Abstract
Soldier differentiations require high juvenile hormone (JH) titers in workers and two molts via a presoldier stage. However, it is still unclear when the endogenous JH titer increases in workers, because identifying an individual before it undergoes the presoldier molt is quite difficult in natural conditions. In an incipient colony of Zootermopsis nevadensis Hagen, the oldest 3rd instar larva (Νo. 1 larva) always differentiates into a presoldier and the second 3rd instar larva (Νo. 2 larva) molts into a 4th instar larva. Consequently, by focusing on the ontogeny of the Νo. 1 larva, the timing of the increase in the JH titer can be determined. Here, we compared the expression levels of JH biosynthetic genes in the heads of Νo. 1 and Νo. 2 larvae. Most genes involved in JH biosynthesis were found in a genome database, and two candidate JH epoxidase (CYP15A1) genes were identified. Expression levels of JH acid methyltransferase (JHAMT) and one candidate CYP15A1, probably involved in the final steps of the JH biosynthesis, were specifically high in Νo. 1 larvae at 2–3 and 4–5 days after the appearance, respectively. Expression patterns of these genes may be used for the prediction of endogenous JH titer changes during presoldier differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect societies are highly sophisticated because of the cooperation and division of labor among castes (Wilson 2000). Among sterile castes, which exhibit altruistic behaviors, the termite soldiers are a unique caste in terms of specific morphology and its role in nest defense (Deligne et al. 1981). All extant species, except for some lineages that have suffered a secondary loss, have a soldier caste (Lo and Eggeleton 2011). Consequently, it is strongly suggested that soldiers evolved just once in the course of eusocial termite evolution (Lo and Eggeleton 2011; Noirot and Pasteels 1987).
Caste differentiation in termites is an example of polyphenism; alternative phenotypes are generated through changes in the developmental process in response to environmental cues during the postembryonic period (Hartfelder and Emlen 2012; Miura 2005; Nijhout 2003; Roisin 2000; Watanabe et al. 2014). Generally, in insects, different phenotypes resulting from alterations in developmental processes are regulated by the endocrine system (Hartfelder and Emlen 2012; Nijhout 1999). For caste differentiation in termites, juvenile hormone (JH) plays a central role (Henderson 1998; Miura and Scharf 2011; Nijhout and Wheeler 1982). Soldiers differentiate from workers by undergoing two molts via a presoldier stage, which is associated with an increasing JH titer in workers (Cornette et al. 2008a). Previous studies reported that JH or JH analog (JHA) treatment of workers induced presoldier differentiation (e.g., Haward and Haverty 1979; Itano and Maekawa 2008; Maekawa et al. 2014; Ogino et al. 1993; Scharf et al. 2003; Toga et al. 2009; Tsuchiya et al. 2008). Based on these presoldier induction methods, soldier-specific morphogenesis and physiological/genetic changes after an increase in the JH titer in workers have been analyzed, and some important changes were identified (e.g., Cornette et al. 2008b, 2013; Koshikawa et al. 2005; Masuoka et al. 2013; Toga et al. 2012, 2013; Watanabe and Maekawa 2012). However, it is still unknown when the genes involved in JH biosynthesis are activated and/or the JH titer increases during soldier differentiation under natural conditions. This is because the soldier ratio in a colony is normally low compared with workers (usually below 10 %; Haverty 1977), and discriminating an individual worker that will differentiate into a presoldier and soldier is quite difficult under natural conditions.
In an incipient colony of the damp-wood termite Zootermopsis nevadensis Hagen (Isoptera: Archotermopsidae), which belongs to one of the basal lineages of termites, the oldest 3rd instar larva (Νo. 1 larva) always differentiates into a presoldier, and the 2nd oldest 3rd instar larva (Νo. 2 larva) molts into a 4th instar worker (Maekawa et al. 2012). When the Νo. 1 larva was artificially removed from the colony, the Νo. 2 larva differentiated into a presoldier, suggesting that presoldier differentiation was not determined genetically. This study was the first case to show that a particular individual differentiates into a presoldier before the molt. By focusing on the Νo. 1 larvae in incipient colonies of this species, the expression patterns of genes involved in JH biosynthesis can be elucidated during presoldier differentiation under natural conditions.
JH is synthesized in the corpora allata, and the biosynthetic pathway mainly comprises two enzymatic pathways: the early steps and late steps of the JH synthesis pathway (Bélles et al. 2005) (Fig. 1). The former is the broadly conserved mevalonate pathway, which includes eight enzymatic reactions. The latter is specific to insects and includes five enzymatic reactions. Based on the EST libraries derived from the corpora allata and corpora cardiaca of three insect species (cockroach Diploptera punctata Eschscholtz and mosquitoes Aedes aegypti L. and Anopheles albimanus Weidemann), JH biosynthetic genes were identified, and the expression of at least six genes was examined (Noriega et al. 2006). The expression of JH biosynthetic genes was also observed in the corpora allata and corpora cardiaca of the honeybee Apis mellifera L. (Bomtorin et al. 2014). Moreover, based on an EST library derived from multiple tissues of the silkworm moth Bombyx mori L., eight genes in the mevalonate pathway were identified, and specific expression in the corpora allata was elucidated (Kinjoh et al. 2007; Ueda et al. 2009). These studies indicated that the expression levels of JH biosynthetic genes influence endogenous changes in the JH titer. In particular, the JH titer may be strongly correlated with the expression levels of JH acid methyltransferase (JHAMT) and JH epoxidase (CYP15A1), both of which are involved in the final steps of JH biosynthesis (Fig. 1). For example, knockdown of JHAMT by RNA interference (RNAi) resulted in a lower level of JH synthesis in the corpora allata in the desert locust Schistocerca gregaria Forsskål (Marchal et al. 2011). A decrease in the JH titer in A. mellifera was also achieved by JHAMT RNAi (Bomtorin et al. 2014). CYP15A1, encoding JH epoxidase (Helvig et al. 2004), converts methyl farnesoate into JH III, and RNAi resulted in an increase in the level of methyl farnesoate in the corpora allata and a decrease in the JH titer in S. gregaria (Marchal et al. 2011). Moreover, knockdown of a JH epoxidase homolog, CYP15F1, by RNAi resulted in a decrease in presoldier differentiation from workers in the rhinotermitid termite Reticulitermes flavipes Kollar (Tarver et al. 2012). Consequently, changes in the endogenous JH titer may also be influenced by the expression levels of these genes in Z. nevadensis.
The genome sequence of Z. nevadensis was published, and genes involved in the JH biosynthetic pathways were predicted (Terrapon et al. 2014). In the present study, we first tried to quantify the JH titer in 3rd instar larvae using high-performance liquid chromatography-mass spectrometry (HPLC-MS) according to the previously described methods (e.g., Maekawa et al. 2010). Then, expression levels of JH biosynthetic genes were compared between the Νo. 1 and Νo. 2 larvae using real-time quantitative PCR (qPCR). Based on the results, we discuss the timing of changes in the endogenous JH titer during presoldier differentiation in Z. nevadensis in natural conditions.
Materials and methods
Incipient colony foundation
Mature colonies of Z. nevadensis were collected from Kawanishi-shi, Hyogo Prefecture, Japan, in April 2013. These colonies were maintained in constant darkness at room temperature in the laboratory. After alates (winged adults) emerged from the colonies, they were collected and separated by sex. To establish incipient colonies, male and female alates from different colonies were paired in 60-mm plastic dishes with crushed pieces of nest wood. These dishes were kept in constant darkness at 25 °C until the 3rd instar larvae (Νo. 1 and 2 larvae) appeared in each colony. The abdomens of the Νo. 1 and Νo. 2 larvae were marked with waterproof inks of different colors to discriminate each other (Maekawa et al. 2012).
JH titer quantification
Experimental details were reported previously (Maekawa et al. 2010; Saiki et al. 2015; Watanabe et al. 2011). Briefly, the 3rd instar larvae for JH extraction were sampled from a mature colony. Five and ten individuals per sample were collected, and each sample was prepared in triplicate. Based on the previous protocols, 30 ng of fenoxycarb (Wako, Osaka, Japan) was used as an internal standard, and JH extraction in the whole body was performed. The JH titer in each sample was quantified with an Agilent 1100 HPLC system with an autosampler (Agilent Technologies, Santa Clara, CA, USA). Mass spectral analysis was performed by electrospray ionization in the positive mode on a microTOF-HS (Bruker Daltonik GmbH, Germany).
Juvenile hormone biosynthetic genes identified from the Z. nevadensis genome database
Terrapon et al. (2014) reported gene sequences involved in the JH biosynthesis pathways, and the following 13 genes were obtained from the database (http://termitegenome.org/): acetoacetyl-CoA thiolase (AcoAT) (Gene ID: Znev_06783), 3-hydroxy-3-methylglutaryl-CoA synthase 1 and 2 (HMGS1, 2) (Znev_08283 and 14140), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) (Znev_02974), mevalonate kinase (MK) (Znev_14029), phosphomevalonate kinase (PK) (Znev_12655), diphosphomevalonate decarboxylase (DD) (Znev_08133), isopentenyl diphosphate isomerase (IPPI) (Znev_09747), farnesyl pyrophosphate synthase (FPS) (Znev_02161), farnesal dehydrogenase 1 and 2 (FD1, 2) (Znev_01798 and 03358), JH acid methyltransferase (JHAMT) (Znev_12145) and JH epoxidase (CYP15A1) (Znev_14299).
Characterization of JHAMT
The sequence length of JHAMT in the genome database was extremely short (171 bp), and the conserved motif (SAM-binding motif, see results) could not be found. Thus, gene-specific PCR primers were newly designed, and subcloning and sequencing were performed.
cDNA preparation
Total RNA was extracted from the whole bodies of Νo. 1 larvae using ISOGEN (NipponGene, Tokyo, Japan). Five different individuals were used for RNA extraction. The extracted total RNA was purified with DNase treatment to remove contaminating DNA. RNA purity and quantity were measured using a NanoVue spectrophotometer (GE Healthcare BioSciences, Tokyo, Japan). The purified RNA was reverse-transcribed for cDNA synthesis using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster, CA, USA).
Subcloning and sequencing
BLAST searches were performed against the Z. nevadensis genome database using D. punctata JHAMT (GenBank accession no. AHZ20738). For RT-PCR, a forward primer (5′-ATG CAC AAT GCG GAA CTC T–3′) and a reverse primer (5′-TTA CGT TTT TCT TGC GTA CGC–3′) were newly designed based on the obtained sequence using Primer3Plus (Untergasser et al. 2007). PCR products were purified using a QIAquick Gel Extraction Kit (Qiagen, Tokyo, Japan) and subcloned into a pGEM easy T-vector (Promega, Madison, WI, USA). The inserted DNA sequence was amplified by PCR, and purified products were sequenced using a BigDye Terminator v3.1 Cycle Sequencing Kit and an automatic DNA Sequencer 3130 Genetic Analyzer (Applied Biosystems). Obtained sequences were subjected to BLAST database searches and compared with other published JHAMT sequences using MUSCLE in MEGA5 (Tamura et al. 2011).
Alignment and phylogenetic analysis of CYP15A1
According to Terrapon et al. (2014), there was only one CYP15A1 gene in the Z. nevadensis genome (Znev_14299). However, BLAST database searches were performed using the published CYP15A1 genes from other insect species [R. flavipes (Tarver et al. 2012; no. FJ792774), D. punctata (Helvig et al. 2004; no. AY509244) and S. gregaria (Marchal et al. 2011; no. HQ634703)] and found another similar gene sequence in Z. nevadensis (gene ID: Znev_06057). Multiple alignment was performed using these two amino acid sequences with seven published sequences derived from six insect species (including CYP306A1 genes from fruit fly Drosophila melanogaster Meigen and from Z. nevadensis) using MUSCLE in MEGA5 (Tamura et al. 2011). For phylogenetic analysis, a maximum likelihood tree was constructed based on the WAG + Gamma distribution model using amino acid sequences (100 bootstrap replications).
Gene expression analyses using real-time quantitative PCR
Gene expression analysis between Νo. 1 and 2 larvae
No. 1 and 2 larvae were collected at three time points (0–1, 2–3 and 4–5 days after the appearance) in each colony. Eighteen different Νo. 1 and 2 larvae were sampled at each time point. Individuals were immersed immediately in liquid nitrogen and stored at −80 °C until use (three individuals in one tube). All individuals were separated into heads and other body parts, and to compare gene expressions between Νo. 1 and 2 larvae, head samples were used for cDNA preparation. Three different individuals were used for each cDNA sample, and in total six cDNA samples were prepared for each time point. In accordance with the procedure cited above, total RNA extraction, DNase treatment and cDNA synthesis were performed. For cDNA preparation, equal concentrations of total RNA (22 ng) were reverse-transcribed.
To perform real-time quantitative PCR, sequence-specific primers were designed against JH biosynthetic genes using Primer3Plus (Table 1). qPCR was performed using a Thunderbird qPCR mix (Toyobo, Osaka, Japan) using a MiniOpticon Real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The suitability of three reference genes [EF-1α, β-actin and NADH-dh from Z. nevadensis (GenBank accession no. AB915828, AB915826 and AB936819, respectively)] was evaluated using GeNorm (Vandesompele et al. 2002) and NormFinder software (Andersen et al. 2004). Because both softwares showed that the expression levels of EF-1α were the most stable, this gene was used as a reference gene. qPCR analysis was performed in biological sextuplicates (number of individuals examined = 3 × 6). Statistical analysis was carried out by two-way ANOVA and Tukey’s test with Mac Statistical Analysis version 2.0 (Esumi, Tokyo, Japan).
Gene expression analysis in each body part of Νo. 1 larvae
For the gene expression analysis in each body part, heads and other body parts of Νo. 1 larvae were used for cDNA preparation. For cDNA preparation, equal concentrations of total RNA (360 ng) were reverse-transcribed. qPCR analysis was performed in biological sextuplicates (number of individuals examined = 3 × 6). In accordance with the method shown above, JHAMT and CYP15A1 gene expression levels were quantified and compared between heads and other body parts.
Results
JH titer quantification using high-performance liquid chromatography-mass spectrometry
We performed JH titer quantification using 3rd instar larvae collected from a mature colony. Each sample consisted of 5 or 10 individuals, and measurement was replicated three times in each sample. Although the level of fenoxycarb could be quantified, the peak of JH III in the chromatograph could not be measured, even in samples extracted from 10 individuals (data not shown).
Identification of JH biosynthetic genes and characterization of JHAMT and CYP15A1
Based on genomic information of Z. nevadensis (Terrapon et al. 2014), 13 JH biosynthetic genes were identified. Of these genes, the sequence length of JHAMT (gene ID: Znev_12145; Supplementary Table) was extremely short (171 bp) compared with those observed in other species [e.g., S. gregaria: 927 bp (no. HQ634702), B. mori: 837 bp (no. AB113578)], and there were no conserved motifs (e.g., SAM-binding motif) in this sequence. After subcloning and sequencing, we identified a 825-bp sequence, including the coding region predicted by Terrapon et al. (2014) with a SAM-binding motif (Fig. 2). The deduced amino acid sequence (275 aa) was subjected to BLASTP searches and found to have 64 and 44 % similarity with JHAMT homologs from D. punctata and S. gregaria, respectively. Thus, we concluded that this sequence is the JHAMT homolog from Z. nevadensis. The determined nucleotide and putative amino acid sequences are available in the DDBJ/EMBL/GenBank databases (accession no. LC049616).
Multiple alignment of JH acid methyltransferase (JHAMT) using the amino acid sequences of Zootermopsis nevadensis (obtained in this study, GenBank accession no. LC049616), Apis mellifera (XM_001119986), Bombyx mori (BAC98835), Drosophila melanogaster (BAC98836), Tribolium castaneum (AB360763), Schistocerca gregaria (ADV17350) and Diploptera punctata (AHZ20738). Amino acids common to each species are indicated by white letters with a black background. The SAM-binding motif is indicated with a line above the sequences
Terrapon et al. (2014) proposed only one sequence as a gene encoding JH epoxidase (CYP15A1) (Znev_14299). We performed homology searches using CYP15A1 genes identified in D. punctata, R. flavipes and S. gregaria by BLASTX against a non-redundant database. As a result, we identified another sequence (Znev_06057) as well as the sequence reported previously (Znev_14299). Multiple alignments and molecular phylogenetic analysis showed that both of these sequences were included in the CYP15 family (Figs. 3, 4), although Znev_14299 lacks conserved P/G rich and Helix-C motifs, both of which contribute to protein conformation and the enzymatic function of CYP15 (Werck-Reichhart and Feyereisen 2000) (Fig. 3).
Multiple alignment of JH epoxidase (CYP15A1) using amino acid sequences of Zootermopsis nevadensis (Znev_14299 and Znev_06057), Apis mellifera (GenBank accession no. XM_623572), Schistocerca gregaria (ADV17351), Diploptera punctata (AAS13464), Anopheles gambiae (XP_315675), Reticulitermes flavipes (ACN93795) and R. flavipes CYP15F1 (ACN93794). Amino acids common to each species are indicated by white letters with a black background. Six conserved motifs are shown with lines above the sequences
Expression levels of genes in the early steps of the juvenile hormone biosynthetic pathway
Expression analyses were performed by qPCR using head samples of Νo. 1 and 2 larvae collected at three time points (0–1, 2–3 and 4–5 days after the molting to the 3rd larval stage). The expression level of AcoAT was not significantly different between Νo. 1 and 2 larvae at each time point (two-way ANOVA followed by Tukey’s test, p < 0.05), although interaction was detected between Νo. 1 and 2 larvae and time points (df = 2, F = 5.599, p = 8.58E−03) (Fig. 5a). Expression levels of HMGR and HMGS1 were not significantly different in all cases (Fig. 5b, c). Statistical differences between Νo. 1 and 2 larvae were observed in HMGS2 (Νo. 1/Νo. 2: df = 1, F = 8.378, p = 7.02E−03), but interaction was not detected between Νo. 1 and 2 larvae and time points (Fig. 5d). Significant differences of DD expression levels were found in all cases (Νo. 1/Νo. 2 larvae: df = 1, F = 6.873, p = 0.0136; days df = 2, F = 14.004, p = 5.06E−05; interaction: df = 2, F = 11.296, p = 2.20E−04). Expression levels of DD in Νo. 1 larvae 2–3 days after molting were significantly higher than those of any other Νo. 1 larvae (two-way ANOVA followed by Tukey’s test, p < 0.05) (Fig. 5e). Statistical differences were also observed in IPPI (Νo. 1/Νo. 2: df = 1, F = 14.497, p = 6.46E−04; interaction: df = 2, F = 3.679, p = 0.0372). Expression levels of IPPI in Νo. 2 larvae 4–5 days after molting were significantly higher than those of Νo. 1 larvae at the same time point (two-way ANOVA followed by Tukey’s test, p < 0.05) (Fig. 5f). The expression level of MK was not significantly different in all cases (Fig. 5g). Statistical differences were observed in PK (Νo. 1/Νo. 2: df = 1, F = 20.368, p = 9.18E− 05; days: df = 2, F = 4.425, p = 0.0207), but interaction was not detected between Νo. 1 and 2 larvae and time points (Fig. 5h).
Expression levels of genes (average ± SD, n = 6) in the early steps of the JH biosynthetic pathway between Νo. 1 and 2 larvae at three time points after molting. a Acetoacetyl-CoA thiolase (AcoAT), b 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), c 3-hydroxy-3-methylglutaryl-CoA synthase 1 (HMGS1), d 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGS2), e diphosphomevalonate decarboxylase (DD), f isopentenyl diphosphate isomerase (IPPI), g mevalonate kinase (MK) and h phosphomevalonate kinase (PK). Expression analyses were performed in the biological sextuplicates, and each data value was normalized to the expression level of EF1α. The black and white columns indicate Νo. 1 and 2 larvae, respectively. Different letters above the bars indicate a significant difference (two-way ANOVA followed by Tukey’s test, p < 0.05)
Expression levels of genes in late steps of the juvenile hormone biosynthetic pathway
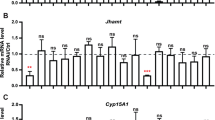
Two-way ANOVA was performed, and interaction was detected in all genes examined except for CYP15A1 (Znev_14299) (FPS: df = 2, F = 4.406, p = 2.10E−02; FD1: df = 2, F = 7.364, p = 2.50E−03; FD2: df = 2, F = 4.966, p = 0.014; JHAMT: df = 2, F = 7.269, p = 2.67E−03; CYP15A1 (Znev_06057): df = 2, F = 7.060, p = 3.07E−03) (Fig. 6). Statistical differences between Νo. 1 and 2 larvae were observed in four genes [FPS: df = 1, F = 39.900, p = 5.77E−07; FD2: df = 1, F = 18.642, p = 1.58E−04; JHAMT: df = 1, F = 12.228, p = 1.49E−03; CYP15A1 (Znev_06057): df = 1, F = 6.852, p = 0.014]. Significant differences were also found among three time points in four genes [FPS: df = 2, F = 6.487, p = 4.56E−03; FD1: df = 2, F = 5.539, p = 8.97E−03; FD2: df = 2, F = 4.585, p = 0.018; CYP15A1 (Znev_06057): df = 2, F = 6.694, p = 3.95E−03]. Expression levels of FPS in Νo. 2 larvae 2–3 and 4–5 days after molting were significantly higher than those of any other Νo. 1 larvae (Fig. 6a). Moreover, FD1 and FD2 expression levels in Νo. 2 larvae 4–5 days after molting were also significantly higher than those of Νo. 1 larvae (Fig. 6b, c). However, expression patterns of JHAMT and CYP15A1 (Znev_06057) were completely different from those of other genes examined in the late steps. Namely, JHAMT expression levels in Νo. 1 larvae 2–3 days after molting were significantly higher than those of any other Νo. 2 larvae (Fig. 6d). CYP15A1 (Znev_06057) expression levels in Νo. 1 larvae 4–5 days after molting were significantly higher and also the highest levels found among all larvae examined (two-way ANOVA followed by Tukey’s test, p < 0.05) (Fig. 6f).
Expression levels of genes (average ± SD, n = 6) in the late steps of the JH biosynthetic pathway between Νo. 1 and 2 larvae at three time points after molting. a Farnesyl pyrophosphate synthase (FPS), b farnesal dehydrogenase 1 (FD1), c farnesal dehydrogenase 2 (FD2), d JH acid methyltransferase (JHAMT), e JH epoxidase (CYP15A1) (Znev_14299) and f JH epoxidase (CYP15A1) (Znev_06057). Expression analyses were performed in the biological sextuplicates, and each data value was normalized to the expression level of EF1α. The black and white columns indicate Νo. 1 and 2 larvae, respectively. Different letters above the bars indicate a significant difference (two-way ANOVA followed by Tukey’s test, p < 0.05)
JHAMT and CYP15A1 gene expression analyses between heads and other body parts
Expression levels of JHAMT in the heads of larvae were significantly higher than those in other body parts (predominantly the thorax and abdomen) of Νo. 1 larvae (Fig. 7a). Although expression levels of both CYP15A1 genes in heads tended to be higher than those in other body parts, there were no significant differences between these levels (Fig. 7b, c) (Student’s t test, p = 0.074 and = 0.301, respectively).
Expression levels of genes (average ± SD, n = 6) in each body part of Νo. 1 larvae. a JH acid methyltransferase (JHAMT), b JH epoxidase (CYP15A1) (Znev_14299) and c JH epoxidase (CYP15A1) (Znev_06057). Expression analyses were performed in the biological sextuplicates, and each data value was normalized to the expression level of EF1α. An asterisk over the bars indicates a significant difference between groups (Welch’s t test, p < 0.05)
Discussion
JH quantification analyses
Because many termite species have been shown to have JH III [e.g., Coptotermes formosanus Shiraki (Park and Raina 2004), Reticulitermes speratus Kolbe (Maekawa et al. 2010) and Hodotermopsis sjostedti Holmgren (Cornette et al. 2008a)], Z. nevadensis is likely to have JH III. In accordance with previous studies on different termite species, JH quantification analyses using HPLC-MS were performed in 3rd instar larvae (5 or 10 individuals) sampled from mature colonies. Itano and Maekawa (2008) reported that the size of the corpora allata of 3rd instar larvae from a mature colony of Z. nevadensis was significantly larger than those from an incipient colony. Their results may indicate that the JH titers of those individuals would be higher. However, the present study fails to detect any significant levels of JH III. This failure may be attributed to the small amount of JH and/or high activity of JH-degrading enzymes during sample processing in Z. nevadensis larvae. The Νo. 1 larva is only a single individual in each incipient colony, and it is difficult to collect more individuals for JH quantification. Consequently, we focus on the expression patterns of JH biosynthetic genes.
Genes involved in juvenile hormone biosynthesis
Because we failed to detect measurable amounts of JH in the whole-body samples, we focused on genes involved in the JH biosynthesis in termites, of which homologs are shown to be expressed in the corpora allata of other insect species (e.g., B. mori, Ae. aegypti, An. albimanus, and D. punctate) (Kinjoh et al. 2007; Noriega et al. 2006). Of 13 genes examined, JHAMT is newly obtained by subcloning and sequencing. Because the obtained amino acid sequence is similar to those of other insects, and the conserved region (SAM-binding motif) is confirmed at the N-terminal site (Fig. 2), it may have enzymatic activity. JHAMT in B. mori was confirmed for the first time as the enzyme that converted JH acid to JH in the corpora allata (Shinoda and Itoyama 2003).
CYP15s belong to the cytochrome P450 gene family and are involved in JH biosynthesis (Feyereisen 2006). Multiple alignments of Znev_14299 and CYP15A1 genes previously described from other insects show that the former lacks two conserved motifs (P/G rich and Helix-C) and has one incomplete motif (PERF). These motifs are important for protein conformation (Werck-Reichhart and Feyereisen 2000). Molecular phylogenetic analysis shows that Znev_14299 is most closely related to R. flavipes CYP15A1, which possesses an N-terminal site truncation and pseudogene defect conserved motif (Tarver et al. 2012). Based on these results, there is a possibility that Znev_14299 may not have sufficient function for JH biosynthesis in Z. nevadensis. Another sequence identified in this study (Znev_06067) is confirmed to have six conserved motifs. Phylogenetic analysis shows that Znev_06067 is more closely related to R. flavipes CYP15F1 than to R. flavipes CYP15A1. CYP15F1 was highly expressed at the head and gut of R. flavipes workers, and CYP15F1 RNAi resulted in a decrease in the presoldier differentiation ratio (Tarver et al. 2012). Although further detailed analyses on these homologs (Znev_14299 and 06057) are required to determine whether they have different roles in JH biosynthesis, the expression levels of these two genes were compared between Νo. 1 and 2 larvae in this study.
Expression patterns of juvenile hormone biosynthetic genes
The present study shows that the expression of DD, JHAMT and CYP15A1 (Znev_06057) is specifically upregulated during presoldier differentiation. In particular, JHAMT and CYP15A1 are involved in the final steps of the JH biosynthetic pathway, and thus high expression levels of these genes may cause an increase in JH titer in Νo. 1 larvae. Expression levels of JHAMT in Νo. 1 larvae are significantly higher than those in Νo. 2 larvae 2–3 days after the appearance, suggesting that the JH biosynthesis in Νo. 1 larvae may be enhanced during this period. The expression level of the putative CYP15A1 (Znev_06057) is remarkably high only in Νo. 1 larvae 4–5 days after the appearance. The JH titer in these individuals probably increases quickly during this period, and then gut purging (a release of the contents of the gut) occurs before the molt into a presoldier. JHAMT expression analysis of each body part in the Νo. 1 larva indicates that its level in heads (including the corpora allata) is extremely high (approximately 60 times higher) compared with other body parts. Although further detailed analyses of expression sites are needed, it is suggested that the JH titer in Νo. 1 larvae is strongly affected by JHAMT expression in the head. In B. mori, JHAMT is involved in the final step of JH biosynthesis, and JH epoxidase (CYP15C1) was constantly expressed before the final molt (Daimon et al. 2012). The present study shows that Znev_14299 is also constantly expressed during presoldier differentiation. On the other hand, the expression level of Znev_06057 is rapidly increased before the presoldier molt. In D. punctata, epoxidase was involved in the final step of JH biosynthesis (Helvig et al. 2004); thus, Znev_06057 may be the key gene to regulate the presoldier differentiation in natural conditions. Further gene function analyses of JHAMT and two epoxidases obtained in this study should be performed to conclude this hypothesis.
There are several genes (IPPI, FPS, FD1 and FD2) with high expression levels until 5 days after the molt only in Νo. 2 larvae. The penultimate larva of the German cockroach Blattella germanica L. had consistently high JH titers during this stage (Treiblmayr et al. 2006). In the dry-wood termite Cryptotermes secundus Hill, JH titers of 3rd instar larvae were higher than those of nymphs (>4th instar) (Korb et al. 2009). In the incipient colony of Z. nevadensis, it took about 7–8 days for Νo. 1 larvae to differentiate into a presoldier, but Νo. 2 larvae molted into 4th instar workers in about 20 days (Maekawa et al. 2012). Thus, the JH titers of Νo. 2 larvae may increase gradually during the latter half of the 3rd larval stage. The fact that several genes (IPPI, FPS, FD1 and FD2) express at high levels on day 4–5 may be related to this delayed increase in the JH titer in Νo. 2 larvae. To examine this possibility, the expression profiles of each gene during the entire 3rd instar larval period should be elucidated.
In the present study, the expression levels of JHAMT and CYP15A1 (Znev_06057) at each point may be used for the prediction of endogenous JH titer changes in 3rd instar larvae of Z. nevadensis. The previous study of this species showed that JHA-induced presoldier differentiation from young instar larvae (3rd or 4th instars) was observed at least 10 days after the JHA treatment (Itano and Maekawa 2008). Although the expression patterns of JHAMT and CYP15A1 (Znev_06057) are different from each other, both genes are highly expressed in the Νo. 1 larva within 5 days after the appearance (Fig. 6d, f), and several days later, a natural presoldier is differentiated (7.3 ± 0.84 days after the appearance; Maekawa et al. 2012). Consequently, the JH biosynthetic activity and endogenous JH titer levels may be rapidly increased much earlier than those after JHA treatment. In general, the JH titer is regulated not only by the JH biosynthetic activity in the corpora allata, but also by the action of other proteins involved in JH degradation and sequestration (Hartfelder 2000). Further research on JH-related genes should be performed to understand the physiological changes via the increased JH titer during presoldier differentiation in natural conditions.
Conclusion
This study clarified that JHAMT and CYP15A1, which are predicted to be involved in the final steps of the JH biosynthetic pathway, are highly expressed during presoldier differentiation. No. 1 larvae of this species received proctodeal trophallactic food from reproductives significantly more often than Νo. 2 larvae (Maekawa et al. 2012). The frequent trophallactic behavior was especially observed 2–4 days after the appearance of Νo. 1 larvae. These periods are nearly equal to those when high expression levels of JH biosynthetic genes are observed in this study. The JH titer and/or JH biosynthetic gene expression is known to be affected by nutritional conditions in M. sexta, Ae. aegypti, D. melanogaster and A. mellifera (Bomtorin et al. 2014; Cymborowski et al. 1982; Noriega 2004; Tu et al. 2005). These results imply a crosstalk between nutritional signals and the JH biosynthetic pathway, and such interactions may also be important mechanisms to regulate presoldier differentiation in Z. nevadensis. Further gene expression and function analyses are to be performed to verify this hypothesis.
References
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Bélles X, Martín D, Piulachs M-D (2005) The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol 50:181–199
Bomtorin AD, Mackert A, Rosa GCC, Moda LM, Martins JR, Bitondi MMG, Hartfelder K, Simões ZLP (2014) Juvenile hormone biosynthesis gene expression in the corpora allata of honey bee (Apis mellifera L.) female castes. PLoS One 9(1):e86923
Cornette R, Gotoh H, Koshikawa S, Miura T (2008a) Juvenile hormone titers and caste differentiation in the damp-wood termite Hodotermopsis sjostedti. J Insect Physiol 54:922–930
Cornette R, Koshikawa S, Miura T (2008b) Histology of the hormone-producing glands in the damp-wood termite Hodotermopsis sjostedti (Isoptera, Termopsidae): a focus on soldier differentiation. Insect Soc 55:407–416
Cornette R, Hayashi Y, Koshikawa S, Miura T (2013) Differential gene expression in response to juvenile hormone analog treatment in the damp-wood termite Hodotermopsis sjostedti (Isoptera, Archotermopsidae). J Insect Physiol 59:509–518
Cymborowski B, Bogus M, Backage NE, Williams CM, Riddiford LM (1982) Juvenile hormone titres and metabolism during starvation-induced supernumerary larval moulting of the tobacco hornworm, Manduca sexta L. J Insect Physiol 28:129–135
Daimon T, Kozaki T, Niwa R et al (2012) Precocious metamorphosis in the juvenile hormone—deficient mutant of the silkworm. Bombyx mori. PLoS Genet 8(3):e1002486
Deligne J, Quennedey A, Blum MS (1981) The enemies and defense mechanisms of termites. In: Hermann HR (ed) Social insects, vol 2. Academic Press, New York, pp 1–76
Feyereisen R (2006) Evolution of insect P450. Biochem Soc Trans 34:1252–1255
Hartfelder K (2000) Insect juvenile hormone: from “status quo” to high society. Braz J Med Biol Res 33:157–177
Hartfelder K, Emlen DJ (2012) Endocrine control of insect polyphenism. In: Gilbert LI (ed) Insect endocrinology. Elsevier, Boston, pp 464–522
Haverty MI (1977) The proportion of soldiers in termite colonies: a list and bibliography. Sociobiology 2:199–216
Haward RW, Haverty MI (1979) Termites and juvenile hormone analogues: a review of methodology and observed effects. Sociobiology 4:269–278
Helvig C, Koener JF, Unnithan GC, Feyereisen R (2004) CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc Natl Acad Sci USA 101:4024–4029
Henderson G (1998) Primer pheromones and possible soldier caste influence on the evolution of sociality in lower termites. In: Meer RKV, Breed MD, Winston ML, Espelie KE (eds) Pheromone communication in social insects. Westview Press, Boulder, pp 314–330
Itano H, Maekawa K (2008) Soldier differentiation and larval juvenile hormone sensitivity in an incipient colony of the damp-wood termite Zootermopsis nevadensis (Isoptera, Termopsidae). Sociobiology 51:1–12
Kinjoh T, Kaneko Y, Itoyama K, Mita K, Hiruma K, Shinoda T (2007) Control of juvenile hormone biosynthesis in Bombyx mori: cloning of the enzymes in the mevalonate pathway and assessment of their developmental expression in the corpora allata. Insect Biochem Mol Biol 37:808–818
Korb J, Hoffmann K, Hartfelder K (2009) Endocrine signatures underlying plasticity in postembryonic development of lower termite, Cryptotermes secundus (Kalotermitidae). Evol Dev 11:269–277
Koshikawa S, Cornette R, Hojo M, Maekawa K, Matsumoto T, Miura T (2005) Screening of genes expressed in developing mandibles during soldier differentiation in the termite Hodotermopsis sjostedti. FEBS Lett 579:1365–1370
Lo N, Eggeleton P (2011) Termite phylogenetics and co-cladogenesis with symbionts. In: Bignell DE, Roisin Y, Lo N (eds) Biology of termite: a modern synthesis. Springer, Heidelberg, pp 27–50
Maekawa K, Ishitani K, Gotoh H, Cornette R, Miura T (2010) Juvenile hormone titre and vitellogenin gene expression related to ovarian development in primary reproductives compared with nymphs and nymphoid reproductives of the termite Reticulitermes speratus. Physiol Entomol 35:52–58
Maekawa K, Nakamura S, Watanabe D (2012) Termite soldier differentiation in incipient colonies is related to parental proctodeal trophallactic behavior. Zool Sci 29:213–217
Maekawa K, Hayashi Y, Lee T, Lo N (2014) Presoldier differentiation of Australian termite species induced by juvenile hormone analogues. Austral Entomol 53:138–143
Marchal E, Zhang J, Badisco L, Verlinden H, Hult EF, Wielendaele PV, Yagi KJ, Tobe SS, Broeck JV (2011) Final steps in juvenile hormone biosynthesis in the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol 41:219–227
Masuoka Y, Miyazaki S, Saiki R, Tsutomu T, Maekawa K (2013) High Laccase2 expression is likely involved in the formation of specific cuticular structures during soldier differentiation of the termite Reticulitermes speratus. Arthropod Struct Dev 42:469–475
Miura T (2005) Developmental regulation of caste-specific characters in social-insect polyphenism. Evol Dev 7:122–129
Miura T, Scharf ME (2011) Molecular basis underlying caste differentiation in termites. In: Bignell DE, Roisin Y, Lo N (eds) Biology of termite: a modern synthesis. Springer, Heidelberg, pp 211–253
Nijhout HF (1999) Control mechanisms of polyphenic development in insects. BioSience 49:181–192
Nijhout HF (2003) Development and evolution of adaptive polyphenisms. Evol Dev 5:9–18
Nijhout HF, Wheeler DE (1982) Juvenile hormone and the physiological basis of insect polymorphisms. Q Rev Biol 57:109–133
Noirot C, Pasteels JM (1987) Ontogenetic development and evolution of the worker caste in termites. Experientia 43:851–952
Noriega FG (2004) Nutritional regulation of JH synthesis: a mechanism to control reproductive maturation in mosquitoes? Insect Biochem Mol Biol 34:687–693
Noriega FG, Ribeiro JMC, Koener JF, Valenzuela JG, Hernandez-Martinez S, Pham VM, Feyereisen R (2006) Comparative genomics of insect hormone biosynthesis. Insect Biochem Mol Biol 36:366–374
Ogino K, Hirono Y, Matsumoto T, Ishikawa H (1993) Juvenile hormone analogue, S-31183, causes a high level induction of presoldier differentiation in the Japanese damp-wood termite. Zool Sci 10:361–366
Park IY, Raina AK (2004) Juvenile hormone III titers and regulation of soldier caste in Coptotermes formosanus (Isoptera: Rhinotermitidae). J Insect Physiol 50:561–566
Roisin Y (2000) Diversity and evolution of caste patterns. In: Abe T, Bignell DE, Higashi M (eds) Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Press, Dortrecht, pp 95–119
Saiki R, Gotoh H, Toga K, Miura T, Maekawa K (2015) High juvenile hormone titer and abdominal activation of the JH signaling may induce reproduction of termite neotenics. Insect Mol Biol 24(4):432–441
Scharf ME, Ratliff CR, Hoteling JT, Pittendrigh BR, Bennett GW (2003) Caste differentiation responses of two sympatric Reticulitermes termite species to juvenile hormone homologs and synthetic juvenoids in two laboratory assays. Insectes Soc 50:346–354
Shinoda T, Itoyama K (2003) Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci USA 100:11986–11991
Tamura K, Peterson D, Perterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tarver MR, Coy MR, Scharf ME (2012) Cyp15F1: a novel cytochrome P450 gene linked to juvenile hormone-dependent caste differentiation in the termite Reticulitermes flavipes. Arch Insect Biochem Physiol 80:92–108
Terrapon N, Li C, Robertson HM et al (2014) Molecular traces of alternative social organization in a termite genome. Nat Commun 5:3636
Toga K, Hojo M, Miura T, Maekawa K (2009) Presoldier induction by a juvenile hormone analog in the nasute termite Nasutitermes takasagoensis (Isoptera: Termitidae). Zool Sci 26:382–388
Toga K, Hojo M, Miura T, Maekawa K (2012) Expression and function of a limb-patterning gene Distal-less in the soldier-specific morphogenesis in the nasute termite Nasutitermes takasagoensis. Evol Dev 4:286–295
Toga K, Saiki R, Maekawa K (2013) Hox gene deformed is likely involved in mandibular regression during presoldier differentiation in the nasute termite Nasutitermes takasagoensis. J Exp Zool 9999:1–8
Treiblmayr K, Pascual N, Piulachs M-D, Keller T, Bélles X (2006) Juvenile hormone titer versus juvenile hormone synthesis in female nymphs and adults of the German cockroach (Blattella germanica). J Insect Sci 6:43
Tsuchiya M, Watanabe D, Maekawa K (2008) Effect on mandibular length of juvenile hormones and regulation of soldier differentiation in the termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Appl Entomol Zool 43:307–314
Tu M-P, Yin C-M, Tatar M (2005) Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen Comp Endocrinol 142:347–356
Ueda H, Shinoda T, Hiruma K (2009) Spatial expression of the mevalonate enzymes involved in juvenile hormone biosynthesis in the corpora allata in Bombyx mori. J Insect Physiol 55:798–804
Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35:W71–W74
Vandesompele J, Preter KD, Pattyn F, Poppe B, Roy NV, Paepe AD, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: research0034
Watanabe D, Maekawa K (2012) Relationships between frontal-gland formation and mandibular modification during JH III-induced presoldier differentiation in the termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Entomol Sci 15:56–62
Watanabe D, Gotoh H, Miura T, Maekawa K (2011) Soldier presence suppresses presoldier differentiation through a rapid decrease of JH in the termite Reticulitermes speratus. J Insect Physiol 57:791–795
Watanabe D, Gotoh H, Miura T, Maekawa K (2014) Social interactions affecting caste development through physiological actions in termites. Front Physiol 5:127
Werck-Reichhart D, Feyereisen R (2000) Cytochromes P450: a success story. Genome Biol 1(6): reviews3003.1–3009.9
Wilson EO (2000) Sociobiology: the new synthesis, Twenty-fifth anniversary. The Belknap Press of Harvard University Press, Cambridge
Acknowledgments
We are grateful to Toru Miura and Hiroki Gotoh (Hokkaido University) for advice on and use of the HPLC–MS system. We also thank Ryota Saiki, Shutaro Hanmoto, Souichiro Kawamura and Ryutaro Suzuki (University of Toyama) for help during both the field and laboratory work. This study was supported in part by Grants-in-Aid for Scientific Research (nos. 24570022 and 25128705 to KM) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yaguchi, H., Masuoka, Y., Inoue, T. et al. Expressions of juvenile hormone biosynthetic genes during presoldier differentiation in the incipient colony of Zootermopsis nevadensis (Isoptera: Archotermopsidae). Appl Entomol Zool 50, 497–508 (2015). https://doi.org/10.1007/s13355-015-0358-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-015-0358-3