Abstract
Mastitis is a major source of economic loss in dairy herds. The objective of this research was to evaluate the association between genotypes within SLC11A1 and CXCR1 candidate genes and clinical mastitis in Holstein dairy cattle using the selective genotyping method. The data set contained clinical mastitis records of 3,823 Holstein cows from two Holstein dairy herds located in two different regions in Iran. Data included the number of cases of clinical mastitis per lactation. Selective genotyping was based on extreme values for clinical mastitis residuals (CMR) from mixed model analyses. Two extreme groups consisting of 135 cows were formed (as cases and controls), and genotyped for the two candidate genes, namely, SLC11A1 and CXCR1, using polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP) and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), respectively. Associations between single nucleotide polymorphism (SNP) genotypes with CMR and breeding values for milk and protein yield were carried out by applying logistic regression analyses, i.e. estimating the probability of the heterogeneous genotype in the dependency of values for CMR and breeding values (BVs). The sequencing results revealed a novel mutation in 1139 bp of exon 11 of the SLC11A1 gene and this SNP had a significant association with CMR (P < 0.05). PCR-RFLP analysis leads to three banding patterns for CXCR1c.735C>G and these genotypes had significant relationships with CMR. Overall, the results showed that SLC11A1 and CXCR1 are valuable candidate genes for the improvement of mastitis resistance as well as production traits in dairy cattle populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mastitis is one of the most predominant diseases in dairy cattle herds and leads to economic losses in the dairy farms. Mastitis as an inflammation of the mammary gland is caused by a wide range of invading pathogens. Many candidate genes for mastitis resistance are being studied in a variety of populations to understand the molecular markers (Shivanand et al. 2011). Genes associated with neutrophil functions are potential genetic markers for mastitis (Paape et al. 2000), such as chemokine (C-X-C motif) receptor 1 (CXCR1). The protein encoded by the CXCR1 gene is a member of the family of G-protein-coupled receptor. This protein is a receptor for interleukin 8 (IL8). It binds to IL8 with high affinity and changes the signal through a G-protein-activated second messenger system. CXCR1 and CXCR2, as chemokine receptors present on neutrophil surfaces, are required for extreme neutrophil function during infection (Murphy and Tiffany 1991). Grosse et al. (1999) mapped the CXCR1 gene on BTA (Bos taurus autosome) 2, approximately 90.3 cM from the centromere. Examination of the CXCR1 sequence in Holstein and Jersey dairy cattle revealed one additional single nucleotide polymorphism (SNP) within a 311-bp fragment of the coding region not present in the beef cattle population. The non-synonymous (G to C) SNP at c.735 results in the glutamine to histidine substitution at amino acid 245. The amino acid is located within the third intracellular loop of CXCR1, an important region involved in mediating calcium signalling and mobilisation, as well as G-protein binding. Hence, the bovine CXCR1 gene is a potential candidate gene for mastitis resistance, as CXCR1 is a critical component during neutrophil migration to the mammary gland during mastitis incidence (Youngerman et al. 2004b). However, controversial findings were reported for the effect of this gene on clinical mastitis in the US Holstein Friesians (Youngerman et al. 2004b), in Canadian Holstein cows (Leyva-Baca et al. 2008) and in German Holstein cattle (Goertz et al. 2009). Because of these disputes, a more thorough understanding of SNPs present in and near the bovine CXCR1 gene is required.
The solute carrier family 11 a1 (SLC11A1), already known as natural resistance-associated macrophage protein (NRAMP1), plays an important role in innate immunity, favouring bacterial killing by macrophages, also its influence on adaptive immunity (Vidal et al. 1995). The SLC11A1 gene encodes a multi-pass membrane protein. The protein functions as a divalent transition metal (iron and manganese) transporter involved in iron metabolism and host resistance to specific pathogens. It is reported that mutations in this gene have been related with susceptibility to infectious diseases such as tuberculosis and leprosy, and inflammatory diseases. The bovine SLC11A1 gene has also been found to be associated with natural resistance against brucellosis in cattle and buffalo (Capparelli et al. 2007; Martínez et al. 2008). This gene maps on chromosome 2 and it composed of 39 exons and 38 introns.
Selection of disease-resistant cattle based on genetic markers related with mastitis would not only enable the development of a more resistant population but would also allow closer examination of mechanisms that contribute to differential disease resistance and could ultimately lead to novel therapeutic strategies against mastitis and other inflammatory diseases (Youngerman et al. 2004a).
As the two CXCR1 and SLC11A1 genes play a key role in mastitis resistance, the objectives of this study were to identify candidate SNPs within a fragment of the bovine CXCR1 and SLC11A1 genes to determine the frequencies of SNPs within dairy Holstein cattle and their association with clinical mastitis.
Materials and methods
Data
The clinical mastitis (CM) records were collected from 3,823 Holstein dairy cattle distributed within two Holstein herds located in two different regions (Tehran and Isfahan) in Iran during 2008 to 2010. CM was diagnosed as described by Gernand et al. (2012), i.e. an obvious infection of the udder, including dolor, rubor, change of colour and, additionally, flakes in the milk. When counting cases of CM, an interval of 5 days was required to consider an occurrence of CM as a new case of CM (Hinrichs et al. 2005; Gernand et al. 2012). More description about the data structure is presented by Bagheri et al. (2013). At the end, 1,647 CM cases were recorded. The pool of cows used for selective genotyping, i.e. the most resistant and the most susceptible group for CM, was extracted based on values for clinical mastitis residuals (CMR). For analysis of variance, the number of CM cases per lactation was analysed by applying the procedure GLM in SAS version 9.1 (SAS 2004), using the following statistical model:
where:
- Yijk :
-
No. of CM cases per lactation for the kth cow
- Hi :
-
Fixed effect of the ith herd-year
- Lj :
-
Fixed effect of the jth lactation
- Xk :
-
305-day lactation milk yield of cow k in previous lactation
- β:
-
Linear regression of the no. of CM cases per lactation on 305-day milk yield
- eijk :
-
Random residual effect of CM cases
305-day lactation milk yield was considered in the statistical model because some authors, e.g. Fleischer et al. (2001), found pronounced associations between 305-day milk yield in previous lactation and occurrence of health disorders in the current lactation. Furthermore, a cow’s production level may encourage breeders for the application of preferential treatment improving both the cow’s health and longevity. Based on values for CMR, two extreme groups including 135 cows per group were extracted for selective genotyping (Bagheri et al. 2013). The significant difference between the genotypes in case and control groups was explored by a two-sample t-test with an alpha level of 0.05.
Genes genotyping
Genomic DNA extraction was carried out by the improved salting out method (Miller et al. 1988). DNA concentration and DNA quality were assessed by microplate spectrophotometer light and 1 % agarose gel electrophoresis. DNA was diluted to 50 ng/μL and stored in the refrigerator at −20 °C. Forward and reverse primers to amplify a 221-bp fragment of exon 11 of the SLC11A1 gene were used according to Zhang et al. (2009), and to amplify a 311-bp fragment of the bovine CXCR1 gene, were based on the study by Goertz et al. (2009). Polymerase chain reactions (PCRs) were carried out in a total volume of 25 μL solution containing 50 ng templates DNA, 2.5 × buffer (Tris-HCl 100 mmol/L, pH 8.3; KCl 500 mmol/L), 1.0 μmol/L primers, 2.0 mmol/L MgCl2, 1.0 mmol/L dNTPs and 0.5 U Taq DNA polymerase. The reaction conditions of the PCR for SLC11A1 were: an initial DNA denaturing of 95 °C for 5 min, followed by 35 cycles of 94 °C for 40 s, 61 °C for 40 s and 72 °C for 30 s, and a final extension at 72 °C for 5 min. For CXCR1, an initial denaturation at 95 °C for 2 min, followed by 35 cycles of denaturing at 95 °C for 1 min, 58.5 °C for 30 s and 68 °C for 1 min, and a final extension at 72 °C for 5 min. The PCR products were checked by agarose gel electrophoresis using 2 % agarose gel in 1× TAE buffer at 100 V for 40 min. The amplified products were visualized using a UV transilluminator.
The polymorphisms at exon 11 of the SLC11A1 gene were investigated by polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP). A total of 6.0 μL PCR product of exon 11 of the SLC11A1 gene was mixed with 12 μL of the denaturation solution (50 mmol/L NaOH, 1 mmol/L EDTA) and 1 μL of the loading buffer containing 0.25 % bromophenol blue and 0.25 % xylene cyanol, denatured for 10 min at 95 °C and rapidly chilled in an ice block for 10 min. The samples were electrophoresed using a 12 % sodium dodecyl sulphate polyacrylamide gel. A thermostatically controlled refrigerated circulator was used to maintain constant temperature (4 °C) of the gels. The gels were run in the following conditions: 250 V, 40 mA, 10 min (pre-electrophoresis) and 150 V, 24 mA, for 4 h. The gels were then stained by silver stain. Fifteen randomly chosen samples of PCR products from homozygote and heterozygote cows were utilised in sequencing. Analyses of sequences were done using Big Dye Terminator v3.1 Cycle Sequencing Kit chemistry on an ABI prism 3130 Genetic Analyzer (USA).
Polymorphisms of CXCR1c.735C>G were detected using polymerase chain reaction-restriction fragment length polymorphisms (PCR-RFLP). In brief, the product of the PCR was digested using a 20-μL reaction containing 1.5 × buffer L, and 5 U Bme1580I and 300 ng PCR products were used at a constant temperature (37 °C) for 12 h. The digested products were electrophoresed using a 3 % agarose gel in 1× TAE buffer at 90 V for 2 h. The patterns of digested products were visualised under a UV transilluminator.
Association analyses
For the estimation of SNP effects, we followed the approach proposed by Henshall and Goddard (1999) for selective genotyping data. At first, logistic regression analyses were carried out using SAS’ GLIMMIX macro (Schabenberger 2007). The statistical model for estimating the probability of e.g. a genotype GC versus a genotype CC was defined as follows:
- πr :
-
Probability of the genotype GC of cow r
- a:
-
Intercept
- Yr :
-
Estimated breeding value (EBV) for production traits or CMR
- b:
-
Linear regression of genotype GC on EBV or CMR
Test of significance of linear regression coefficients b was based on sum of square type I tests (Wald-type tests), as implemented in the GLIMMIX macro (König et al. 2005). In a second step, the contrast α of the heterozygous genotype to the homozygous genotype, e.g. the effect of genotype GC in contrast to genotype CC, was estimated as described by Henshall and Goddard (1999) or by Sharma et al. (2006) using the equation:
where σ 2 X denotes the variance of EBV or CMR in the unselected base population of 3,823 cows.
Both loci analysed were bi-allelic, resulting in three different genotypes. Following the example given above, both homozygous genotypes (GG and CC) were contrasted to the heterozygous genotype GC in two consecutive runs, i.e. first contrasting GC to GG and, in a second run, contrasting GC to CC.
Results
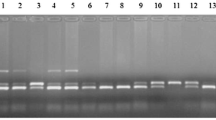
A polymorphism was identified within a 221-bp fragment of exon 11 of the SLC11A1 gene using PCR-SSCP (Fig. 1). The SSCP analysis and sequencing results also verified a new mutation at position 1139 C>G. Blast of different genotypes of the SLC11A1 gene is shown in Fig. 2. For the CXCR1 gene, G to C polymorphism was recognised using PCR-RFLP, with which the GG genotype was identified by the presence of 19- and 292-bp fragments. The CC genotype was recognised with the occurrence of 19-, 103- and 189-bp fragments. The heterozygous (GC) genotype was detected by the existence of 19-, 103-, 189- and 292-bp fragments.
The allele and genotype frequencies for both genes are given in Table 1. Values from the Chi-square (χ2) test for genotype frequencies indicate that all the studied loci deviate from Hardy–Weinberg equilibrium significantly (P < 0.05). Frequencies of homozygous genotype in both groups (susceptible and resistance cows) were almost the same for the SLC11A1 gene. However, the heterozygous genotype in the susceptible group had a higher frequency. At this locus, the GG genotype was rare. The CC genotype had the highest frequency in the resistant group, while the heterozygous genotype had the greatest frequency in the susceptible group. In the CXCR1 gene, the frequency of the C allele was significantly higher in the susceptible group, whereas the G allele had a higher frequency in the resistant group.
Differences in EBVs for production traits and for CMR when comparing the heterozygous genotype to both homozygous genotypes of the SNPs SLC11A1g.1139 C>G and CXCR1c.735 C>G are given in Table 2. The difference between genotypes CG and GG of SLC11A1 was not significant for production and CMR traits, whereas a significant difference was observed between genotypes CG and CC for the CMR trait. The CXCR1 gene has a significant relationship with resistance to clinical mastitis. It seems that the G allele of the CXCR1 gene is associated with resistance to clinical mastitis. The results of association were also in agreement with our expectation from the heterozygous frequency.
Discussion
The results of sequencing indicated that the SSCP difference in SLC11A1 was attributed to a novel mutation in g.1139 (C>G). Recently, three polymorphisms (g.5828 C>T, g.7325 G>A, g.7571 C>G) within the coding regions of the SLC11A1 gene were identified in Bos taurus and Bos indicus breeds by SSCP and DNA sequencing (Martínez et al. 2008), and an SNP at g.1066 near the SNP in our study has already been detected by Zhang et al. (2009). Martínez et al. (2008) explained that a moderate variation in the coding sequence of the SLC11A1 gene would be a sign of selection pressure on the SLC11A1 gene. Zhang et al. (2009) used the somatic cell score (SCS) as an indicator of the udder health, but, here, we studied the effects of these candidate genes on clinical mastitis incidence in a case–control study. This study followed the procedure of Sharma et al. (2006), with the difference that, in the current study, the contrast between heterozygous with both homozygous genotypes were surveyed. Also, in a previous study, the selective genotyping procedure in combination with logistic regression analysis introduced some useful markers in the studied genes for clinical mastitis (Bagheri et al. 2013).
In addition to the CXCR1c.735C>G position as a quantitative trait locus for SCS and its function in the immune response, the CXCR1 chemokine receptor gene located on Bos taurus autosome 2 is an auspicious candidate gene for udder health in dairy cattle. Chen et al. (2011), using the PCR-SSCP technique, identified four SNPs, −1830A>G, −1768T>A, −344T>C and 783C>A, at the 5′ upstream and coding region of CXCR1. Rambeaud and Pighetti (2007) compared bovine genome sequences with the human CXCR1 and CXCR2 sequences and revealed the gene CXCR2 formerly associated with actually CXCR1. In an association analysis study, Chen et al. (2011) reported that the genotypes −1830AA, −1768TT and −344TT of the CXCR1 gene were correlated significantly with SCS. Beecher et al. (2010) reported that CXCR1 had a significant association (P < 0.05) with fat yield and no significant association with subclinical mastitis. Galvão et al. (2011) demonstrated that CXCR1c.735C>G genotypes are associated with the incidence rate of clinical mastitis in Holstein cows. In the latter study, cows with the GG genotype had an increased incidence rate of clinical mastitis compared with CC and GC cows. These findings were different from our study. We observed a decrease in CMR and an increase in milk and protein yield for GG cows. Our observations were in line with a recent work on dairy cows (Verbeke et al. 2014). In the German Holstein Friesian cattle, the association between the SNP at position c.735C>G within the CXCR1 gene and SCS was not statistically significant (Goertz et al. 2009). Further researches with a large number of samples are required in order to confirm the association between this mutation and SCS.
As mentioned by other studies, MHC genes such as CXCR1 should be considered in selection strategies for improving udder health traits in dairy cattle populations. The selection of specific alleles of important genes would improve udder health but the correlated response for other economic traits should also be considered. In most breeding programs for Holstein cattle around the world, yield traits have great importance, including 50 % emphasis in combination with other breeding goals (Miglior et al. 2005). According to the quantitative genetics methodology, the genetic correlation between protein yield or milk production and resistance to clinical mastitis are antagonistic (Gernand et al. 2012). In a selection experiment in Scandinavia in which sire selection was done based on high breeding values for production traits, parallel to breeding values for these traits, the clinical mastitis increased too (Heringstad et al. 2007).
Simultaneous responses of two traits with unfavourable genetic correlation result in the decrease of economic weights of two traits. Although lack of sufficient phenotypic data for these traits prevent considering this idea in breeding experiments, direct selection of genes or markers which carry favourable alleles for two traits in small selection groups could be practical in dairy cattle breeding programmes. Such ideas for animal genotyping is introduced by Moe et al. (2009), which were based on selection against defective sires when considering fertility.
Conclusions
The sequencing results revealed a novel mutation in 1139 bp of exon 11 of the SLC11A1 gene. In contrast to Zhang et al. (2009), this single nucleotide polymorphism (SNP) has a significant relationship with clinical mastitis residuals (CMR). Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis leads to three banding patterns for CXCR1c.735C>G and these genotypes had significant relationships with CMR. Considering the well-known unfavourable relationship between clinical mastitis and production traits, direct selection of the SLC11A1 and CXCR1 genes would have potential application in the improvement of dairy cattle breeding programmes.
References
Bagheri M, Miraie-Ashtiani R, Moradi-Sharhrbabak M, Nejati-Javaremi A, Pakdel A, von Borstel UU, Pimentel ECG, König S (2013) Selective genotyping and logistic regression analyses to identify favorable SNP-genotypes for clinical mastitis and production traits in Holstein dairy cattle. Livest Sci 151:140–151
Beecher C, Daly M, Childs S, Berry DP, Magee DA, McCarthy TV, Giblin L (2010) Polymorphisms in bovine immune genes and their associations with somatic cell count and milk production in dairy cattle. BMC Genet 11:99
Capparelli R, Alfano F, Amoroso MG, Borriello G, Fenizia D, Bianco A, Roperto S, Roperto F, Iannelli D (2007) Protective effect of the Nramp1 BB genotype against Brucella abortus in the water buffalo (Bubalus bubalis). Infect Immun 75:988–996
Chen R, Yang Z, Ji D, Mao Y, Chen Y, Zhang Y, Hamza, Wang X, Li Y (2011) SNPs of CXCR1 gene and its associations with somatic cell score in Chinese Holstein Cattle. Anim Biotechnol 22:133–142
Fleischer RC, Perry EA, Muralidharan K, Stevens EE, Wemmer CM (2001) Phylogeography of the Asian elephant (Elephas maximus) based on mitochondrial DNA. Evolution 55:1882–1892
Galvão KN, Pighetti GM, Cheong SH, Nydam DV, Gilbert RO (2011) Association between interleukin-8 receptor-α (CXCR1) polymorphism and disease incidence, production, reproduction, and survival in Holstein cows. J Dairy Sci 94:2083–2091
Gernand E, Rehbein P, von Borstel UU, König S (2012) Incidences of and genetic parameters for mastitis, claw disorders, and common health traits recorded in dairy cattle contract herds. J Dairy Sci 95:2144–2156
Goertz I, Baes C, Weimann C, Reinsch N, Erhardt G (2009) Association between single nucleotide polymorphisms in the CXCR1 gene and somatic cell score in Holstein dairy cattle. J Dairy Sci 92:4018–4022
Grosse WM, Kappes SM, Laegreid WW, Keele JW, Chitko-McKown CG, Heaton MP (1999) Single nucleotide polymorphism (SNP) discovery and linkage mapping of bovine cytokine genes. Mamm Genome 10:1062–1069
Henshall JM, Goddard ME (1999) Multiple-trait mapping of quantitative trait loci after selective genotyping using logistic regression. Genetics 151(2):885–894
Heringstad B, Klemetsdal G, Steine T (2007) Selection responses for disease resistance in two selection experiments with Norwegian red cows. J Dairy Sci 90:2419–2426
Hinrichs D, Stamer E, Junge W, Kalm E (2005) Genetic analyses of mastitis data using animal threshold models and genetic correlation with production traits. J Dairy Sci 88:2260–2268
König S, Sharifi AR, Wentrot H, Landmann D, Eise M, Simianer H (2005) Genetic parameters of claw and foot disorders estimated with logistic models. J Dairy Sci 88:3316–3325
Leyva-Baca I, Schenkel F, Martin J, Karrow NA (2008) Polymorphisms in the 5′ upstream region of the CXCR1 chemokine receptor gene, and their association with somatic cell score in Holstein cattle in Canada. J Dairy Sci 91:407–417
Martínez R, Dunner S, Barrera G, Cañon J (2008) Novel variants within the coding regions of the SLC11A1 gene identified in Bos taurus and Bos indicus breeds. J Anim Breed Genet 125:57–62
Miglior F, Muir BL, Van Doormaal BJ (2005) Selection indices in Holstein cattle of various countries. J Dairy Sci 88:1255–1263
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Moe M, Lien S, Aasmundstad T, Meuwissen THE, Hansen MHS, Bendixen C, Grindflek E (2009) Association between SNPs within candidate genes and compounds related to boar taint and reproduction. BMC Genet 10:32
Murphy PM, Tiffany HL (1991) Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science 253:1280–1283
Paape MJ, Shafer-Weaver K, Capuco AV, Van Oostveldt K, Burvenich C (2000) Immune surveillance of mammary tissue by phagocytic cells. Adv Exp Med Biol 480:259–277
Rambeaud M, Pighetti GM (2007) Differential calcium signaling in dairy cows with specific CXCR1 genotypes potentially related to interleukin-8 receptor functionality. Immunogenetics 59:53–58
SAS (2004) SAS/STAT 9.1 user’s guide. SAS Institute Inc., Cary
Schabenberger O (2007) Growing up fast: SAS 9.2 enhancements to the GLIMMIX procedure. SAS Global Forum, Citeseer
Sharma BS, Jansen GB, Karrow NA, Kelton D, Jiang Z (2006) Detection and characterization of amplified fragment length polymorphism markers for clinical mastitis in Canadian Holsteins. J Dairy Sci 89:3653–3663
Shivanand DM, Ahlawat SPS, Bhusan B, Tiwari AK, Sonawane A, Kumar P, Inamdar B, Dutt T (2011) PCR-SSCP and sequencing of CXCR2 receptor gene in Vrindavani cattle. J Adv Vet Res 1:52–56
Verbeke J, Van Poucke M, Peelman L, Piepers S, De Vliegher S (2014) Associations between CXCR1 polymorphisms and pathogen-specific incidence rate of clinical mastitis, test-day somatic cell count, and test-day milk yield. J Dairy Sci 97(12):7927–7939
Vidal S, Tremblay ML, Govoni G, Gauthier S, Sebastiani G, Malo D, Skamene E, Olivier M, Jothy S, Gros P (1995) The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med 182:655–666
Youngerman SM, Saxton AM, Pighetti GM (2004a) Novel single nucleotide polymorphisms and haplotypes within the bovine CXCR2 gene . Immunogenetics 56:355–359
Youngerman SM, Saxton AM, Oliver SP, Pighetti GM (2004b) Association of CXCR2 polymorphisms with subclinical and clinical mastitis in dairy cattle. J Dairy Sci 87:2442–2448
Zhang CL, Wang YH, Chen H, Gu CW, Fang XT (2009) SLC11A1 gene polymorphisms are not associated to somatic cell score and milk yield in Chinese Holstein. Vet Immunol Immunopathol 127:389–392
Acknowledgements
Data and blood samples were kindly obtained from two dairy cattle farms (Foka and Taliseh). The authors are thankful for the help of these farms’ owners and staff.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Maciej Szydlowski
Rights and permissions
About this article
Cite this article
Bagheri, M., Moradi-Sharhrbabak, M., Miraie-Ashtiani, R. et al. Case–control approach application for finding a relationship between candidate genes and clinical mastitis in Holstein dairy cattle. J Appl Genetics 57, 107–112 (2016). https://doi.org/10.1007/s13353-015-0299-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13353-015-0299-0