Abstract
Cryptolaemus montrouzieri Mulsant (Coleoptera: Coccinellidae) is a ladybird native to Australia, preying on mealybugs and soft scales, and has been utilized worldwide as a biological control agent. It has long been recognized that C. montrouzieri that was introduced into the main island of Japan had failed to become established. The present study monitored yearly and seasonal occurrence of C. montrouzieri adults in citrus groves at Shizuoka Prefectural Fruit Tree Research Center in Shizuoka City, central Japan in 2008–2012 by using sticky traps and beating citrus trees. Adults of C. montrouzieri were continuously captured for 5 and 4 years in a pesticide-free citrus grove and a neighboring reduced-pesticide grove, respectively. Larvae of C. montrouzieri were observed consuming a cottony scale, Pulvinaria aurantii Cockerell, on citrus trees. These results provide unequivocal evidence for the ladybird’s establishment in central Japan. The number of trapped ladybird adults exhibited four peaks a year: in mid-April, early to late June, mid-August, and late September to early October. Adult numbers in each grove varied largely across years, showing a great increase followed by a rapid decline during a period of 4 years. Factors affecting the seasonal/yearly occurrence of C. montrouzieri adults in citrus groves are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryptolaemus montrouzieri Mulsant (Coleoptera: Coccinellidae) that is indigenous to eastern Australia (Booth and Pope 1986) has been introduced around the world to control mealybugs and soft scale insects on various crops since its first release into citrus groves in California, USA, in 1891 (DeBach and Hagen 1964; Kairo et al. 2013). The exotic ladybird has become established in natural environments in many countries, exclusively in tropical and subtropical regions (see a review by Kairo et al. 2013).
In Japan, C. montrouzieri was introduced in 1934 from Saipan into the Ogasawara Islands (also known as the Bonin Islands) which are located in a subtropical region (Sakimura 1935), and has become established on the islands (Kurosawa et al. 1985). On the other hand, into Honshu Island (the main island of Japan), located in a temperate zone, the ladybird had been introduced twice so far (Furuhashi and Nishino 1980; Ishii 1955). First, the ladybird imported from Hawaii in 1931 was released into a citrus grove in Okayama Prefecture, located in the western portion of the main island of Japan, to suppress mealybugs, but was unable to overwinter (Ishii 1955), possibly due to low temperature in winter (Bartlett 1974; Maes et al. 2015). Then, C. montrouzieri sent from California in 1979 was introduced into a pesticide-free citrus grove in Shizuoka City, the central portion of the main island in 1980, but afterwards the ladybird had not been detected in the grove (Furuhashi and Fushimi 2001). Therefore, it has long been recognized that C. montrouzieri had failed to become established on the main island of Japan (Murakami 1997).
Recently, however, C. montrouzieri has been recorded at many sites in the central portion of the main island of Japan (Furuhashi and Fushimi 2001; Ishikawa 2011; Kawashita and Matsubara 2002; Ohashi 2001; Tanaka and Saito 2001; Yahiro 2008). In the citrus grove in Shizuoka City where C. montrouzieri was released in 1980, adults and larvae of the ladybird were found in 2001 (Furuhashi and Fushimi 2001). In addition, C. montrouzieri adults and larvae have been noted since 1999 in several non-agricultural fields in central Japan, including the prefectures of Osaka (Ohashi 2001), Hyogo (Tanaka and Saito 2001), Shiga (Yahiro 2008), and Kanagawa (Kawashita and Matsubara 2002). However, all these studies were conducted for only 1 or 2 years, and hence could not completely prove the ladybird’s establishment on the main island of Japan. Long-term studies on the occurrence of C. montrouzieri at particular sites on the main island of Japan therefore need to be carried out to provide unequivocal evidence for the ladybird’s permanent establishment on the island. In addition, long-term monitoring would also document yearly change and seasonal prevalence of the abundance of C. montrouzieri in fields in Japan. This information would help elucidate factors determining population dynamics of the ladybird and relationships between the ladybird and its prey insects on the main island of Japan.
The present study monitored yearly and seasonal occurrence of adults of C. montrouzieri during the 5 years of 2008–2012 in experimental citrus groves, including the one where the ladybird was introduced in 1980 and rerecorded in 2001 (Furuhashi and Fushimi 2001), at Shizuoka Prefectural Fruit Tree Research Center in Shizuoka City by using sticky traps set inside citrus tree canopies and beating citrus leaves. Furuhashi and Fushimi (2001) implied the establishment of the ladybird in the citrus grove based on a single-month survey. Multiple-year monitoring is necessary to supply more convincing evidence for the ladybird’s establishment in the grove. In addition, insect species that C. montrouzieri larvae were feeding on was recorded in the citrus groves. Monitoring of C. montrouzieri adults was also conducted in nine commercial citrus groves in Shizuoka City during the 5 years using sticky traps to detect its occurrence in the city. Based on the results obtained, this study discusses the possibility of the ladybird’s reproduction and establishment in the citrus groves on the main island of Japan, the ladybird’s possible prey insects on citrus, and factors influencing its yearly/seasonal population changes in citrus groves in central Japan.
Materials and methods
Ladybird
The body of the C. montrouzieri adult is 3.8–4.2 mm in length (Kurosawa et al. 1985). The head and pronotum are light red–orange in color. The elytra are dark brown–black (ground color), with a light red–orange hind apex. The pronotum and elytra are densely covered with short silver hairs.
Yearly and seasonal occurrence at Shizuoka Fruit Tree Research Center
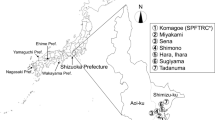
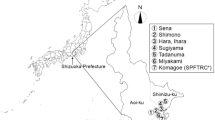
Occurrence of C. montrouzieri adults was monitored in experimental groves (each more than 0.05 ha in area) cultivating Satsuma mandarin, Citrus unshiu Marc, at Shizuoka Prefectural Fruit Tree Research Center in Komagoe, located in the southern, coastal area of Shimizu-ku, Shizuoka City (Fig. 1, No. 7); the research center was moved to a different site called Mobata in the city in 2015. Figure 2 shows the surveyed groves (sites A–E), including one where no pesticide had been applied for over 30 years [hereafter referred to as pesticide-free grove: KO-A(f)], ones where petroleum oil was only sprayed in June [hereafter referred to as organic grove: KO-B(o), KO-E(o)], one where pesticide application was reduced approximately 50% less than that in conventionally managed groves [hereafter referred to as reduced-pesticide grove: KO-C(r)], and one that was conventionally managed [hereafter referred to as conventional grove: KO-D(c)].
Map showing experimental citrus groves where Cryptolaemus montrouzieri adults were monitored using sticky traps and beating method in Komagoe (Shizuoka Prefectural Fruit Tree Research Center) in Shizuoka City in 2008–2012. A pesticide-free, B organic, C reduced-pesticide, D conventional, E organic grove
Monitoring using sticky traps was conducted in four and five groves in 2008 (from May through December) and 2009–2012 (each from January through December), respectively (Table 1). Three (in 2008) or five (in 2009–2012) citrus trees, which were approximately 5 m apart from one another, were selected at the center of each grove. Inside each tree canopy, a single piece of double-sided, yellow sticky card trap (a yellow adhesive sheet (‘IT-sheet’, Sankei Chemical CO., LTD) of 100 × 200 mm attached on each side of a yellow acrylic plate of 200 × 200 mm) was set in a vertical position 1.3–1.5 m above the ground using a wooden pole and metal fittings. These traps were replaced at an interval of 6–8 days (generally 7 days). The number of C. montrouzieri adults caught on the collected traps was counted under a binocular microscope (OLYMPUS SZX12); identification of the ladybird was based on the external morphology described above.
Survey by beating citrus leaves was carried out in four and five groves in 2008 (from May through October) and 2009–2012 (each from April through November), respectively (Table 2). This was done twice a month: early (on 6–8th, generally 7th) and late (on 22–24th, generally 23rd) in each month. Ten citrus trees were chosen randomly at the center of each grove; citrus trees inside which sticky traps were set were not included. Then, two portions (each involving approximately 100–200 leaves) on opposite sides (180° apart) were selected on each tree. Citrus leaves and twigs of each portion were beaten 10 times using a wooden pole (1.5 cm in diameter, 1 m in length) and insects falling from the leaves were caught using an insect net (20 cm in diameter) held under each portion. Thus, a total of 200 times of beating were done in each grove. Adults of C. montrouzieri captured were counted under the binocular microscope.
Identification of prey insects
To find C. montrouzieri larvae and its prey insects, five citrus trees were chosen randomly at the center of each citrus grove when replacing sticky traps, and then the leaves and twigs of each tree were roughly observed for about 2 min. Insects that many C. montrouzieri larvae were consuming were collected from citrus trees only in KO-C(r) in mid-September 2011 and then were identified in the laboratory.
Occurrence in Shizuoka City
Monitoring of C. montrouzieri adults using sticky traps was performed in commercial C. unshiu groves (each more than 0.3 ha in area), located in the southern part of Shizuoka City, mostly in Shimizu-ku, one of the major citrus-producing areas in Shizuoka Prefecture (Fig. 1, nos. 1–6); a total of two, five, six, nine, and nine groves in 2008 (from July through November), 2009, 2010 (both from May through November), 2011, and 2012 (both from April through November), respectively (Table 3). The groves included ones in Sena-1, Shimono, and Sugiyama that were organically managed [hereafter referred to as SE-1(o), SH(o), and SU(o)], one in Tadanuma-1 that was a reduced-pesticide grove [TA-1(r)], and ones in Sena-2, Hara, Ihara, Tadanuma-2, and Miyakami that were conventionally managed [SE-2(c), HA(c), IH(c), TA-2(c), and MI(c)]. These groves were selected because different levels of pesticide application might affect the occurrence of C. montrouzieri directly and/or indirectly through reducing abundance of its prey. In 2008, adults were surveyed in one reduced-pesticide, and one conventional grove. In 2009, adults were monitored in one reduced-pesticide, and four conventional groves. In 2010, monitoring was performed in one organic, one reduced-pesticide, and four conventional groves. In 2011 and 2012, this was done in three organic, one reduced-pesticide, and five conventional groves.
Three citrus trees, approximately 5 m apart from one another, were chosen at the center of each grove. A piece of double-sided, yellow sticky card trap was set inside each tree canopy, as stated above. These traps were replaced at an interval of 12–16 days (generally 14 days), and the number of C. montrouzieri adults captured on the collected traps was counted under a binocular microscope (OLYMPUS SZX12).
Results
Yearly adult occurrence
In a pesticide-free citrus grove [KO-A(f)], the number of C. montrouzieri adults caught on sticky traps was greatest among the five groves surveyed at Shizuoka Fruit Tree Research Center (Table 1). The ladybird adults were continuously trapped for the 5 years examined, but the numbers varied largely across the years, greatly increasing from 2008 to 2009, reaching a peak in 2010, and then decreasing rapidly. In a reduced-pesticide grove [KO-C(r)], C. montrouzieri adults were captured for three consecutive years (2009–2011), with the numbers exhibiting a small peak in 2010. In an organic grove [KO-B(o)] that was adjacent to KO-A(f), some adults were recorded in 2009 and 2010. In a conventional grove [KO-D(c)] and another organic grove [KO-E(o)], adults were rarely detected.
In KO-A(f), C. montrouzieri adults collected by beating citrus leaves were also most abundant among the five groves, showing the same yearly change in numbers as the adults caught on sticky traps (Table 2). In KO-C(r), adults were noted for 4 years straight (2009–2012), and the numbers increased largely from 2009 to 2010, reaching a peak in 2011.
No clear tendency was found for the effect of pesticide application levels on the ladybird abundance.
Seasonal adult occurrence
Adults of C. montrouzieri were captured on sticky traps at the research center mainly from early April through June and from mid-August through October, with the largest numbers in late September to early October (Fig. 3). In each of 2009 and 2010 when many adults were detected, adult numbers showed four peaks: in mid-April, early to late June, mid-August, and late September to early October.
Many adults were caught by beating citrus leaves from early April to early June and from late August through November, with more adults in October (Fig. 4). In both monitoring methods, adults were more abundant in autumn. Beating citrus leaves collected many adults in November, but sticky traps did not.
Seasonal changes in the number of Cryptolaemus montrouzieri adults caught by beating citrus leaves in each experimental citrus grove in Komagoe (Shizuoka Prefectural Fruit Tree Research Center) in Shizuoka City in 2008–2012. No ladybird was captured in 2008 (see Table 2)
Prey insects
Insects that many larvae of C. montrouzieri were consuming on citrus trees in KO-C(r) in mid-September 2011 were identified as larvae of a cottony scale, Pulvinaria aurantii Cockerell (Hemiptera: Coccidae).
Occurrence in Shizuoka City
Among nine commercial citrus groves in Shizuoka City, a single C. montrouzieri adult was captured on sticky traps only in an organic grove [Sena-1(o)] in 2012 (Table 3).
Discussion
Adults of the exotic ladybird C. montrouzieri were caught for five and four consecutive years in a pesticide-free citrus grove and a neighboring reduced-pesticide citrus grove, respectively, in Shizuoka City, located in the central part of the main island of Japan (Tables 1, 2). Larvae of the ladybird were also observed in the latter grove, preying on P. aurantii on citrus trees. These results indicate that C. montrouzieri had continuously passed many generations through reproduction in the citrus groves during the 5 or 4 years; this means that the ladybird has become established in the groves. Several studies have detected so far the occurrence of the ladybird in central Japan (Furuhashi and Fushimi 2001; Ishikawa 2011; Kawashita and Matsubara 2002; Ohashi 2001; Tanaka and Saito 2001; Yahiro 2008), but they all carried out 1- or 2-year surveys. Therefore, the present long-term (5-year) study provides the first unequivocal evidence for the establishment of C. montrouzieri on the main island of Japan.
The geographical distribution of C. montrouzieri in Japan had been limited to the Ogasawara Islands, located in a subtropical region, before the 1990s (Kurosawa et al. 1985). The present study proves the establishment of the ladybird on the main island of Japan, and other studies (Ishikawa 2011; Kawashita and Matsubara 2002; Ohashi 2001; Tanaka and Saito 2001; Yahiro 2008) suggest its wide distribution in central Japan. The present study also detected its presence at one site in Shizuoka City that was ca. 10 km away from the citrus groves where the ladybird has become established (Table 3). Several species of exotic predacious ladybirds that had intruded into Japan, such as Adalia bipunctata (Linnaeus) and Platynaspidius maculosus (Weise), are also now expanding their distributions on the main island (Kaneko 2013; Toda and Sakuratani 2006). Some alien aphidophagous ladybird species have caused declines in the abundance of indigenous ladybird species (e.g., Brown et al. 2011; Elliott et al. 1996; Evans 2004). Increased abundance of C. montrouzieri on the main island of Japan might negatively affect abundance of native ladybirds, such as Hyperaspis japonica (Crotch) and Nephus phosphorus (Lewis) that attack mealybugs and soft scales. Therefore, we need to carefully monitor the spread of distributions and abundance of exotic ladybirds, including C. montrouzieri, in Japan. Setting sticky traps inside tree canopies would be a useful method for surveying ladybirds, as shown by the present study and others (Kaneko et al. 2006; Kaneko 2013).
The present study documented by beating citrus leaves that many C. montrouzieri adults existed on citrus trees in late November and early April at Shizuoka Fruit Tree Research Center that is located in the southern, coastal area of central Japan (Figs. 1, 4). Sticky traps set inside citrus trees, on the other hand, collected few C. montrouzieri adults in those seasons (Fig. 3), which might be due to lower foraging (especially flying) activities of the ladybird adults at lower temperatures. The result of beating citrus trees implies that the ladybird overwintered in the adult stage in the citrus groves or neighborhood. The ladybird is unable to survive cold winters in temperate zones (Bartlett 1974; Maes et al. 2015). Bartlett (1974) experimentally showed that half of the C. montrouzieri adults died when exposed to an air temperature of −3.9 °C for about 7 h. Meteorological data at an AMeDAS point named ‘Shimizu’ (the nearest AMeDAS point from Shizuoka Fruit Tree Research Center) indicate that the lowest air temperature was never lower than −3.9 °C during 2008–2012 (the lowest temperature during the period was −3.5 °C on 3 February 2012; Japan Meteorological Agency 2016a). This suggests that the relatively warmer winter might allow C. montrouzieri adults to overwinter at the research center even though it is located in a temperate zone. Microhabitats where the ladybird adults stay in winter (e.g., on citrus/other trees, beneath leaf litter, or under rocks) are unclear at the site, thus need to be determined.
Cryptolaemus montrouzieri attacks a wide range of hemipteran species (Kairo et al. 2013). The present study found C. montrouzieri larvae feeding on a cottony scale P. aurantii on citrus trees. Predation by the ladybird on P. aurantii on citrus was also shown by Bozorg-Amirkalaee et al. (2015) in Iran. Ohashi (2001) observed that C. montrouzieri adults and larvae consumed P. aurantii on oleander, Nerium oleander L., in Japan; this is the only study that reported the prey insect of C. montrouzieri in the country. Thus, P. aurantii would be one of important prey insects for the exotic ladybird in Japan.
Seasonal occurrence of adults of coccidophagous ladybirds is likely to be associated with that of their prey insects in the case that ladybird larvae preferentially feed on particular developmental stages of prey (Kaneko et al. 2006). Adult females of C. montrouzieri lay eggs into the egg sacs of mealybugs and soft scales, and then hatched ladybird larvae consume eggs, crawlers, and young larvae of the prey insects (Shylesha and Mani 2016). Adult females of P. aurantii form cottony egg sacs in mid-May and mid- to late August in Japan, producing crawlers of the following generation in late May to early June and early to mid-September (Kawai 1980). The present study indicated that the number of C. montrouzieri adults trapped in the citrus groves in late summer to autumn exhibited two peaks: in mid-August and late September to early October (Fig. 3). It might be expected that in the groves C. montrouzieri females search for and oviposit into egg sacs of P. aurantii females in mid-August and then hatched larvae grow by preying on P. aurantii eggs and larvae, finally becoming adults in late September to early October. Average air temperature over 1981–2010 at an AMeDAS point named ‘Shimizu’ (the nearest AMeDAS point from the citrus groves) was approximately 25 °C in mid-August to late September (Japan Meteorological Agency 2016b). Cryptolaemus montrouzieri requires ca. 47 days from deposited egg to adult emergence under laboratory conditions of 25 °C (Babu and Azam 1987; Kairo et al. 2013). These facts may explain the duration between the two peaks of the ladybird adult numbers detected in the citrus groves in late summer to autumn. On the other hand, two peaks of the ladybird adult numbers were also noted in mid-April and early to late June (Fig. 3). However, egg sac formation by P. aurantii females in spring occurs in mid-May (Kawai 1980), which cannot account for abundant ladybird adults in mid-April; this might be because the ladybird exploited prey insects other than P. aurantii in the citrus groves in spring to early summer. Seasonal changes in the occurrence of C. montrouzieri larvae and P. aurantii larvae and their interactions on citrus trees therefore need to be documented to assess influence of P. aurantii on the seasonal occurrence of C. montrouzieri adults in citrus groves.
Abundance of C. montrouzieri adults in each citrus grove varied largely across the 5 years examined, greatly increasing and then decreasing rapidly during a period of 3 to 4 years (Tables 1, 2). In the citrus grove KO-C(r), abundance of P. aurantii on citrus trees showed a similar pattern to that of the ladybird, being much higher in 2010–2011 and extremely low in 2012 (S. Kaneko, personal observation). This implies that the drastic change in C. montrouzieri adult abundance in the citrus groves might be connected with the change in P. aurantii density. On the other hand, mealybugs and soft scales also occurred in the citrus groves, and hence there remains a possibility that the ladybird exploited insects other than P. aurantii there. Composition of insect species that C. montrouzieri attacks on citrus trees and its seasonal difference in Japan need to be investigated in order to determine factors affecting yearly/seasonal population dynamics of the ladybird and to evaluate its role in controlling populations of insect pests in citrus groves.
References
Babu TR, Azam KM (1987) Biology of Cryptolaemus montrouzieri Mulsant, (Coccinellidae: Coleoptera) in relation with temperature. Entomophaga 32:381–386
Bartlett BR (1974) Introduction into California of cold-tolerant biotypes of the mealybug predator, Cryptolaemus montrouzieri, and laboratory procedures for testing natural enemies for cold-hardiness. Environ Entomol 3:553–556
Booth RG, Pope RD (1986) A review of the genus Cryptolaemus (Coleoptera: Coccinellidae) with particular reference to the species resembling C. montrouzieri Mulsant. Bull Entomol Res 76:701–717
Bozorg-Amirkalaee M, Fathi SAA, Golizadeh A, Mahdavian SE (2015) Performance of Cryptolaemus montrouzieri feeding on Pulvinaria aurantii ovisacs on citrus plants. Biocontrol Sci Technol 25:207–222
Brown PMJ, Frost R, Doberski J, Sparks T, Harrington R, Roy HE (2011) Decline in native ladybirds in response to the arrival of Harmonia axyridis: early evidence from England. Ecol Entomol 36:231–240
DeBach P, Hagen KS (1964) Manipulation of entomophagous species. In: DeBach P, Schlinger EI (eds) Biological control of insect pests and weeds. Chapman and Hall, London, pp 429–458
Elliott N, Kieckhefer R, Kauffman W (1996) Effects of an invading coccinellid on native coccinellids in an agricultural landscape. Oecologia 105:537–544
Evans EW (2004) Habitat displacement of North American ladybirds by an introduced species. Ecology 85:637–647
Furuhashi K, Fushimi N (2001) The establishment of natural enemy, Cryptolaemus montrouzieri of mealybugs in Shizuoka Prefecture. Plant Prot 55:572–573 (in Japanese)
Furuhashi K, Nishino M (1980) Reintroduction of Cryptolaemus montrouzieri into Japan and its predation effect on mealybugs. Proc Kansai Plant Prot Soc 22:28–29 (in Japanese)
Ishii T (1955) Applied zoology in Japan for 25 years: 5. Natural enemies. Jpn J Appl Zool 20:25–28 (in Japanese)
Ishikawa H (2011) New record of Platynaspidius maculosus from Shizuoka Prefecture in Japan. Gekkan-Mushi 487:46 (in Japanese)
Japan Meteorological Agency (2016a) http://www.data.jma.go.jp/obd/stats/etrn/view/annually_a.php?prec_no=50&block_no=1243&year=&month=&day=&view= Accessed 1 December 2016 (in Japanese)
Japan Meteorological Agency (2016b) http://www.data.jma.go.jp/obd/stats/etrn/view/nml_amd_10d.php?prec_no=50&block_no=1243&year=&month=&day=&view=p1 Accessed 1 December 2016 (in Japanese)
Kairo MTK, Paraiso O, Gautam RD, Peterkin DD (2013) Cryptolaemus montrouzieri (Mulsant) (Coccinellidae: Scymninae): a review of biology, ecology, and use in biological control with particular reference to potential impact on non-target organisms. CAB Rev 8(5):1–20
Kaneko S (2013) Occurrence of the exotic predatory ladybird Platynaspidius maculosus (Coleoptera: Coccinellidae) in citrus groves in Shizuoka City, Central Japan: seasonal prevalence of adults captured on sticky traps. Appl Entomol Zool 48:189–194
Kaneko S, Ozawa A, Saito T, Tatara A, Katayama H, Doi M (2006) Relationship between the seasonal prevalence of the predacious coccinellid Pseudoscymnus hareja (Coleoptera: Coccinellidae) and the mulberry scale Pseudaulacaspis pentagona (Hemiptera: Diaspididae) in tea fields: monitoring using sticky traps. Appl Entomol Zool 41:621–626
Kawai S (1980) Scale insects of Japan in colors. Zenkoku Noson Kyoiku Kyokai, Tokyo (in Japanese)
Kawashita T, Matsubara Y (2002) Record of Cryptolaemus montrouzieri from Yokohama City in Japan. Gekkan-Mushi 382:11–12 (in Japanese)
Kurosawa Y, Hisamatsu S, Sasaji H (1985) The Coleoptera of Japan in color, vol III. Hoikusha Publishing, Osaka (in Japanese)
Maes S, Grégoire JC, De Clercq P (2015) Cold tolerance of the predatory ladybird Cryptolaemus montrouzieri. Biocontrol 60:199–207
Murakami Y (1997) Natural enemies of the chestnut gall wasp: approaches to biological control. Kyushu University Press, Fukuoka (in Japanese)
Ohashi K (2001) Occurrence of the ladybird beetle Cryptolaemus montrouzieri Mulsant (Coleoptera: Coccinellidae) in Osaka, Japan. Jpn J Environ Entomol Zool 12:185–186
Sakimura K (1935) Transportation of predaceous coccinellids from Saipan to Bonin Islands and Formosa. Kontyû 9:76–82
Shylesha AN, Mani M (2016) Natural enemies of mealybugs. In: Mani M, Shivaraju C (eds) Mealybugs and their management in agricultural and horticultural crops. Springer, New Delhi, pp 149–172
Tanaka I, Saito T (2001) Record of Cryptolaemus montrouzieri in the Kinki region in Japan. Gekkan-Mushi 366:46–47 (in Japanese)
Toda Y, Sakuratani Y (2006) Expansion of the geographical distribution of an exotic ladybird beetle, Adalia bipunctata (Coleoptera: Coccinellidae), and its interspecific relationships with native ladybird beetles in Japan. Ecol Res 21:292–300
Yahiro K (2008) Record of Cryptolaemus montrouzieri from Shiga Prefecture in Japan. Came-Mushi 148:7 (in Japanese)
Acknowledgements
This work was partially supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (no. 1111 in ‘Selection of functional biodiversity indicators and development of assessment methods’ in 2008–2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaneko, S. Establishment and yearly/seasonal occurrence of the exotic coccidophagous ladybird Cryptolaemus montrouzieri (Coleoptera: Coccinellidae) in citrus groves in Shizuoka City, central Japan: a 5-year survey on adult numbers. Appl Entomol Zool 52, 231–240 (2017). https://doi.org/10.1007/s13355-016-0471-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-016-0471-y