evaluate the effectiveness of using them to control important pests (Ueno and Nakai 2009). Most species of the genus Trissolcus Ashmead (Hymenoptera: Scelionidae) are egg parasitoids of bugs of the superfamily Pentatomoidea (Talamas et al. 2015, 2017). Trissolcus plautiae (Watanabe) is the most dominant egg parasitoid of P. stali and it has been investigated regarding its life history (Ohno 1987; Toyama and Mishiro 2010). Trissolcus mitsukurii (Ashmead), Tr. plautiae, and Tr. comperei (Crawford) are known parasitoids of H. halys, and they have been studied regarding the influence of temperature on their development (Arakawa and Namura 2002). Matsuo et al. (2016) reported that Tr. japonicus (Ashmead) is the main natural enemy of G. subpunctatus. Therefore, it is possible that Tr. japonicus is effective as a natural measure to control G. subpunctatus, but its life history is poorly understood.
In this study, we investigated the relationship between the temperature and developmental period of Tr. japonicus to estimate their developmental zero and effective cumulative temperatures. Furthermore, to elucidate the fecundity of Tr. japonicus, ovarian development and the oviposition rate at 25°C were determined.
Materials and methods
Insects
A stock culture of Tr. japonicus was established from adults collected in Ogi City, Saga Prefecture in August 2014 using G. subpunctatus egg masses. Laboratory colonies were maintained in 50-mL plastic tubes (3.0-cm diameter and 11.5-cm height) at 25 ± 1°C and 60 ± 10% RH with a 16L: 8D photoperiod (MIR-153, SANYO Electric Co., Ltd, Tokyo, Japan). A drop of undiluted honey was applied to the inner wall every 5 days and we provided newly laid egg masses of G. subpunctatus for oviposition. Newly emerged wasps were kept together for 2 days to ensure mating, and they were used for testing when they were 3–5 days old. Since males emerge earlier than females, we obtained unmated females by artificially removing males before females emerged, and they were also used for testing when they were 3–5 days old.
A stock culture of G. subpunctatus was established from adults collected in Mie Prefecture in October 2015, November 2016, and October 2017. Adults were continuously reared on raw peanuts (Arachis hypogaea L.) and water containing 0.05% L-ascorbic acid sodium salt and 0.025% l-cysteine hydrochloride monohydrate in glass petri dishes (123-mm diameter × 23-mm height) at 25 ± 1°C and 60 ± 10% RH with a 16L:8D photoperiod. Food and water were replaced every 3–4 days. Newly laid egg masses were transferred to rearing cases for nymphs (135-mm diameter × 76-mm height) with a 3-cm diameter, mesh-covered hole containing a Morella rubra Sieb. et Zucc. twig (Fagales: Myricaceae) (Shimizu 2010) under the same conditions. Newly emerged adults were collected from the cages and we evenly distributed male and female pairs among adult rearing cages to obtain egg masses.
Developmental period and thermal requirements of Tr. japonicus
The effect of temperature on the developmental period and thermal requirements of Tr. japonicus were studied under six constant temperatures: 18, 22, 25, 28, 31, and 34°C. All temperature treatments were conducted with a 16L: 8D photoperiod. To determine the developmental period of Tr. japonicus, 1-day-old G. subpunctatus egg masses (14 eggs per egg mass) were exposed to 3–5-day-old mated or unmated females for 6 h at 25°C in 50-mL tubes. Many parasitoid Hymenoptera are known to reproduce parthenogenetically using arrhenotoky, i.e., females arise from fertilized and males from unfertilized eggs (Colazza 1993). Offspring laid by unmated females are only male; therefore, we supplied male offspring by this sex determination. Females were then removed, and the egg masses were incubated in a multiple chamber (LH-30-8CT, Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan) at each experimental temperature and checked every 12 h until adult emergence. Host eggs from which neither parasitoids nor host nymphs emerged were dissected to identify their contents under a microscope after 1 week. Eggs containing parasitoid adults were recorded as unemerged adults, while eggs containing neither parasitoid adults nor host nymphs were regarded as failures. Eggs from which host nymphs emerged were regarded as parasitized eggs and excluded from the analysis. We calculated the rate of parasitism on grouping male and female adults together because it was not possible to identify the sex of unemerged adults.
The development rate from oviposition to adult emergence and thermal requirements of Tr. japonicus were evaluated. Developmental zero temperatures and effective cumulative temperatures [degree days (DD)] were determined from a linear regression of the developmental rate (the reciprocal of time spent in days from oviposition to adult parasitoid emergence) on temperature.
Fecundity of Tr. japonicus
The number of mature eggs in ovaries of Tr. japonicus at different ages after emergence was investigated. Under the 25°C condition, female parasitoid emergence was observed every day and the females were distributed to the 50-mL tubes over time with a drop of undiluted honey attached to the inner wall. Each female was dissected in a drop of physiological saline solution placed on a petri dish. After the ovary had been fully dissected, a drop of 0.3% methylene blue that stains only mature eggs (Ohno 1987) was added. Each treatment was replicated 5–9 times (females). The number of mature eggs was determined under a microscope.
Daily reproduction of Tr. japonicus was investigated as follows. Newly emerging males and females were transferred to 50-mL tubes and kept in the incubator (MIR-153, SANYO Electric Co., Ltd., Tokyo, Japan) at 25 ± 1 °C and 60 ± 10% RH with a 16L: 8D photoperiod and a drop of undiluted honey attached to the inner wall for 1 day for mating. Thereafter, a female was transferred to a 15-mL tube with a drop of undiluted honey attached to the inner wall and three G. subpunctatus egg masses (approximately 3 × 14 = 42 eggs) were supplied on the first day, and then two egg masses (approximately, 2 × 14 = 28 eggs) daily from the second day until death. Females were allowed to oviposit for 24 h. Host eggs exposed to females were kept under the same conditions as for incubation. They were checked daily until the adult emergence. Each treatment was replicated 8 times (females). The number and sex of emerged parasitoids were examined. Host eggs from which neither parasitoids nor host nymphs emerged were dissected to clarify their contents under a microscope. The net reproductive rate (R0, female/female) and intrinsic rate of increase (rm, female/female/day) were determined from the length of the developmental period (in days) of Tr. japonicus under the 25 °C condition (Table 2) using the methods of Fujisaki (2000).
Statistical analyses
Numbers of eggs in ovaries at different ages were analyzed using Tukey–Kramer’s test after ANOVA. Developmental periods between females and males and were compared using the t test corrected by the Bonferroni method. The PC software “Excel Statistics, ver. 2.15” (SSRI Co., Ltd.) was used for the calculations. Linear regression of the developmental rate versus temperature was carried out using PC software “Microsoft Excel 2016” (Microsoft Corporation.).
Results and discussion
Developmental period and thermal requirements of Tr. japonicus
Ratios of emerged adults were high at the temperatures of 22, 25, 28, and 31 °C for both females and males (Table 1). Ratios of failures to develop to adulthood and unemerged adults were comparatively high at 18 and 34 °C, respectively. Under the condition of 34 °C, a few dead adults (n = 4) with the head exposed outside the eggshell were observed, although under other temperature conditions, the whole body of unemerged adults was inside the eggshell. Such dead adults have been reported in several species of Trissolcus when parasitized egg masses are incubated under dry conditions (20–30% humidity) (Nishimoto et al. 2015). However, the test was not conducted under such dry conditions because a container with water was kept in a multiple chamber, we considered that the high temperature affected adult emergence. Under the 18 °C condition, a few cases of failure to develop (n = 8) were observed. Such cases whereby no parasitoid developed were shown with other Scelionidae wasps under 15 °C conditions (Canto-Silva et al. 2005), and we considered that the low temperature affected larval development.
The developmental period of both females and males became shorter as the temperature increased over the range of 18–31 °C (Table 2). Shortening of the developmental period accompanying an increase in temperature has been recorded for several species of Trissolcus (James and Warren 1991; Müjan and Nihal 2006; Toyama and Mishiro 2010), and it has been suggested that Tr. japonicus exhibits similar characteristics. However, there were no definite differences in the developmental period between 31 and 34 °C for both females and males. This result suggests that the developmental period reaches its peak at 34 °C; therefore, we excluded data for the 34 °C condition when calculating the thermal requirements. The developmental rates increased linearly with increasing temperature for both males and females (Fig. 1). A linear regression using these temperatures yielded a coefficient of determination (r2) of about 0.99 for both females and males, which indicated a stronger correlation. The developmental zero temperatures for females and males were estimated to be 12.43 and 11.65 °C, respectively. Males, which develop faster than females, required 140.85 DD for development, whereas 153.85 DD was estimated to be the thermal requirement for female development. According to a field survey in Saga Prefecture in 2013, the oviposition period of G. subpunctatus was mid-June to early September (Tsunashima et al. 2017). Using temperature means for Saga City, Saga Prefecture from June 11, 2013 to September 11, 2013 (Japan Meteorological Agency 2013) and developmental zero temperatures and effective cumulative temperatures obtained in this study, 9–10 generations of Tr. japonicus could occur during this period, and this is nearly three times the number of generations produced in the same period by its host G. subpunctatus (Tsunashima et al. 2017). It follows that Tr. japonicus produced several generations per one generation of its host.
Fecundity of Tr. japonicus
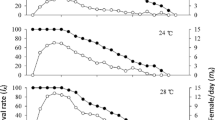
Newly emerged (0-day-old) females possessed about five mature eggs in their ovary, and this increased and reached its peak at 3 days, and thereafter did not change (Fig. 2). Ten-day-old females had the most mature eggs (35.8 eggs), but there was no significant difference from the 3-day-old females. This indicates that Tr. japonicus can have 14 eggs, equivalent to 1 egg mass of G. subpunctatus from the first day after emergence, and can lay 2 or more egg masses after 3 days. Ohno (1987) indicated that Tr. plautiae was a synovigenic species, and that newly emerged females had only a few mature eggs in their ovaries, and this increased as the female aged. Since Tr. japonicus showed a similar pattern, it was suggested to be a synovigenic species.
The number of eggs laid by mated females was the highest (19.9 eggs) on the first day, and it declined as the female aged (Fig. 3). As the reason for the number of eggs laid on the first day after emergence exceeding the number of mature eggs in their ovary on that day, we considered that female oviposition behavior induces egg production. Lifetime production was 80.4 (119–27, SE = 10.4) eggs and the longevity of females that produced host eggs daily was 15.5 (30–11, SE = 2.2) days. This indicates that one female can lay eggs in 5–6 G. subpuctatus egg masses consisting of 14 eggs throughout its lifetime. Out of the number of emerged and unemerged adults described above, the number of female offspring per mated female was the highest on the first day, and it declined as females aged, while the number of male offspring did not fluctuate so much (Fig. 4). That is, the sex ratio was higher as females aged. Similar results were reported for other species, Tr. plautiae and Tr. grandis Thomson (Ohno 1987; Amir-Maafi and Parker 2011). The net reproductive rate (R0) and intrinsic rate of natural increase (rm) were 46.1 and 0.25, respectively. Trissolcus japonicus had lower values of both R0 and rm than other Scelionidae; Tr. grandis had R0 of 147.9 and rm of 0.342 on eggs of Eurygaster integriceps Puton (Hemiptera: Scutelleridae) under 25 °C conditions (Amir-Maafi and Parker 2011) and Tr. plautiae had R0 103 under 25 °C (Ohno 1987), although Tr. semistriatus Nees had similar values for both R0 48.8 and rm 0.27 under 26 °C conditions (Müjan and Nihal 2006). Arakawa et al. (2004) indicated that the fecundity of Tr. mitsukurii was affected by the host species. Trissolcus japonicus parasitizes not only G. subpunctatus, but also P. stali and H. halys (Matsuo et al. 2014, 2016); therefore, it is necessary to investigate whether these stink bugs affect the parasitoid life history.
References
Amir-Maafi M, Parker BL (2011) Biological parameters of the egg parasitoid Trissolcus grandis (Hymenoptera: Scelionidae) on Eurygaster integriceps (Hemiptera: Scutelleridae). J Entomol Soc Iran 30:67–81
Arakawa R, Namura Y (2002) Effects of temperature on development of three Trissolcus spp. (Hymenoptera: Scelionidae), egg parasitoids of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Entomol Sci 5:215–218
Arakawa R, Miura M, Fujita M (2004) Effects of host species on the body size, fecundity, and longevity of Trissolcus mitsukurii (Hymenoptera: Scelionidae), a solitary egg parasitoid of stink bugs. Appl Entomol Zool 39:177–181. https://doi.org/10.1303/aez.2004.177
Canto-Silva CR, Romanowski HP, Redaelli LR (2005) Effect of temperature on the development and viability of Gryon gallardoi (Brethes) (Hymenoptera: Scelionidae) parasitizing Spartocera dentiventris (Berg) (Hemiptera: Coreidae) eggs. Braz J Biol 65:415–421. https://doi.org/10.1590/S1519-69842005000300006
Colazza S (1993) Factors influencing brood sex ratio in the egg parasitoid Trissolcus basalis (Woll.) (Hymenoptera: Scelionidae). Entomol Bari 27:211–219. https://doi.org/10.15162/0425-1016/645
Fujisaki K (2000) Ecology of population and community. In: Nakasuji F, Naito C, Ishii M, Fujisaki K, Sasaki M (eds) Basic applied entomology. Asakura Publishing, Tokyo, pp 77–106 (in Japanese)
James DG, Warren GN (1991) Effect of temperature on development, survival, longevity and fecundity of Trissolcus oenone Dodd (Hymenoptera: Scelionidae). J Aust Entomol Soc 30:303–306. https://doi.org/10.1111/j.1440-6055.1991.tb00441.x
Japan Meteorological Agency (2013). Search past weather data. http://www.data.jma.go.jp/obd/stats/etrn/view/daily_s1.php?prec_no=85&block_no=47813&year=2013&month=6. Accessed 30 April 2019. (in Japanese)
Kiritani K (2007) The impact of global warming and land-use change on the pest status of rice and fruit bugs (Heteroptera) in Japan. Glob Change Biol 13:1586–1595. https://doi.org/10.1111/j.1365-2486.2007.01397.x
Matsuo K, Hirose Y, Johnson NF (2014) A taxonomic issue of two species of Trissolcus (Hymenoptera: Platygastridae) parasitic on eggs of the brown-winged green bug, Plautia stali (Hemiptera: Pentatomidae): resurrection of T. plautiae, a cryptic species of T. japonicus revealed by morphology, reproductive isolation and molecular evidence. Appl Entomol Zool 49:385–394. https://doi.org/10.1007/s13355-014-0260-4
Matsuo K, Honda T, Itoyama K, Toyama M, Hirose Y (2016) Discovery of three egg parasitoid species attacking the shield bug Glaucias subpunctatus (Hemiptera: Pentatomidae). Jpn J Appl Entomol Zool 60:43–45. https://doi.org/10.1303/jjaez.2016.43 (in Japanese with English summary)
Müjan K, Nihal K (2006) Age-specific fecundity and life table of Trissolcus semistriatus, an egg parasitoid of the sunn pest Eurygaster integriceps. Entomol Sci 9:39–46. https://doi.org/10.1111/j.1479-8298.2006.00152.x
Nishimoto H, Fujita T, Tanaka T, Katou S (2015) Interspecific competition between two scelionid egg parasitoids, the non-native, invasive Trissolcus basalis (Wollaston) and the native Trissolcus mitsukurii (Ashmead) on the southern green stink bug Nezara viridula (Linnaeus) in Japan. Ann Rept Kansai Pl Prot 57:37–48. https://doi.org/10.4165/kapps.57.37 (in Japanese with English summary)
Oda M, Sugiura T, Nakanishi Y, Shibata E, Uesumi Y (1981) The ecology on the occurrence of Japanese cedar and Japanese cypress of the brown-winged green bug, Plautia stali Scotto and brown-marmorated stink bug, Halyomorpha mista Uhler. Bull Nara Agric Exp Stn 12:120–130 (in Japanese with English summary)
Ohno K (1987) Ovarian development, fecundity and sex ratio of Trissolcus plautiae (Watanabe) (Hymenoptera: Scelionidae), an egg parasitoid of the brown-winged green bug, Plautia stali Scott. Jpn J Appl Entomol Zool 31:385–390. https://doi.org/10.1303/jjaez.31.385
Shimizu K (2010) The trophic effect of twigs of Chinese strawberry tree (Myrica rubra) on the development and oviposition of fruit-piercing stink bugs. Ann Rept Kanto-tosan Pl Prot Soc 57:87–90. https://doi.org/10.11337/ktpps.2010.87 (in Japanese)
Suzuki K (2005) Occurrence of the fruit-piercing stink bug Glaucias subpunctatus (Walker) (Hemiptera: Pentatomidae) in central and southern Mie prefecture. Ann Rept Kansai Pl Prot 47:161–162. https://doi.org/10.4165/kapps.47.161 (in Japanese)
Takimoto M, Ogasawara (2003) Forecasting the population density of Plautia crossota stali and the time of coming to an orchard. Res Bull Aichi Agric Res Ctr 35:131–134 (in Japanese with English summary)
Talamas EJ, Johnson NF, Buffington M (2015) Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae). J Hymenopt Res 43:45–110. https://doi.org/10.3897/JHR.43.8560
Talamas EJ, Buffington ML, Hoelmer K (2017) Revision of Palearctic Trissolcus Ashmead (Hymenoptera, Scelionidae). J Hymenopt Res 56:3–185. https://doi.org/10.3897/jhr.56.10158
Tanaka K (1979) Life history of Plautia crossata stali SCOTT in the middle part of Mie Prefecture. Proc Kansai Pl Prot Soc 21:3–7. https://doi.org/10.4165/kapps1958.21.0_3 (in Japanese with English summary)
Toyama M, Mishiro K (2010) Effect of temperature on the life history of Trissolcus plautiae (Hymenoptera: Scelionidae), an egg-parasitoid wasp of the fruit-piercing stink bugs (Heteroptera: Pentatomidae). Bull Natl Inst Fruit Tree Sci 11:17–23 (in Japanese with English summary)
Tsunashima A, Honda T, Ozawa Y, Kuchiki Itoyama K (2017) Estimation of the lower developmental threshold temperature, the effective accumulative temperature and the annual generation number of Glaucias subpunctatus Walker (Hemiptera: Pentatomidae) in Saga prefecture. Kyushu Pl Prot Res 63:102–107. https://doi.org/10.4241/kyubyochu.63.102 (in Japanese)
Tsutsumi T (2001) A method for inspecting stylet sheaths of stink bugs on the cone of Japanese cypress. Pl Prot 55:560–562 (in Japanese)
Tsutsumi T (2003) Fruit-piercing stink bugs. Rural Culture Association, Tokyo, p 126 (in Japanese)
Ueno T, Nakai M (2009) Basics of biological control. In: Nakai M, Ohno K, Tanaka T (eds) biological control. Asakura Publishing, Tokyo, pp 1–3 (in Japanese)
Acknowledgements
This research was supported by a Research Project Grant (B) from the Institute of Science and Technology, Meiji University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuki, D., Wada, Y., Tsunashima, A. et al. Developmental properties and parasitism capacity of the egg parasitoid Trissolcus japonicus (Hymenoptera: Scelionidae), reared on eggs of Glaucias subpunctatus (Hemiptera: Pentatomidae). Appl Entomol Zool 54, 307–312 (2019). https://doi.org/10.1007/s13355-019-00627-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-019-00627-z